Progress of Methylation of C6-8~Arene with Methanol: Mechanism, Catalysts, Kinetic/Thermodynamics and Perspectives
Abstract
:1. Introduction
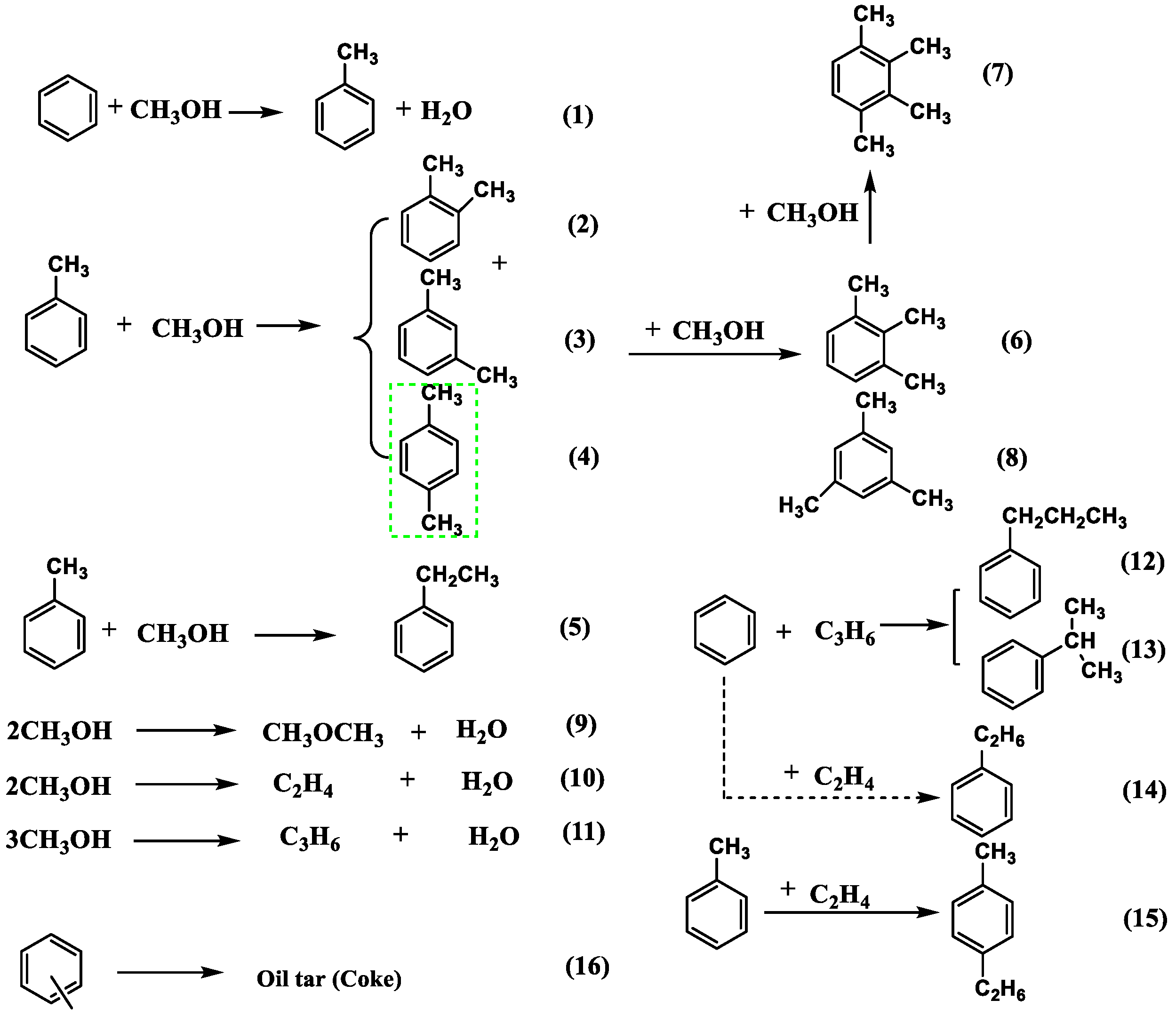
2. Methylation Reaction Network
3. Thermodynamic Analysis of Methylation Reaction
3.1. Thermodynamic Calculation Method for the Methylation Series Reactions of Benzene
3.2. Methylation Series Reaction System
3.3. Data Involved in Methylation Reaction System
3.4. Enthalpy Change, Entropy Change, Gibbs Free Energy and Equilibrium Constant of Methylation Reaction System
3.4.1. Analysis of Enthalpy Change and Entropy Change of Methylation Reaction System
3.4.2. Gibbs Free Energy Analysis of Methylation Reaction System
3.4.3. The Equilibrium Constant of Methylation Reaction System
4. Mechanism of Methylation Reaction
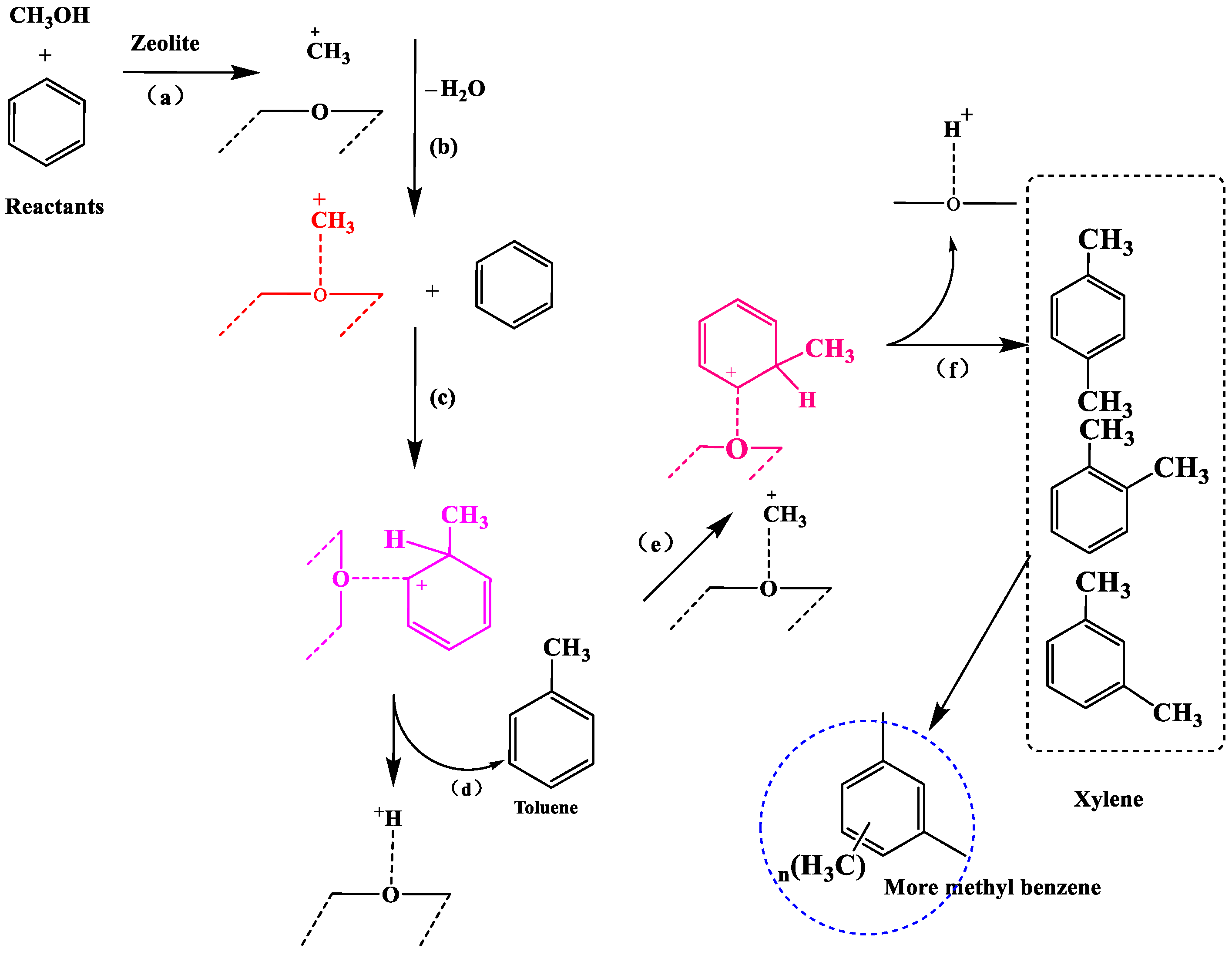
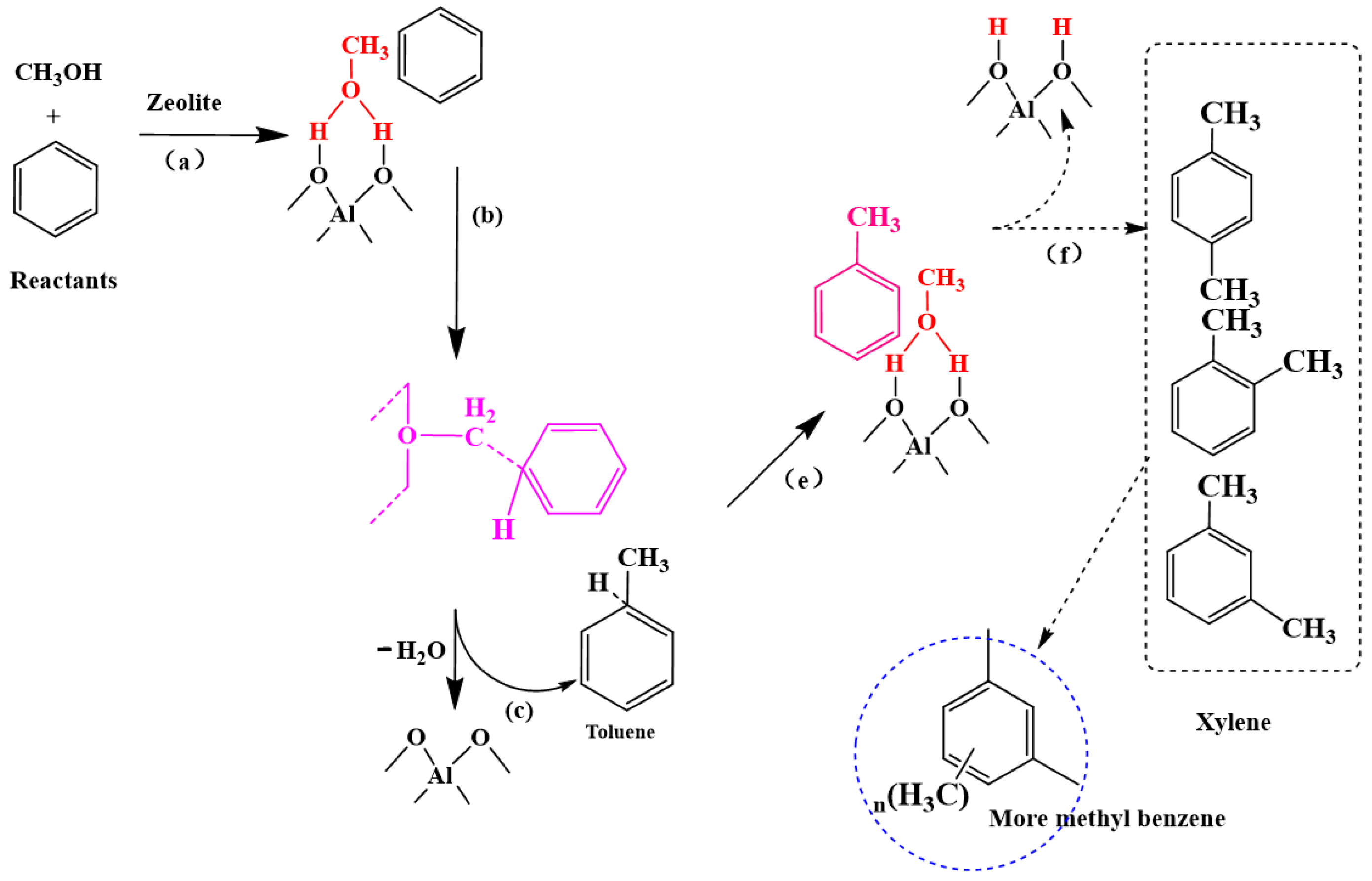
4.1. Aromatic Methylation Reaction
4.2. Gas-Phase Products in the Methylation Reaction
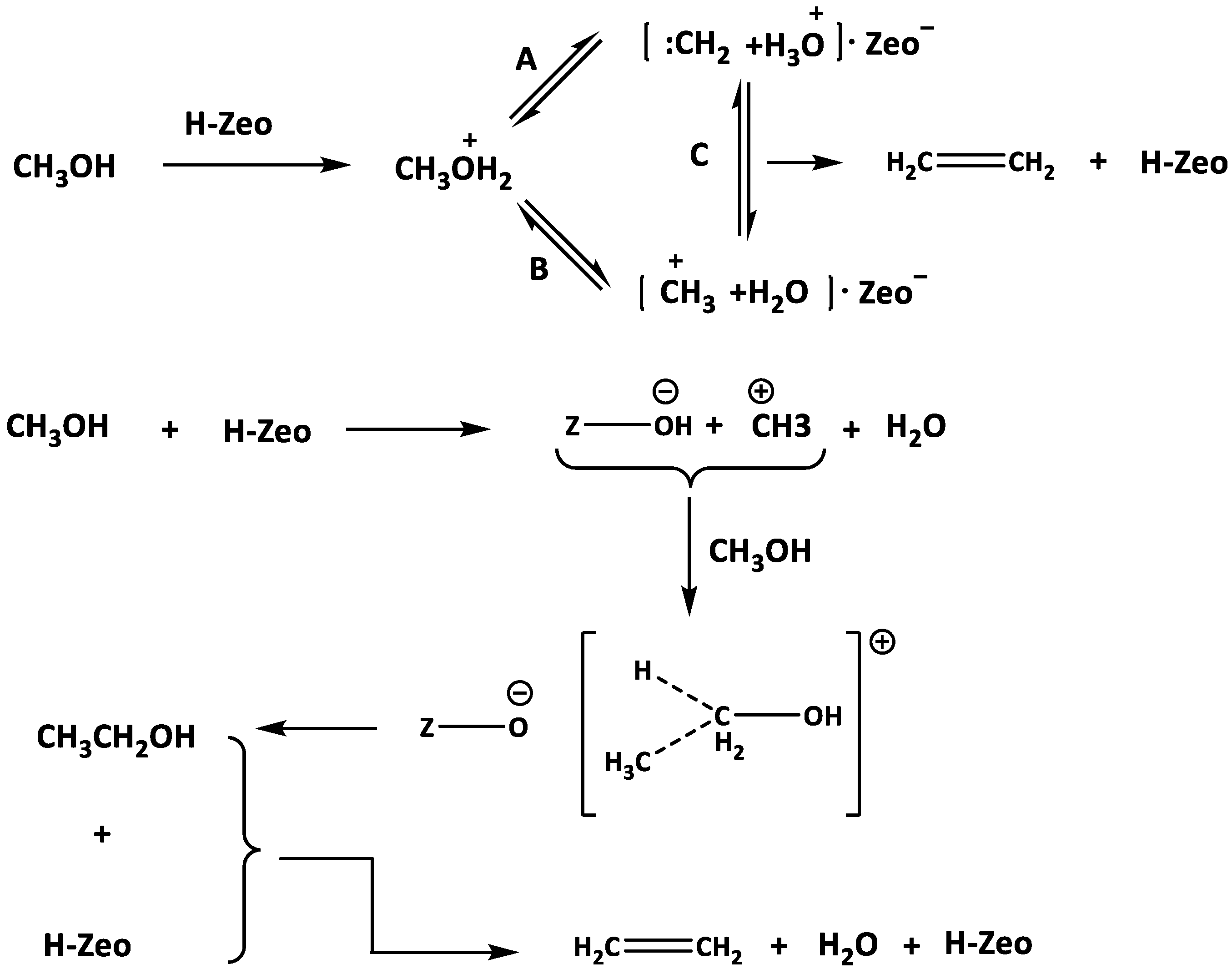
- (1)
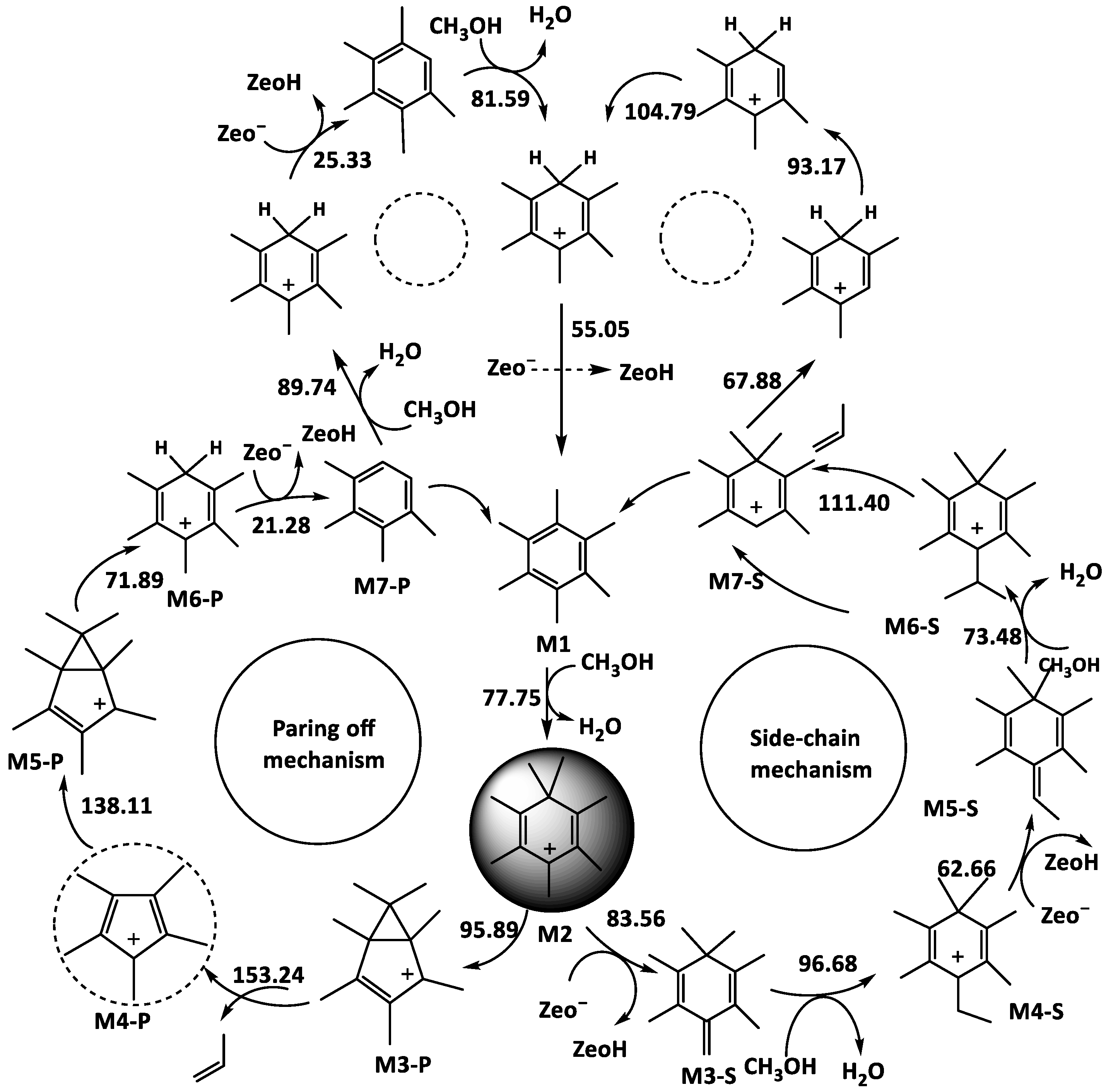
- Side chain methylation mechanism
- (2)
- Paring mechanism
- (3)
- MTH/MTA reaction mechanism
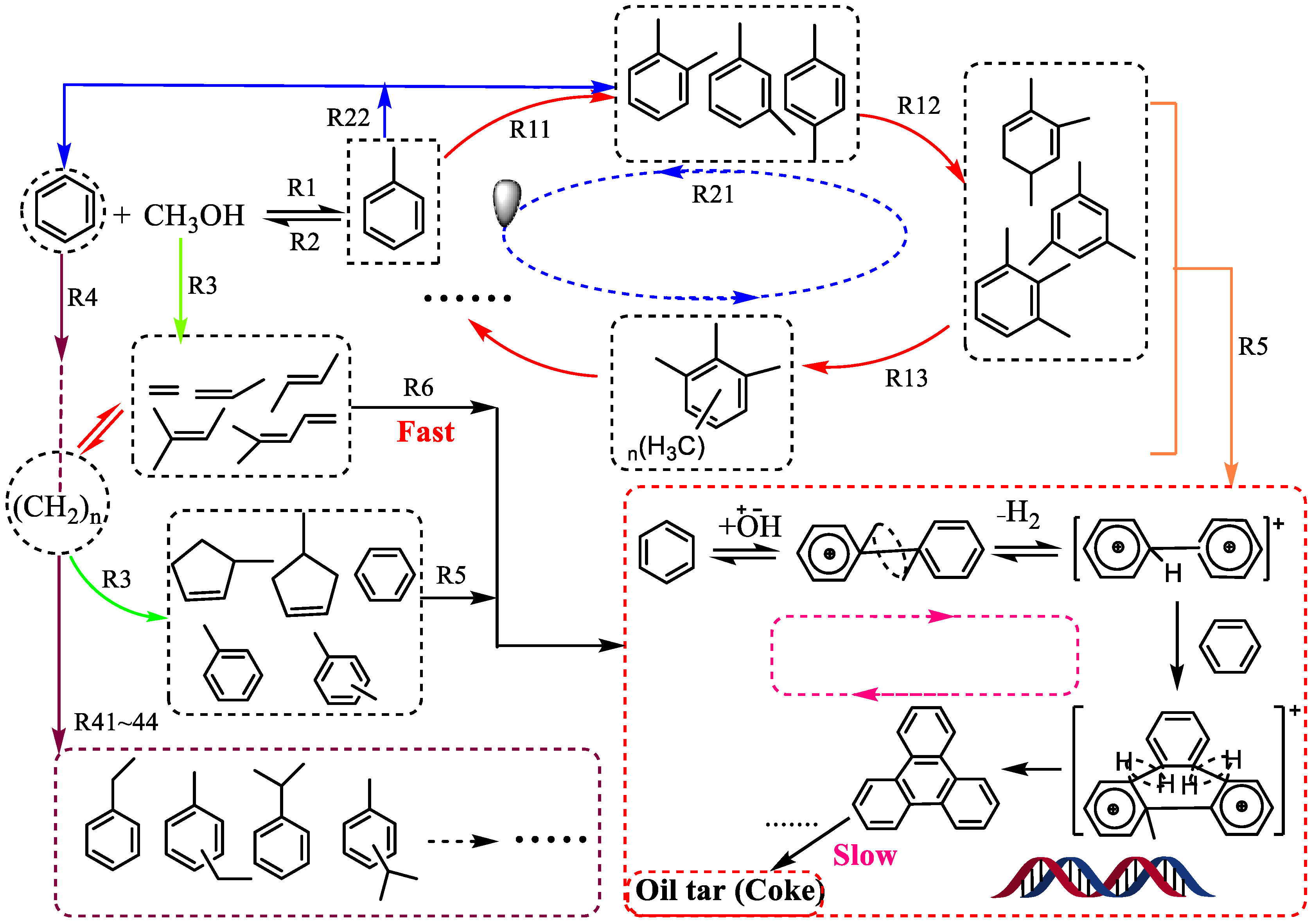
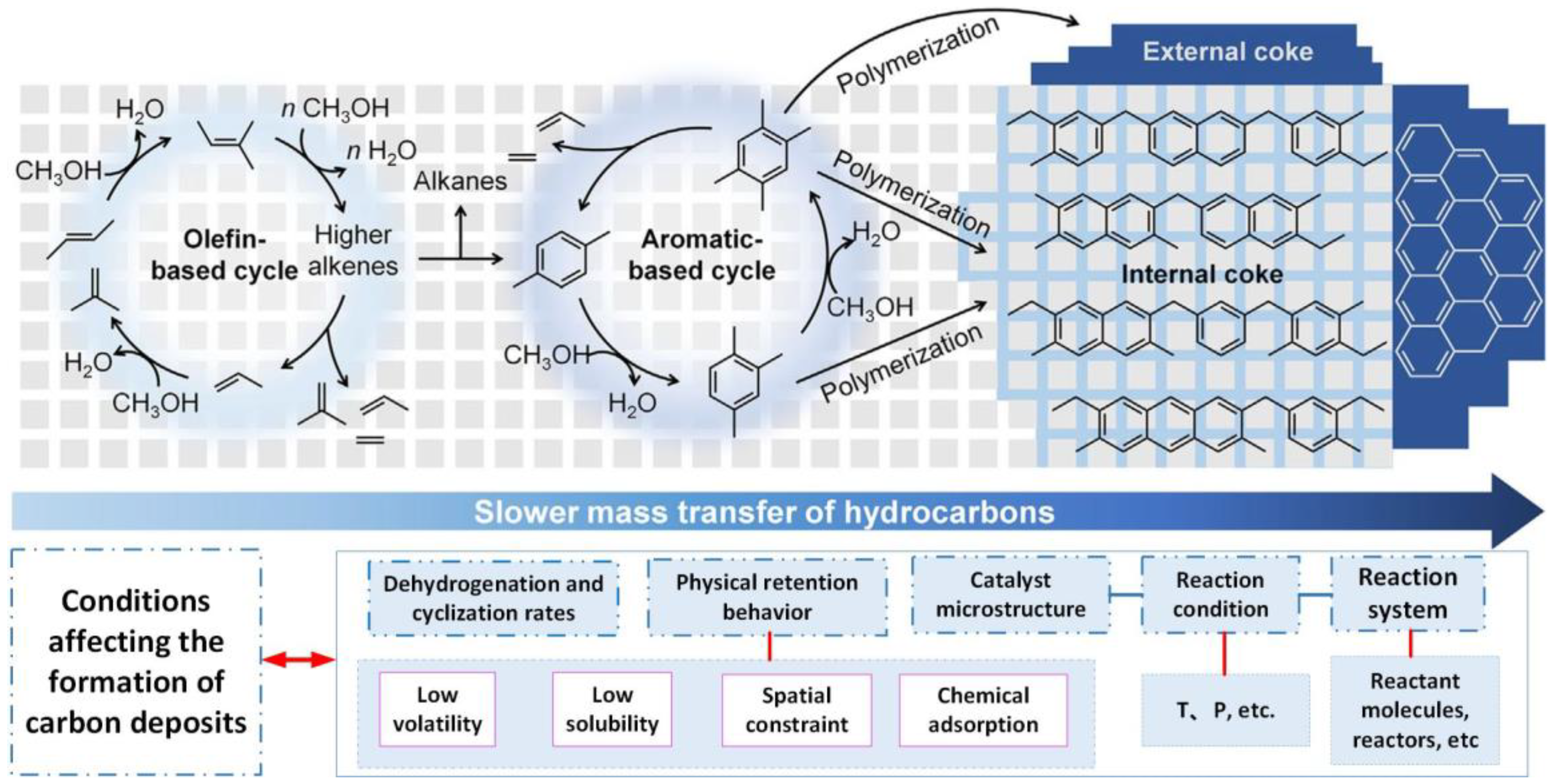
4.3. Deactivation Mechanism of Carbon Deposition
5. Research Progress in the Methylation of Benzene with Methanol
5.1. Benzene Methylation Catalyst
5.1.1. Zeolites
5.1.2. Shape-Selectivity of Catalyst
5.1.3. Catalyst Acidity
5.2. Effects of Different Reaction Environments on the Methylation of Benzene with Methanol
5.3. Inhibition of By-Products in the Methylation of Benzene with Methanol
5.4. Process Conditions for the Methylation Reaction of Benzene with Methanol
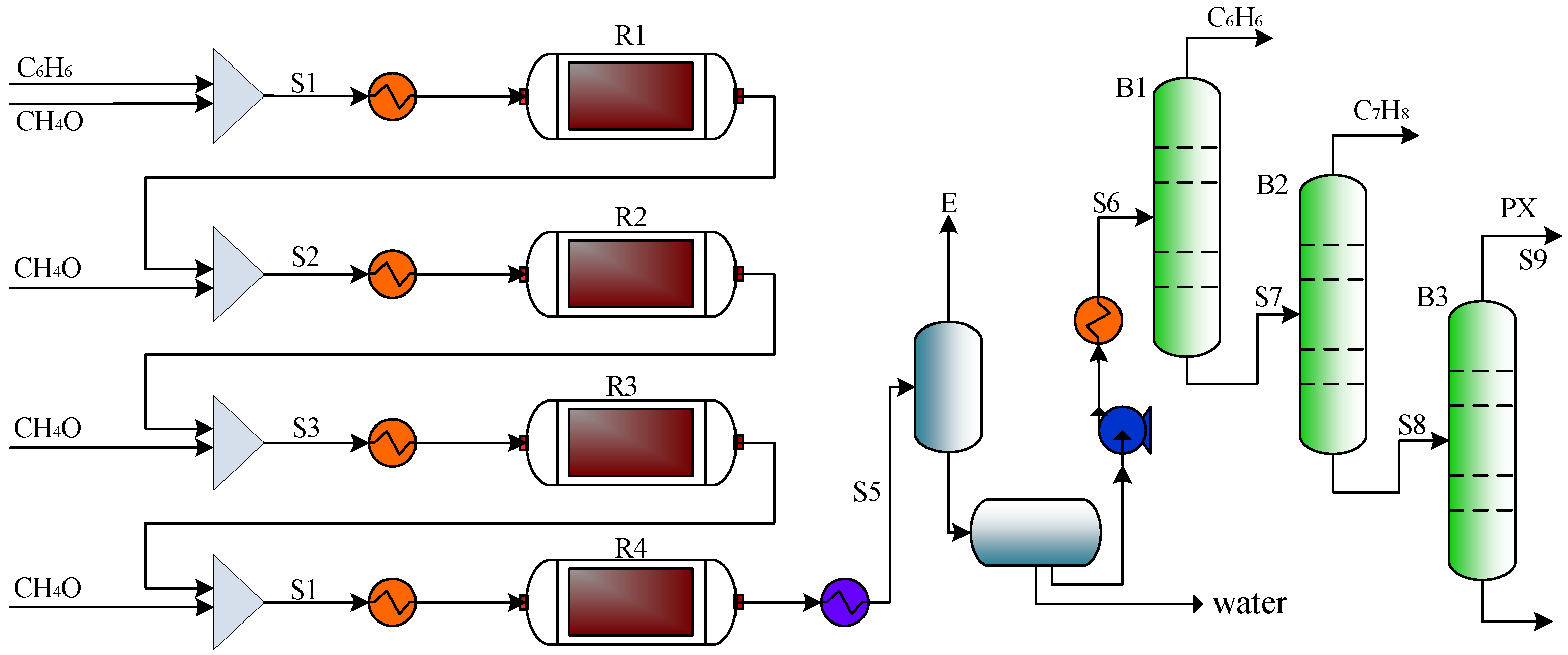
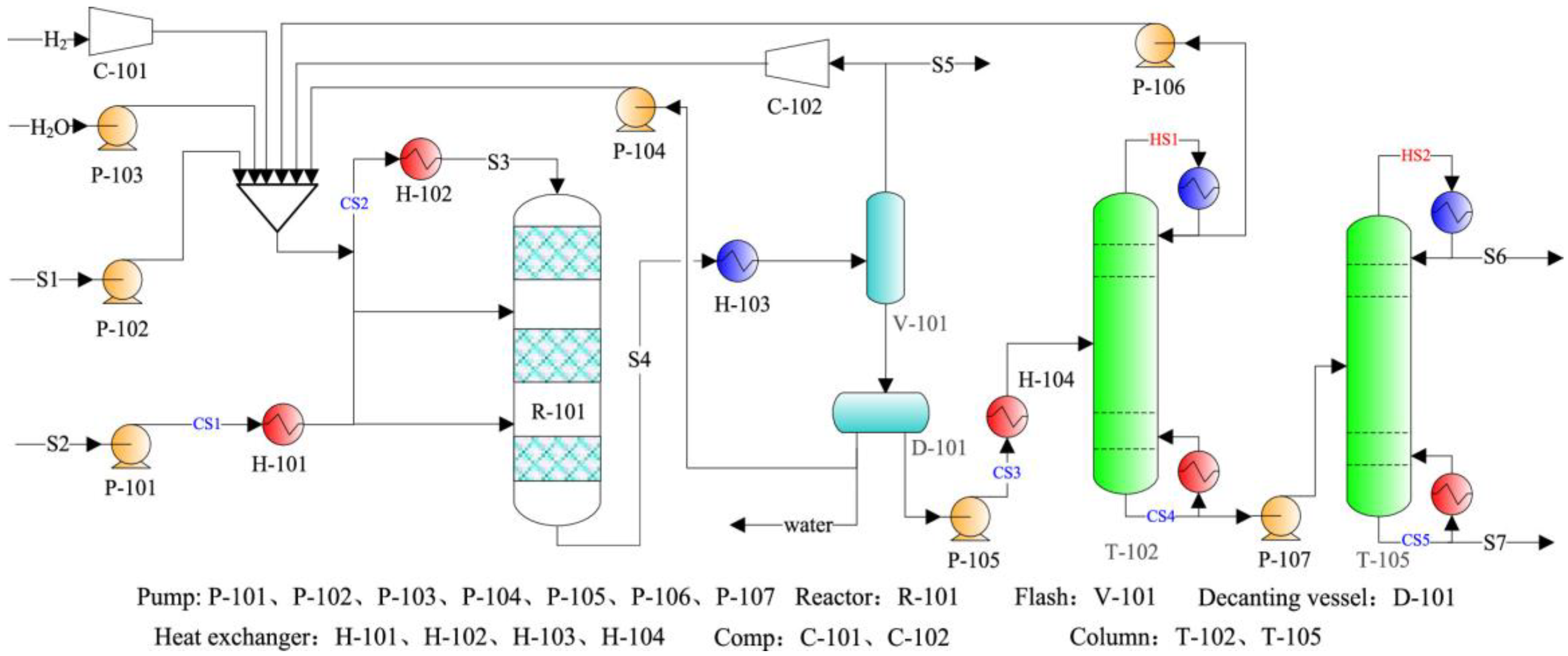
5.5. Process Comparison for the Methylation Reaction of Benzene with Methanol
6. Research Progress of Toluene–Methanol Methylation Reaction
6.1. The Methylation Reaction System of Toluene with Methanol
6.2. Toluene–Methanol Methylation Catalyst
6.2.1. Shape Selectivity of Catalyst
6.2.2. Catalyst Acidity
6.2.3. Other Aspects
6.3. Effects of Different Reaction Environments on the Methylation of Toluene with Methanol
6.4. Process Comparison for the Methylation Reaction of Toluene with Methanol
7. Progress in Preparation of Trimethylbenzene by the Methylation Reaction of Xylene with Methanol
7.1. Catalyst for Synthesis of Trimethylbenzene
7.2. Process Comparison of Trimethylbenzene Synthesis
8. Kinetics of Methylation Reactions
9. Summary and Prospect
9.1. Perspectives of BM-XP/TM-PX Development
9.2. Perspectives of MX-TMB Development
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Li, Y.F.; Chen, L.; Au, C.T.; Yin, S.F. A new catalytic process for the synthesis of para-xylene through benzene methylation with CH3Br. Catal. Commun. 2014, 54, 6–10. [Google Scholar] [CrossRef]
- Feng, Z.W.; Hu, B.; Guo, X.Q. Thermodynamic analysis of methanol and benzene alkylation by ASPEN PLUS. SHANXI Chem. Ind. (China) 2019, 1, 16–21. [Google Scholar]
- Xu, Y.R.; Xu, X.L.; Zhu, X.D. Thermodynamic analysis on alkylation reaction of benzene and methanol. Chem. React. Eng. Technol. 2015, 31, 475–480. [Google Scholar]
- Wang, D.L.; Zhang, J.Q.; Dong, P.; Li, G.X.; Fan, X.Y.; Yang, Y. Novel short process for p-xylene production based on the selectivity intensification of toluene methylation with methanol. ACS Omega 2022, 7, 1211–1222. [Google Scholar] [CrossRef]
- Stian, S.; Melina, V.; Unni, O.; Saepurahman; Bjørgen, M. Mechanistic aspects of the zeolite catalyzed methylation of alkenes and aromatics with methanol: A review. Top. Catal. 2011, 54, 897–906. [Google Scholar]
