Skin-Whitening and Antiwrinkle Proprieties of Maackia amurensis Methanolic Extract Lead Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Experimental Procedures
2.3. Plant Materials and Sample Preparation
2.4. Cell Culture
2.5. Cell Viability
2.6. Melanin Content Assay
2.7. Western Blot Analysis
2.8. Soluble Collagen Assay
2.9. Extraction and Isolation of Compounds
2.10. HPLC Analysis
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of M. amurensis Branch Extract on Cell Viability in B16F1 Cells
3.2. Effect of M. amurensis Branch Extract on Melanin Contents in B16F1 Cells
3.3. Effect of M. amurensis Branch Extract on Melanogenic Protein Expression in B16F1 Cells
3.4. Effect of M. amurensis Branch Extract on Cell Viability in CCD-986Sk Cells
3.5. Effect of M. amurensis Branch Extract on Soluble Collagen in CCD-986sk Cells
3.6. Effect of M. amurensis Branch Extract on MMP-1 Expression in CCD-986sk Cells
3.7. Structural Elucidation of Isolated Compounds
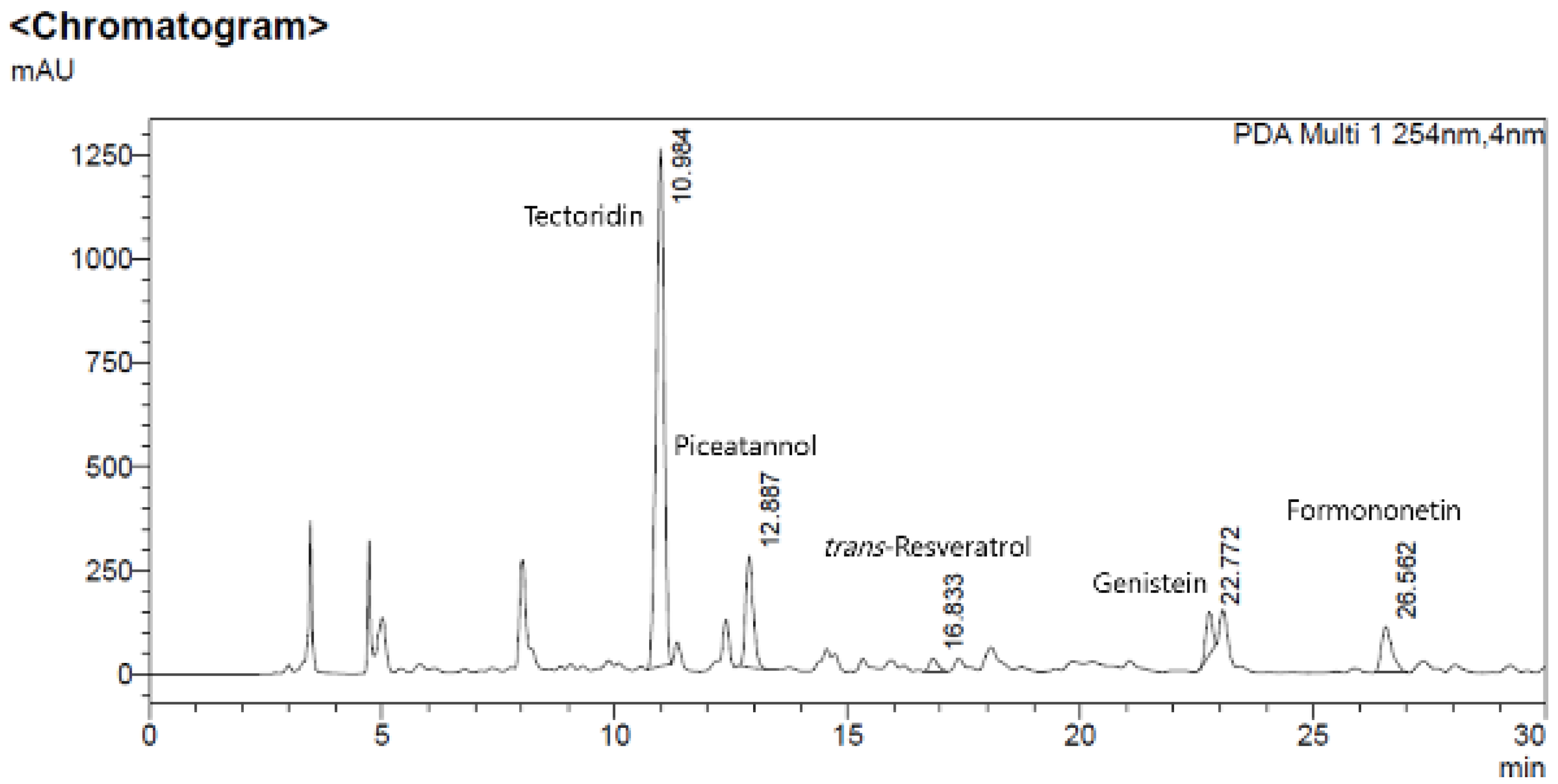
3.8. HPLC Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.-W.; Kim, H.-A.; Yoon, H.-R.; Jeon, T.-Y. Establishment of seaweed fermentation process for cosmetic material research. J. Korea Acad.-Ind. Coop. Soc. 2019, 20, 14–19. [Google Scholar]
- Chermahini, S.H.; Majid, F.A.A.; Sarmidi, M.R. Cosmeceutical value of herbal extracts as natural ingredients and novel technologies in anti-aging. J. Med. Plants Res. 2011, 5, 3074–3077. [Google Scholar]
- Kim, H.-S. A Study on the Trends of the Natural UV Protection Materials Related to Skin Beauty. J. Korean Appl. Sci. Technol. 2021, 38, 107–117. [Google Scholar]
- Rodrigues, F.; de la Luz Cádiz-Gurrea, M.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.; Carretero, A.S. Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 393–427. [Google Scholar]
- Lim, Y.-J.; Lee, E.H.; Kang, T.H.; Ha, S.K.; Oh, M.S.; Kim, S.M.; Yoon, T.-J.; Kang, C.; Park, J.-H.; Kim, S.Y. Inhibitory effects of arbutin on melanin biosynthesis of α-melanocyte stimulating hormone-induced hyperpigmentation in cultured brownish guinea pig skin tissues. Arch. Pharm. Res. 2009, 32, 367–373. [Google Scholar] [CrossRef]
- Liu-Smith, F.; Poe, C.; Farmer, P.J.; Meyskens, F.L., Jr. Amyloids, melanins and oxidative stress in melanomagenesis. Exp. Dermatol. 2015, 24, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Bhawan, J.; Gonzalez-Serva, A.; Nehal, K.; Labadie, R.; Lufrano, L.; Thorne, E.G.; Gilchrest, B.A. Effects of tretinoin on photodamaged skin: A histologic study. Arch. Dermatol. 1991, 127, 666–672. [Google Scholar] [CrossRef]