- Chen, Q.T.; Liu, J.; Yang, B. Identifying the key steps determining the selectivity of toluene methylation with methanol over HZSM-5. Nat. Commun. 2021, 12, 3725. [Google Scholar] [CrossRef]
- Ma, P.S. The Experimental Data Manual of Organic Compound; Chemical Industry Press: Beijing, China, 2006. [Google Scholar]
- Dong, P.; Li, Z.Y.; Wang, D.L.; Wang, X.R.; Guo, Y.Q.; Li, G.X.; Zhang, D.Q. Alkylation of benzene by methanol: Thermodynamics analysis for designing and designing for enhancing the selectivity of toluene and para-xylene. Catal. Lett. 2019, 149, 248–258. [Google Scholar] [CrossRef]
- Zhao, Y.; Tan, W.; Wu, H.Y. Effect of Pt on stability of nano-scale ZSM-5 catalyst for toluene alkylation with methanol into p-xylene. Catal. Today 2011, 160, 179–183. [Google Scholar] [CrossRef]
- Venuto, P.B.; Landis, P.S. Organic catalysis over crystalline aluminosilicates. Adv. Catal. 1968, 18, 259–371. [Google Scholar]
- Kaeding, W.W.; Chu, C.; Young, L.B.; Weinstein, B.; Butter, S.A. Selective alkylation of toluene with methanol to produce para-Xylene. J. Catal. 1981, 67, 159–174. [Google Scholar] [CrossRef]
- Odedairo, T.; Al-Khattaf, S. Comparative study of zeolite catalyzed alkylation of benzene with alcohols of Different chain length: H-ZSM-5 versus mordenite. Catal. Today 2013, 204, 73–84. [Google Scholar] [CrossRef]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Ichikaw, S.; Egashira, Y.; Ueyama, K. Catalytic activities and structures of silicalite-1/H-ZSM-5 zeolite composites. Microporous Mesoporous Mater. 2008, 115, 106–112. [Google Scholar] [CrossRef]
- Hu, H.L.; Lyu, J.H.J.; Rui, Y.; Cen, J.; Zhang, Q.F.; Wang, Q.T.; Han, W.W.; Li, X.N. The effect of Si/Al ratio on the catalytic performance of hierarchical porous ZSM-5 for catalyzing benzene alkylation with methanol. Catal. Sci. Technol. 2016, 6, 2647. [Google Scholar] [CrossRef]
- Jeroen, V.D.M.; Melina, V.; Unni, O.; Beato, P.; Bjørgen, M.; Speybroeck, V.V.; Svelle, S. Methylation of benzene by methanol: Single-site kinetics over H-ZSM-5 and H-beta zeolite catalysts. J. Catal. 2012, 292, 201–212. [Google Scholar]
- Wen, Z.H.; Yang, D.; Yang, F.; Wei, Z.H.; Zhu, X.D. Methylation of toluene with methanol over HZSM-5: A periodic density functional theory investigation. Chinese J. Catal. 2016, 37, 1882–1890. [Google Scholar] [CrossRef]
- Wang, G.R. Catalyst and Catalysis; Dalian University of Technology Press: Dalian, China, 2015; pp. 104–110. [Google Scholar]
- Dong, P.; Meng, J.L.; Yun, H.F.; Li, G.X. Alkylation of benzene with methanol to toluene over Na3-H-Y: Analysis of four aspects for obtaining reaction mechanism. J. Incl. Phenom. Macrocycl. Chem. 2020, 97, 147–157. [Google Scholar] [CrossRef]
- Tan, W.; Liu, M.; Zhao, Y.; Hou, K.K.; Wu, H.Y.; Zhang, A.F.; Liu, H.O.; Wang, Y.R.; Song, C.S.; Guo, X.W. Para-selective methylation of toluene with methanol over nano-sized ZSM-5 catalysts: Synergistic effects of surface modifications with SiO2, P2O5 and MgO. Microporous. Mesoporous. Mater. 2014, 196, 18–30. [Google Scholar] [CrossRef]
- Xu, S.T.; Zhi, Y.C.; Han, J.F.; Zhang, W.N.; Wu, X.Q.; Sun, T.T.; Wei, Y.X.; Liu, Z.M. Chapter two-advances in catalysis for methanol-to-olefins conversion. Adv. Catal. 2017, 61, 37–122. [Google Scholar]
- Haw, J.F.; Song, W.G.; Marcus, D.M.; Nicholas, J.B. The mechanism of methanol to hydrocarbon catalysis. Acc. Chem. Res. 2003, 36, 317–326. [Google Scholar] [CrossRef]
- Lesthaeghe, D.; Van, D.M.J.; Vandichel, M.; Waroquier, M.; Speybroeck, V.V. Full theoretical cycle for both ethene and propene formation during methanol-to-olefin conversion in H-ZSM-5. ChemCatChem 2011, 3, 208–212. [Google Scholar] [CrossRef]
- Lesthaeghe, D.; Van, S.V.; Marin, G.B.; Waroquier, I.M. Understanding the failure of direct C-C coupling in the zeolite-catalyzed methanol-to-olefin process. Angew. Chem. Int. Ed. 2006, 45, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Lesthaeghe, D.; Speybroeck, V.V.; Marin, G.B. The rise and fall of direct mechanisms in methanol-to-olefin catalysis: An overview of theoretical contributions. Ind. Eng. Chem. Res. 2007, 46, 8832–8838. [Google Scholar] [CrossRef]
- Lesthaeghe, D.; Speybroeck, V.V.; Marin, G.B.; Waroquier, M. What role do oxonium ions and oxonium ylides play in the ZSM-5 catalysed methanol-to-olefin process? Chem. Phys. Lett. 2006, 417, 309–315. [Google Scholar] [CrossRef]
- Song, W.; Marcus, D.M.; Fu, H.; Ehresmann, J.O.; Haw, J.F. An oft-studied reaction that may never have been: Direct catalytic conversion of methanol or dimethyl ether to hydrocarbons on the solid acids HZSM-5 or HSAPO-34. J. Am. Chem. Soc. 2002, 124, 3844–3845. [Google Scholar] [CrossRef]
- Wu, X.Q.; Xu, S.T.; Zhang, W.N.; Huang, J.D.; Li, J.Z.; Yu, B.B.; Wei, Y.X.; Liu, Z.M. Direct mechanism of the first carbon–carbon bond formation in the methanol-to-hydrocarbons process. Angew. Chem. Int. Ed. 2017, 56, 9039–9043. [Google Scholar] [CrossRef]
- Liu, Y.; Muller, S.; Berger, D.; Jelic, J.; Reuter, K.; Tonigold, M.; Sanchez-Sanchez, M.; Lercher, J.A. Formation mechanism of the first carbon-carbon bond and the first olefin in the methanol conversion into hydrocarbons. Angew. Chem. Int. Ed. 2016, 55, 5723. [Google Scholar] [CrossRef]
- Teketel, S.; Olsbye, U.; Lillerud, K.P.; Beato, P.; Svelle, S. Selectivity control through fundamental mechanistic insight in the conversion of methanol to hydrocarbons over zeolites. Microporous Mesoporous Mater. 2010, 136, 33–41. [Google Scholar] [CrossRef]
- Ilias, S.; Bhan, A. Tuning the selectivity of methanol-to-hydrocarbons conversion on H-ZSM-5 by co-processing olefin or aromatic compounds. J. Catal. 2012, 290, 186–192. [Google Scholar] [CrossRef]
- Lukyanov, D.B.; Vazhnova, T. Selective and stable benzene alkylation with methane into toluene over PtH-MFI bifunctional catalyst. J. Mol. Catal. A Chem. 2009, 305, 95–99. [Google Scholar] [CrossRef]
- Lee, S.; Choi, M. Unveiling coke formation mechanism in MFI zeolites during methanol-to-hydrocarbons conversion. J. Catal. 2019, 375, 183–192. [Google Scholar] [CrossRef]
- Zhu, Z.R.; Chen, Q.L.; Xie, Z.K.; Yang, W.M.; Li, C. The roles of acidity and structure of zeolite for catalyzing toluene alkylation with methanol to xylene. Microporous Mesoporous Mater. 2006, 88, 16–21. [Google Scholar] [CrossRef]
- Vos, A.M.; Rozanska, X.R.; Schoonheydt, A.; Santen, R.V. A theoretical study of the alkylation reaction of toluene with methanol catalyzed by acidic mordenite. J. Am. Chem. Soc. 2001, 123, 2799–2809. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.T.; Han, W.W.; Hu, H.L.; Lyu, J.H.; Xu, X.L.; Zhang, Q.F.; Wang, H.J.; Li, X.N. Influence of the post-treatment of HZSM-5 zeolite on catalytic performance for alkylation of benzene with methanol. Chin. J. Chem. Eng. 2017, 25, 1777–1783. [Google Scholar] [CrossRef]
- Rao, S.M.; Saraçi, E.; Gläser, R.; Coppens, M.O. Coppens surface barriers as dominant mechanism to transport limitations in hierarchically structured catalysts-application to the zeolite-catalyzed alkylation of benzene with ethylene. Chem. Eng. J. 2017, 329, 45–55. [Google Scholar] [CrossRef]