- Jin, M.L.; Park, S.Y.; Kim, Y.H.; Park, G.; Son, H.-J.; Lee, S.-J. Suppression of α-MSH and IBMX-induced melanogenesis by cordycepin via inhibition of CREB and MITF, and activation of PI3K/Akt and ERK-dependent mechanisms. Int. J. Mol. Med. 2012, 29, 119–124. [Google Scholar]
- Jeon, N.-J.; Kim, Y.-S.; Kim, E.-K.; Dong, X.; Lee, J.-W.; Park, J.-S.; Shin, W.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Inhibitory effect of carvacrol on melanin synthesis via suppression of tyrosinase expression. J. Funct. Foods 2018, 45, 199–205. [Google Scholar] [CrossRef]
- Kameyama, K.; Sakai, C.; Kuge, S.; Nishiyama, S.; Tomita, Y.; Ito, S.; Wakamatsu, K.; Hearing, V.J. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment Cell Res. 1995, 8, 97–104. [Google Scholar] [CrossRef]
- Jung, S.K.; Lee, K.W.; Kim, H.Y.; Oh, M.H.; Byun, S.; Lim, S.H.; Heo, Y.-S.; Kang, N.J.; Bode, A.M.; Dong, Z. Myricetin suppresses UVB-induced wrinkle formation and MMP-9 expression by inhibiting Raf. Biochem. Pharmacol. 2010, 79, 1455–1461. [Google Scholar] [CrossRef] [Green Version]
- Buechner, N.; Schroeder, P.; Jakob, S.; Kunze, K.; Maresch, T.; Calles, C.; Krutmann, J.; Haendeler, J. Changes of MMP-1 and collagen type Iα1 by UVA, UVB and IRA are differentially regulated by Trx-1. Exp. Gerontol. 2008, 43, 633–637. [Google Scholar] [CrossRef]
- Shim, M.; Bae, J.Y.; Lee, Y.J.; Ahn, M.J. Tectoridin from Maackia amurensis modulates both estrogen and thyroid receptors. Phytomedicine 2014, 21, 602–606. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Xia, M.-Y.; Wang, Z.-h.; Wang, W.-N.; Cui, Z. Cytotoxic prenylated flavonoids from the stem bark of Maackia amurensis. Chem. Pharm. Bull. 2009, 57, 302–306. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.M.; Jang, H.-J.; Kim, W.J.; Kang, M.-G.; Baek, S.C.; Lee, J.P.; Park, D.; Oh, S.-R.; Kim, H. Calycosin and 8-O-methylretusin isolated from Maackia amurensis as potent and selective reversible inhibitors of human monoamine oxidase-B. Int. J. Biol. Macromol. 2020, 151, 441–448. [Google Scholar] [CrossRef]
- Li, X.; Wang, D.; Cui, Z. A new cytisine-type alkaloid from the stem bark of Maackia amurensis. Nat. Prod. Res. 2010, 24, 1499–1502. [Google Scholar] [CrossRef]
- Kim, G.-S.; Chang, J.-P.; Doh, E.-S.; Kil, K.-J.; Yoo, J.-H. Stem bark of Maackia amurensis extract according to extraction solvent. Korea J. Herbol. 2016, 31, 43–48. [Google Scholar] [CrossRef]