- Zhang, W.S.; Zhang, S.; Xin, W.J.; Liu, H.; Shang, Y.C.; Zhu, X.X.; Liu, S.L.; Xu, L.Y. Shaped binderless ZSM-11 zeolite catalyst prepared via a dry-gel conversion method: Characterization and application for alkylation of benzene with dimethyl ether. J. Energy Chem. 2017, 26, 380–389. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Lu, G.Z.; Miao, C.X.; Wu, X.; Wu, W.H.; Sun, Q. Catalytic performance of X zeolites modified by alkali metal ions for the side-chain alkylation of toluene with methanol. Microporous Mesoporous Mater. 2013, 167, 213–220. [Google Scholar] [CrossRef]
- Yashima, T.; Yamazaki, K.; Ahmad, H.; Katsuta, M.; Hara, N. Alkylation on synthetic zeolites: II. Selectivity p-xylene formation. J. Catal. 1970, 17, 151–156. [Google Scholar] [CrossRef]
- Wang, Y.A.; Gao, Y.; Xie, S.J.; Liu, S.L.; Chen, F.C.; Xin, W.J.; Zhu, X.X.; Li, X.J.; Jiang, N.; Xu, L.Y. Adjustment of the Al siting in MCM-22 zeolite and its effect on alkylation performance of ethylene with benzene. Catal. Today 2018, 316, 71–77. [Google Scholar] [CrossRef]
- Shi, Y.C.; Xing, E.H.; Xie, W.H.; Zhuang, F.M.; Mu, X.H.; Shu, X.T. Shape selectivity of beta and MCM-49 zeolites in liquid-phase alkylation of benzene with ethylene. J. Mol. Catal. A Chem. 2016, 418, 86–94. [Google Scholar] [CrossRef]
- Raj, K.J.A.; Malar, E.J.P.; Vijayaraghavan, V.R. Shape-selective reactions with AEL and AFI type zeolites alkylation of benzene, toluene and ethylbenzene with ethanol, 2-propanol, methanol and t-butanol. J. Mol. Catal. A Chem. 2006, 243, 99–105. [Google Scholar] [CrossRef]
- Alabi, W.; Atanda, L.; Jermy, R.; Al-Khattaf, S. Kinetics of toluene alkylation with methanol catalyzed by pure and hybridized HZSM-5 catalysts. Chem. Eng. J. 2012, 195, 276–288. [Google Scholar] [CrossRef]
- Wang, C.F.; Zhang, L.; Huang, X.; Zhu, Y.F.; Li, G.; Gu, Q.F.; Chen, J.Y.; Ma, L.G.; Li, X.J.; He, Q.H.; et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene. Nat. Commun. 2019, 10, 4348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Tanaka, H.; Jentys, A.; Lercher, J.A. Novel model explaining teluene diffusion in HZSM-5 after surface modification. J. Phys. Chem. B 2004, 108, 1337–1343. [Google Scholar] [CrossRef]
- Hu, H.L.; Zhang, Q.F.; Cen, J.; Li, X.N. High suppression of the formation of ethylbenzene in benzene alkylation with methanol over ZSM-5 catalyst modified by platinum. Catal. Commun. 2014, 57, 129–133. [Google Scholar] [CrossRef]
- Bjørgen, M.; Joensen, F.; Holm, M.S.; Olsbye, U.; Lillerud, K.P.; Svelle, S. Methanol to gasoline over zeolite H-ZSM-5: Improved catalyst performance by treatment with NaOH. Appl. Catal. A Gen. 2008, 345, 43–50. [Google Scholar] [CrossRef]
- Deng, W.; He, X.; Zhang, C.; Gao, Y.Y.; Zhu, X.D.; Zhu, K.K.; Huo, Q.S.; Zhou, Z.J. Promoting xylene production in benzene methylation using hierarchically porous ZSM-5 derived from a modified dry-gel route. Chin. J. Chem. Eng. 2014, 22, 921–929. [Google Scholar] [CrossRef]
- Anderson, J.R.; Foger, K.; Mole, T.; Sanders, J.V. Reactions on ZSM-5-type zeolite catalysts. J. Catal. 1979, 58, 114–130. [Google Scholar] [CrossRef]
- Dong, P.; Zhang, Y.F.; Li, Z.Y.; Yong, H.F.; Li, G.X.; Ji, D. Enhancement of the utilization of methanol in the alkylation of benzene with methanol over 3-aminopropyltriethoxysilane modified HZSM-5. Catal. Commun. 2019, 123, 6–10. [Google Scholar] [CrossRef]
- Li, B.; Chen, H.; Li, S.; Li, N. Distribution of Mo in Mo/ZSM-5 catalyst prepared by impregnation method. Chinese J. Catal. 2005, 26, 769–774. [Google Scholar]
- Gao, K.; Li, S.Z.; Wang, L.; Wang, W.Y. Study of the alkylation of benzene with methanol for the selective formation of toluene and xylene over Co3O4-La2O3/ZSM-5. RSC Adv. 2015, 5, 45098. [Google Scholar] [CrossRef]
- Liu, H.; Wei, H.J.; Xin, W.J.; Song, C.; Xie, S.J.; Liu, Z.N.; Liu, S.L.; Xu, L.Y. Differences between Zn/HZSM-5 and Zn/HZSM-11 zeolite catalysts in alkylation of benzene with dimethyl ether. J. Energy Chem. 2014, 23, 617–624. [Google Scholar] [CrossRef]
- Barthos, R.; Bánsági, T.; Zakar, T.S.; Solymosi, F. Aromatization of methanol and methylation of benzeneover Mo2C/ZSM-5 catalysts. J. Catal. 2007, 247, 368–378. [Google Scholar] [CrossRef]
- Ji, X.F.; Qin, Z.F.; Dong, M.; Wang, G.F.; Dou, T.; Wang, J.G. Friedel-Crafts acylation of anisole and toluene with acetic anhydride over nano-sized Beta zeolites. Catal. Lett. 2007, 117, 171–177. [Google Scholar] [CrossRef]
- Chakinala, N.; Chakinala, A.G. Process design strategies to produce p-xylene via toluene methylation: A review. Ind. Eng. Chem. Res. 2021, 60, 5331–5351. [Google Scholar] [CrossRef]
- Dong, P.; Li, Z.; Ji, D.; Wang, X.R.; Yun, H.F.; Du, Z.S.; Bian, J.; Li, G.X. Catalytic benzene mono-alkylation over three catalysts: Improving activity and selectivity with M-Y catalyst. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 149–155. [Google Scholar] [CrossRef]
- Venuto, P.B.; Landis, P.S. Organic Catalysis over Crystalline Aluminosilieates; Academic Press: New York, NY, USA, 1968; pp. 2–17. [Google Scholar]
- Wen, Z.H.; Yang, D.Q.; He, X.; Li, Y.S.; Zhu, X.D. Methylation of benzene with methanol over HZSM-11 and HZSM-5: A density functional theory study. J. Mol. Catal. A Chem. 2016, 424, 351–357. [Google Scholar] [CrossRef]
- Xu, W.Y.; Miller, S.J.; Agrawal, P.K.; Jones, C.W. Positive effect of water on zeolite BEA catalyzed alkylation of phenol with propylene. Catal. Lett. 2014, 144, 434–438. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, Z.; Wang, Y. Shape selective catalysis in methylation of toluene: Development, challenges and perspectives. Front. Chem. Sci. Eng. 2018, 12, 103–112. [Google Scholar] [CrossRef]
- Fen, Z.W. Production technology of para-xylene and research progress. Mod. Chem. Ind. (China) 2019, 39, 58–62. [Google Scholar]
- Yashima, T. Alkylation on synthetic zeolites I. Alkylation of toluene with methanol. J. Catal. 1970, 16, 273–280. [Google Scholar] [CrossRef]
- Al-Khattaf, S. Xylenes reactions and diffusions in ZSM-5 zeolite-based catalyst. Ind. Eng. Chem. Res. 2007, 46, 59–69. [Google Scholar] [CrossRef]
- Tsai, T.C.; Wang, I.; Huang, C.K.; Sheng, D.L. Study on ethylbenzene and xylene conversion over modified ZSM-5. Appl. Catal. A Gen. 2007, 321, 125–134. [Google Scholar] [CrossRef]
- Kim, J.H.; Namba, S.; Yashima, T. Para-selectivity of zeolites with MFI structure: Difference between disproportionation and alkylation. Appl. Catal. A Gen. 1992, 83, 51–58. [Google Scholar] [CrossRef]
- Yi, D.; Meng, X.; Xu, X.; Liu, N.; Shi, L. Catalytic performance of modified ZSM-5 designed with selectively passivated external surface acidity by phosphorus. Ind. Eng. Chem. Res. 2019, 58, 10154–10163. [Google Scholar] [CrossRef]
- Ghosal, D.; Basu, J.K.; Sengupta, S. Application of La-ZSM-5 Coated Silicon Carbide Foam Catalyst for Toluene Methylation with Methanol. Bull. Chem. React. Eng. Catal. 2015, 10, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Breen, J.P.; Burch, R.; Kulkarni, M.; McLaughlin, D.; Collier, P.J.; Golunski, S.E. Improved Selectivity in the Toluene Alkylation Reaction through Understanding and Optimising the Process Variables. Appl. Catal. A 2007, 316, 53–60. [Google Scholar] [CrossRef]
- Breen, J.; Burch, R.; Kulkarni, M.; Collier, P.; Golunski, S. Enhanced Para-Xylene Selectivity in the Toluene Alkylation Reaction at Ultralow Contact Time. J. Am. Chem. Soc. 2005, 127, 5020–5021. [Google Scholar] [CrossRef]
- Cavallaro, S.; Pino, L.; Tsiakaras, P.; Giordano, N.; Rao, B.S. Alkylation of Toluene with Methanol III: Para-Selectivity on Modified ZSM-5 Zeolites. Zeolites 1987, 7, 408–411. [Google Scholar] [CrossRef]