- Radziejewska, I.; Borzym-Kluczyk, M.; Leszczynska, K. Lotus tetragonolobus, Ulex europaeus, Maackia amurensis, and Arachis hypogaea (peanut) lectins influence the binding of Helicobacter pylori to gastric carbohydrates. Adv. Clin. Exp. Med. 2018, 27, 807–811. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chou, Y.-C.; Wu, C.-Y.; Chang, T.-M. [8]-Gingerol inhibits melanogenesis in murine melanoma cells through down-regulation of the MAPK and PKA signal pathways. Biochem. Biophys. Res. Commun. 2013, 438, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.O.; Shin, K.R.; Jang, B.-C.; Kim, Y.C. Action mechanism of anti-wrinkle effect of Rhamnus yoshinoi methanol extract in human dermal fibroblast and keratinocyte cell lines. Toxicol. Res. 2020, 36, 69–77. [Google Scholar] [CrossRef]
- Strzępek-Gomółka, M.; Gaweł-Bęben, K.; Angelis, A.; Antosiewicz, B.; Sakipova, Z.; Kozhanova, K.; Głowniak, K.; Kukula-Koch, W. Identification of mushroom and murine tyrosinase inhibitors from Achillea biebersteinii Afan. extract. Molecules 2021, 26, 964. [Google Scholar] [CrossRef]
- Park, H.-J.; Cho, J.-H.; Hong, S.-H.; Kim, D.-H.; Jung, H.-Y.; Kang, I.-K.; Cho, Y.-J. Whitening and anti-wrinkle activities of ferulic acid isolated from Tetragonia tetragonioides in B16F10 melanoma and CCD-986sk fibroblast cells. J. Nat. Med. 2018, 72, 127–135. [Google Scholar] [CrossRef]
- Ishikawa, M.; Kawase, I.; Ishii, F. Glycine inhibits melanogenesis in vitro and causes hypopigmentation in vivo. Biol. Pharm. Bull. 2007, 30, 2031–2036. [Google Scholar] [CrossRef] [Green Version]
- Aramwit, P.; Kanokpanont, S.; De-Eknamkul, W.; Kamei, K.; Srichana, T. The effect of sericin with variable amino-acid content from different silk strains on the production of collagen and nitric oxide. J. Biomater. Sci. Polym. Ed. 2009, 20, 1295–1306. [Google Scholar] [CrossRef]
- Korting, H.C.; Schindler, S.; Hartinger, A.; Kerscher, M.; Angerpointner, T.; Maibach, H.I. MTT-assay and neutral red release (NRR)-assay: Relative role in the prediction of the irritancy potential of surfactants. Life Sci. 1994, 55, 533–540. [Google Scholar] [CrossRef]
- Sugimoto, K.; Nishimura, T.; Nomura, K.; Sugimoto, K.; Kuriki, T. Syntheses of arbutin-α-glycosides and a comparison of their inhibitory effects with those of α-arbutin and arbutin on human tyrosinase. Chem. Pharm. Bull. 2003, 51, 798–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, J.-H.; Lee, B.M. Inhibitory effects of plant extracts on tyrosinase, L-DOPA oxidation, and melanin synthesis. J. Toxicol. Environ. Health Part A 2007, 70, 393–407. [Google Scholar] [CrossRef]
- Kubínová, R.; Spačková, V.; Svajdlenka, E.; Lučivjanská, K. Antioxidant activity of extracts and HPLC analysis of flavonoids from Capsella bursa-pastoris (L.) Medik. Ceska A Slov. Farm. Cas. Ceske Farm. Spol. A Slov. Farm. Spol. 2013, 62, 174–176. [Google Scholar]