- Giordano, N.; Pino, L.; Cavallaro, S.; Vitarelli, P.; Rao, B.S. Alkylation of Toluene with Methanol on Zeolites. The Role of Electronegativity on the Chain or Ring Alkylation. Zeolites 1987, 7, 131–134. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zou, W.; Wang, C.; Li, L.; Liu, Z.; Zheng, A.; Kong, D.; Yang, W.; Xie, Z. Mass Transfer Advantage of Hierarchical Zeolites Promotes Methanol Converting into ParaMethyl Group in Toluene Methylation. Ind. Eng. Chem. Res. 2017, 56, 9310–9321. [Google Scholar] [CrossRef]
- Aboul-Gheit, A.K.; Aboul-Fotouh, S.M.; Emam, E.A.; Ahmed, S.M. Catalytic Para-Xylene Maximization. V. Toluene Methylation with Methanol and Disproportionation of Toluene Using Pt/ZSM-5 and Pt/Mordenite Catalysts. J. Chin. Chem. Soc. 2004, 51, 817–826. [Google Scholar] [CrossRef]
- Aboul-Gheit, A.K.; Aboul-Enein, A.A.; Awadallah, A.E.; Ghoneim, S.A.; Emam, E.A. Para-Xylene Maximization Part VIII: Promotion of H-ZSM-5 Zeolite by Pt and HF Doping for Use as Catalysts in Toluene Alkylation with Methanol. Chin. J. Catal. 2010, 31, 1209–1216. [Google Scholar] [CrossRef]
- Aboul-Gheit, A.K.; Abdel-Hamid, S.M.; El-Desouki, D.S. Catalytic Para-Xylene Maximization. IV. Hydroisomerization of MetaXylene on Catalysts Containing Platinum on Differently Steamed HZSM-5. Appl. Catal. A 2001, 209, 179–191. [Google Scholar] [CrossRef]
- Kaeding, W.W.; Chu, C.; Young, L.B.; Butter, S.A. Shape-selective reactions with zeolite catalysts: II. Selective disproportionation of toluene to produce benzene and p-xylene. J. Catal. 1981, 69, 392–398. [Google Scholar] [CrossRef]
- Tan, W.; Hou, K.; Liu, M.; Li, W. Effect of NiO modification on stability of nano-sized ZSM-5 catalyst for the para-selective methylation of toluene with methanol. Pet. Process. Sect. 2015, 31, 503–522. [Google Scholar]
- Liu, J.; Yang, Y.; Wei, S.A.; Shen, W.; Rakovitis, N.; Li, J. Intensified p-xylene production process through toluene and methanol alkylation. Ind. Eng. Chem. Res. 2018, 57, 12829–12841. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, M.T.; Chebbi, R.; Darwish, N.A. Process of p-Xylene Production by Highly Selective Methylation of Toluene. Ind. Eng. Chem. Res. 2013, 52, 13730–13737. [Google Scholar] [CrossRef]
- Tan, Y.T.; Zhu, R.; Zhang, X.; Tang, Y.; Zeng, Z. Kinetic model of toluene alkylation with methanol to para-xylene. Chem. React. Eng. Technol. 2016, 32, 120–128. [Google Scholar]
- Palomino, J. Alquilacion de Tolueno con Metanol Mediante Catalizadores de Zeolite ZSM-5 Modificados; Complutense University of Madrid: Madrid, Spain, 1991. [Google Scholar]
- Zhang, X.M.; Zhang, W.J.; Zhu, J.; Wang, F.D.; Jian, C.G. Study on new synthesis technology of mesitylene. Chin. J. Chem. Eng. 2002, 30, 70–73. [Google Scholar]
- Smith, L.; Cass, O.W. The mechanism of alkylation of xylene. J. Am. Chem. Soc. 1932, 54, 1603. [Google Scholar] [CrossRef]
- Norris, J.F.; Gordon, T.V. The rearrangement of the xylene: Kinetics and chloride. Nature 1939, 161, 2131. [Google Scholar]
- Wang, K.Y.; Wang, X.S. Comparison of catalytic performances on nanoscale HZSM-5 and microscale HZSM-5. Microporous Mesoporous Mater. 2008, 112, 187–192. [Google Scholar] [CrossRef]
- Zhang, W.J.; Zhang, X.M.; Zhu, J.; Wang, F.D.; Zhang, J.B.; Jian, C.G. Study on a new technology of synthesis of 1,3,5-trimethylbenzene from xylene. Chem. Ind. & Eng. Pro. (China) 2001, 10, 26–31. [Google Scholar]
- Song, L.P. Synthesis of 1,3,5-trimethylbenzene. Synth. Chem. (China) 1993, 1, 27–30. [Google Scholar]
- Hill, I.; Malek, A.; Bhan, A. Kinetics and mechanism of benzene, toluene, and xylene methylation over H-MFI. ACS Catal. 2013, 3, 1992–2001. [Google Scholar] [CrossRef]