- Masahiro, T.; Risa, M.; Harutaka, Y.; Kazuhiro, C. Novel antioxidants isolated from Perilla frutescens Britton var. crispa (Thunb.). Biosci. Biotechnol. Biochem. 1996, 60, 1093–1095. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-S.; Lee, S.-M.; Lin, C.-C.; Liu, C.-Y. Hispolon decreases melanin production and induces apoptosis in melanoma cells through the downregulation of tyrosinase and microphthalmia-associated transcription factor (MITF) expressions and the activation of caspase-3,-8 and-9. Int. J. Mol. Sci. 2014, 15, 1201–1215. [Google Scholar] [CrossRef]
- Kim, B.Y.; Park, S.H.; Park, B.J.; Kim, J.J. Whitening effect of Androsace umbellata extract. J. Soc. Cosmet. Sci. Korea 2015, 41, 21–26. [Google Scholar]
- Hanelt, M.; Gareis, M.; Kollarczik, B. Cytotoxicity of mycotoxins evaluated by the MTT-cell culture assay. Mycopathologia 1994, 128, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Baumann, L. Skin ageing and its treatment. J. Pathol. A J. Pathol. Soc. Great Br. Irel. 2007, 211, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Ham, S.A.; Kang, E.S.; Yoo, T.; Lim, H.H.; Lee, W.J.; Hwang, J.S.; Paek, K.S.; Seo, H.G. Dalbergia odorifera Extract Ameliorates UVB-Induced Wrinkle Formation by Modulating Expression of Extracellular Matrix Proteins. Drug Dev. Res. 2015, 76, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Takema, Y.; Hattori, M.; Aizawa, K. The relationship between quantitative changes in collagen and formation of wrinkles on hairless mouse skin after chronic UV irradiation. J. Dermatol. Sci. 1996, 12, 56–63. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Zaręba, I.; Surażyński, A.; Skrzydlewska, E. Comparison of protective effect of ascorbic acid on redox and endocannabinoid systems interactions in in vitro cultured human skin fibroblasts exposed to UV radiation and hydrogen peroxide. Arch. Dermatol. Res. 2017, 309, 285–303. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.A.; Kim, D.H.; Yu, J.M.; Park, C.B.; Park, B.J. Anti-wrinkle effects of extracts and solvent fractions from Nymphoides peltata on CCD-986sk. J. Appl. Biol. Chem. 2017, 60, 357–362. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.H.; Song, C.-K. Antioxidant and metalloproteinase inhibitory activities of ethanol extracts from Lespedeza cuneata G. don. Korean J. Environ. Agric. 2017, 36, 263–268. [Google Scholar] [CrossRef] [Green Version]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Song, X.-z.; Xia, J.-p.; Bi, Z.-g. Effects of (-)-epigallocatechin-3-gallate on expression of matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 in fibroblasts irradiated with ultraviolet A. Chin. Med. J. 2004, 117, 1838–1841. [Google Scholar]
- Kinjo, J.-E.; Furusawa, J.-I.; Baba, J.; Takeshita, T.; Yamasaki, M.; Nohara, T. Studies on the constituents of Pueraria lobata. III. Isoflavonoids and related compounds in the roots and the voluble stems. Chem. Pharm. Bull. 1987, 35, 4846–4850. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.S.; Sung, S.H.; Park, J.H.; Kim, Y.C. Flavonoids fromSpatholobus suberectus. Arch. Pharmacal Res. 2004, 27, 589–592. [Google Scholar] [CrossRef]
- Lee, Y.-Y.; Kwon, S.-H.; Kim, H.-J.; Park, H.-J.; Yang, E.-J.; Kim, S.-K.; Yoon, Y.-H.; Kim, C.-G.; Park, J.-W.; Song, K.-S. Isolation of oleanane triterpenes and trans-resveratrol from the root of peanut (Arachis hypogaea). J. Korean Soc. Appl. Biol. Chem. 2009, 52, 40–44. [Google Scholar] [CrossRef]
- Young Han, S.; Suck Lee, H.; Hye Choi, D.; Woon Hwang, J.; Mo Yang, D.; Jun, J.-G. Efficient Total Synthesis of Piceatannol via (E)-Selective Wittig–Horner Reaction. Synth. Commun. 2009, 39, 1425–1432. [Google Scholar] [CrossRef]
- Han, T.; Cheng, G.; Liu, Y.; Yang, H.; Hu, Y.-T.; Huang, W. In vitro evaluation of tectoridin, tectorigenin and tectorigenin sodium sulfonate on antioxidant properties. Food Chem. Toxicol. 2012, 50, 409–414. [Google Scholar] [CrossRef]
- Kim, J.M.; Ko, R.K.; Jung, D.S.; Kim, S.S.; Lee, N.H. Tyrosinase inhibitory constituents from the stems of Maackia fauriei. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2010, 24, 70–75. [Google Scholar] [CrossRef]
- Jiafeng, Z.; Dayuan, Z. Chemical constituents of the roots of Maackia tenuifolia (Leguminosae). Acta Bot. Sin. 1999, 41, 997–1001. [Google Scholar]
- Mu, H.; Bai, Y.-H.; Wang, S.-T.; Zhu, Z.-M.; Zhang, Y.-W. Research on antioxidant effects and estrogenic effect of formononetin from Trifolium pratense (red clover). Phytomedicine 2009, 16, 314–319. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Wang, J.; Ren, B.; Zhang, L.; Li, W. Formononetin, an isoflavone from Astragalus membranaceus inhibits proliferation and metastasis of ovarian cancer cells. J. Ethnopharmacol. 2018, 221, 91–99. [Google Scholar] [CrossRef]
- Khan, I.; Avery, M.; Burandt, C.; Goins, D.; Mikell, J.; Nash, T.; Azadegan, A.; Walker, L. Antigiardial activity of isoflavones from Dalbergia frutescens bark. J. Nat. Prod. 2000, 63, 1414–1416. [Google Scholar] [CrossRef]
- Surendhiran, D.; Karthiga, J.; Nirmala, S.; Sirajunnisa, A.R. Isolation of genistein from Acalypha fruticosa and studying its antibacterial activity by inhibition of bacterial DNA and protein. J. Omedicine. Toxicol. 2011, 5, 87–96. [Google Scholar]
- Jang, D.S.; Kim, J.M.; Lee, Y.M.; Kim, Y.S.; Kim, J.-H.; Kim, J.S. Puerariafuran, a new inhibitor of advanced glycation end products (AGEs) isolated from the roots of Pueraria lobata. Chem. Pharm. Bull. 2006, 54, 1315–1317. [Google Scholar] [CrossRef] [Green Version]