- Moors, S.L.C.; Wispelaere, K.D.; Mynsbrugge, J.V.; Waroquier, M.; Speybroeck, V.V. Molecular dynamics kinetic study on the zeolite-catalyzed benzene methylation in ZSM-5. ACS Catal. 2013, 3, 2556–2567. [Google Scholar] [CrossRef]
- Bhat, Y.S.; Halgeri, A.B.; Prasada Rao, T.S.R. Kinetics of toluene alkylation with methanol on H-ZSM-8 zeolite Catalyst. Ind. Eng. Chem. Res. 1989, 28, 890–894. [Google Scholar] [CrossRef]
- Ramakrishna, M.; Subhash, B.; Musti, S.R. Kinetics of deactivation of methylation of toluene over H-ZSM-5 and hydrogen mordenite catalyst. Ind. Eng. Chem. Res. 1991, 30, 281–286. [Google Scholar]
- Stelo, J.L.; Uguina, M.A.; Valverde, J.L.; Serrano, D.P. Kinetics of toluene alkylation with methanol over Mg-modified ZSM-5. Ind. Eng. Chem. Res. 1993, 32, 2548–2554. [Google Scholar] [CrossRef]
- Al-Khattaf, S.; Rabiu, S.; Tukur, N.M.; Alnaizy, R. Kinetics of toluene methylation over USY-zeolite catalyst in a riser simulator. Chem. Eng. J. 2008, 139, 622–630. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Xu, S.L.; Ye, M. A numerical investigation on alkylation of toluene with methanol in fluidized bed reactor. Chem. Ind. Eng. Pro. (China) 2020, 39, 5057–5065. [Google Scholar]
- Wei, J. A mathematical theory of enhanced para-xylene selectivity in molecular sieve catalysts. J. Catal. 1982, 76, 433–439. [Google Scholar] [CrossRef]
- Wu, C. The Research on Preparation and Catalytic Properties of Shape-Selective Catalysts for Alkylation of Toluene with Methanol; Lanzhou University of Technology: Lanzhou, China, 2020. [Google Scholar]





| Catalyst | Performance | Surface Chemical Modification | Range of Conversions and Product Selectivity | Comments/Observations | Ref. |
|---|---|---|---|---|---|
| HZSM-5 | Shape selectivity | None | SPX > 99% | Here, inverse Al zoned HZSM-5 with sinusoidal channels predominantly opened to their external surfaces is constructed to maximize the shape-selectivity of HZSM-5 sinusoidal channels. | [44] |
| SiO2, P2O5, MgO | SPX∼98% | The combined modification with SiO2, P2O5 and MgO in a proper sequence can lead to a synergistic effect for tailoring the acid property and pore mouth of the catalyst. | [19] | ||
| Pt or Pd | SX ≈ 40% | Narrowing the pore opening. | [45] | ||
| Pt | CB = 46.9%; SX = 22.6% | Pt modified ZSM-5 catalyst was proved to have high suppressionability towards the formation of ethylbenzene and could improve the stability of the catalyst significantly. | [46] | ||
| NaOH | Ctotal by 3.3 times; S by 1.7 times | The results are well-rationalized by alterations of acidic properties, mesopore formation and improved diffusivity. | [47] | ||
| Hexadecyltrimethoysilane | CB = 59.5%; SX = 39.0%; SEB < 0.1% | The directly adjusting the Si/Al ratio of the catalyst can successfully suppress EB formation by suppressing the side reaction of methanol to olefins. | [14] | ||
| Si/Al | SX = 34.9% (Si/Al = 180) | The presence of additional porosity not only accelerates benzene methylation but also contributes to the faster sequential methylation of primary product toluene to xylenes. | [48] | ||
| Acidity | MgO, CaO | CB was reduced and SPX was improved | The basicity and size of basic cations that determine its catalytic performance. | [49] | |
| P2O5-ZnO | CB = 43.6%; SPX = 91.2% | It is found that the isomerization of p-xylene occurs on the strong acid sites rather than on the weak acid sites, and benzene conversion is subject to the influence of strong acid sites. | [1] | ||
| P2O5, Fe2O3, B2O3, MgO | SPX = 94.3%(P2O5) > 82.5% (B2O3) > 67.1% (MgO) > 57.0% (Fe2O3) | It was found that the strong acid could cause the disproportionation reaction of BTX, so the strong acid had a great influence on the conversion rate of catalyst. | [16] | ||