- Ma, K.; Ishikawa, T.; Seki, H.; Furihata, K.; Ueki, H.; Narimatsu, S.; Higuchi, Y.; Chaichantipyuth, C. Isolation of new isoflavonolignans, butesuperins A and B, from a Thai miracle herb, Butea superba. Heterocycles-Sendai Inst. Heterocycl. Chem. 2005, 65, 893–900. [Google Scholar] [CrossRef]
- Park, J.-S.; Kim, D.H.; Lee, J.K.; Lee, J.Y.; Kim, D.H.; Kim, H.K.; Lee, H.-J.; Kim, H.C. Natural ortho-dihydroxyisoflavone derivatives from aged Korean fermented soybean paste as potent tyrosinase and melanin formation inhibitors. Bioorganic. Med. Chem. Lett. 2010, 20, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Widodo, W.S.; Widowati, W.; Ginting, C.N.; Lister, I.; Armansyah, A.; Girsang, E. Comparison of antioxidant and anti-collagenase activity of genistein and epicatechin. Pharm. Sci. Res. 2019, 6, 6. [Google Scholar]
- Kukrić, Z.Z.; Topalić-Trivunović, L.N. Antibacterial activity of cis-and trans-resveratrol isolated from Polygonum cuspidatum rhizome. Acta Period. Technol. 2006, 37, 131–136. [Google Scholar] [CrossRef]
- Harba, A.H.; abu Zargab, M.; Abdallaa, S. Effects of Trans-Resveratrol, Isolated from Smilax Aspera, on Smooth Muscle, Blood Pressure, and Inflammation in Rats and Nociceptionin Mice. Jordan J. Biol. Sci. 2009, 2, 69–76. [Google Scholar]
- Lee, T.H.; Seo, J.O.; Baek, S.-H.; Kim, S.Y. Inhibitory effects of resveratrol on melanin synthesis in ultraviolet B-induced pigmentation in Guinea pig skin. Biomol. Ther. 2014, 22, 35. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Oh, J.; Averilla, J.N.; Kim, H.J.; Kim, J.S.; Kim, J.S. Grape peel extract and resveratrol inhibit wrinkle formation in mice model through activation of Nrf2/HO-1 signaling pathway. J. Food Sci. 2019, 84, 1600–1608. [Google Scholar] [CrossRef]
- Duarte, N.; Kayser, O.; Abreu, P.; Ferreira, M.J.U. Antileishmanial activity of piceatannol isolated from Euphorbia lagascae seeds. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22, 455–457. [Google Scholar]
- Oh, S.-J.; Baek, N.-I.; Kim, H.-Y. Piceatannol, Antioxidant Compound Isolated from the Root of Rheum undulatum L. Appl. Biol. Chem. 2001, 44, 208–210. [Google Scholar]
- Kitada, M.; Ogura, Y.; Maruki-Uchida, H.; Sai, M.; Suzuki, T.; Kanasaki, K.; Hara, Y.; Seto, H.; Kuroshima, Y.; Monno, I. The effect of piceatannol from passion fruit (Passiflora edulis) seeds on metabolic health in humans. Nutrients 2017, 9, 1142. [Google Scholar] [CrossRef] [Green Version]
- Yokozawa, T.; Kim, Y.J. Piceatannol inhibits melanogenesis by its antioxidative actions. Biol. Pharm. Bull. 2007, 30, 2007–2011. [Google Scholar] [CrossRef] [Green Version]
- Maruki-Uchida, H.; Kurita, I.; Sugiyama, K.; Sai, M.; Maeda, K.; Ito, T. The protective effects of piceatannol from passion fruit (Passiflora edulis) seeds in UVB-irradiated keratinocytes. Biol. Pharm. Bull. 2013, 36, 845–849. [Google Scholar] [CrossRef] [Green Version]
- Jeong, G.-S.; An, R.-B.; Oh, S.-H.; Kang, D.-G.; Lee, H.-S.; Kim, Y.-C. Cytoprotective activity of Belamcanda chinensis rhizome against glutamate-induced oxidative injury in HT22 cells. Nat. Prod. Sci. 2007, 13, 101–104. [Google Scholar]
- Bhat, G.A.; Mir, F.; Shawl, A.S.; Ganai, B.A.; Kamili, A.N.; Masood, A.; Tantry, M.A. Crocetenone, a new rotenoid with an unusual trans-fused ring system from Iris crocea. Nat. Prod. Commun. 2015, 10, 503–504. [Google Scholar] [CrossRef] [Green Version]
- Ahn, Y.J.; Chang, Y.H.; Lee, S.Y.; Jin, M.H. A study on the whitening effects of Pueraria thomsonii extract and its three tectorigenin derivatives. J. Soc. Cosmet. Sci. Korea 2019, 45, 49–56. [Google Scholar]
- Kim, S.B.; Hwang, S.H.; Wang, Z.; Yu, J.M.; Lim, S.S. Rapid identification and isolation of inhibitors of rat lens aldose reductase and antioxidant in Maackia amurensis. BioMed Res. Int. 2017, 2017, 1–10. [Google Scholar]
- Utkina, N.; Kulesh, N. Antioxidant activity of polyphenols and polyphenol complex from the far-eastern tree Maackia amurensis. Pharm. Chem. J. 2012, 46, 488–491. [Google Scholar] [CrossRef]
- Park, W.S.; Bae, J.-Y.; Kim, H.J.; Kim, M.G.; Lee, W.-K.; Kang, H.-L.; Baik, S.-C.; Lim, K.M.; Lee, M.K.; Ahn, M.-J. Anti-Helicobacter pylori compounds from Maackia amurensis. Nat. Prod. Sci. 2015, 21, 49–53. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-G.; Park, G.-K.; Jang, W.; Kim, B.-Y.; Kim, S.-K.; Kim, Y.-A.; Park, S.-H.; Park, B. Skin-Whitening and Antiwrinkle Proprieties of Maackia amurensis Methanolic Extract Lead Compounds. Processes 2022, 10, 855. https://doi.org/10.3390/pr10050855
Kim J-G, Park G-K, Jang W, Kim B-Y, Kim S-K, Kim Y-A, Park S-H, Park B. Skin-Whitening and Antiwrinkle Proprieties of Maackia amurensis Methanolic Extract Lead Compounds. Processes. 2022; 10(5):855. https://doi.org/10.3390/pr10050855
Chicago/Turabian StyleKim, Ju-Gyeong, Gwee-Kyo Park, Wookju Jang, Bo-Yun Kim, Seul-Ki Kim, You-Ah Kim, Sung-Ha Park, and Byoungjun Park. 2022. "Skin-Whitening and Antiwrinkle Proprieties of Maackia amurensis Methanolic Extract Lead Compounds" Processes 10, no. 5: 855. https://doi.org/10.3390/pr10050855
APA StyleKim, J.-G., Park, G.-K., Jang, W., Kim, B.-Y., Kim, S.-K., Kim, Y.-A., Park, S.-H., & Park, B. (2022). Skin-Whitening and Antiwrinkle Proprieties of Maackia amurensis Methanolic Extract Lead Compounds. Processes, 10(5), 855. https://doi.org/10.3390/pr10050855