| Silanization | CB = 75.7%; SPX = 42.2% | DMCS20-HZSM-5 has an optimal level of acidity for catalyzing benzene alkylation with methanol. | [50] | ||
| Mo | Improved conversion rate | The B acid sites of the Mo/ZSM-5 catalyst decreased obviously compared with the parent HZSM-5 zeolite. On the contrary, the amount of L acid sites increase slightly in the Mo/ZSM-5 catalyst-[Mo5O12]6+. | [51] | ||
| Co3O4-La2O3 | SX = 38.07%; CB = 51.36% | Co3O4 and La2O3 incorporation in the zeolite has a significant role in activating the catalysts, which, together with the acidic sites of ZSM-5, produce the best acidic environment. | [52] | ||
| Zn | SX = 27.97%; CB = 46.04% | For 6Zn/HZSM-11 and 6Zn/HZSM-5, benzene can easily contact both of the active catalyst centers. Zn(OH)+ could increase L acid active sites at the expense of B acid. | [53] | ||
| Mo2C | CB = 2.5%; SX = 22.3% | The deposition of Mo2C on ZSM-5 markedly enhances the formation of aromatics. The highest yield of the formation of aromatics is measured at 5% for Mo2C/ZSM-5. | [54] |
| Catalyst | Performance | Surface Chemical Modification | Range of Conversions and Product Selectivity | Comments/Observations | Ref. |
|---|---|---|---|---|---|
| HZSM-5 | Shape selectivity | Phosphorus | CT = 25–30%; SPX = 98% | Selectively depositing phosphorus on the external surface and pore entrance of zeolites was shown to be effective in terms of p-xylene selectivity; these modified catalysts showed improved selectivity with less loss of catalytic activity. | [67] |
| SiO2, P2O5, MgO | SPX < 90%; CT = 30% | The deposition of MgO was found to be efficient in passivating the acid sites and narrowing the pore opening when compared with Si or P modification; para-selectivity did not exceed 90%, even at the maximum loadings with single modification; however, a synergetic effect of multiple modification in suitable sequence led to ∼98% para-selectivity. | [19] | ||
| La, Ce, Nb | CT = 12–36%; SX = 98% | Toluene conversion and para-selectivity was reported to be better using foam coated with lanthanum-modified ZSM-5; kinetic studies were reported with a foam-coated catalyst in which the Langmuir–Hinshelwood model with a dual-site surface-reaction-controlling step was fitted and the activation energy was reported to be 47 kJ/mol. | [68] | ||
| B, P, Mg, Fe, Ga, Pr, Tb, Sn, Al | CT = 0.3–11.2%; SPX = 23–99.9% | The most selective catalyst was found to be 10% B/ZSM-5 with >99.9% selectivity and 5.5% conversion. The Mg/ZSM-5 was less selective than B but had slightly higher activity (6.5%); the stability of BZSM-5 catalyst was noticed to be a major concern due to the loss of B during the reaction. | [69,70] | ||
| Acidity | Fe, B, P | CT = 14–28%; SPX = 49–62% | Activity and selectivities were dictated by the acidity and electronegativity of the zeolites, with the most electropositive ones producing ethylbenzene and styrene and the most electronegative catalyzing the formation of xylenes. | [71,72] | |
| MgO | CB = 13–18%; SPX = 35–88% | Surface modification of the zeolite catalysts with MgO suppressed the mass transport of all the reactions; the degree of utilization of isomerization decreased because of the removal of acid sites, and the MTH had a distinct decline; these results suggest that, by constructing appropriate mesopores, followed by covering up the external acid sites, isomerization and MTH reactions can be retained while maintaining a high methylation rate; decreasing the effective diffusion length was determined to favor the methanol usage for methylation. | [73] | ||
| Pd, Pt, HF | CT = 22–65%; SPX = 38% | The selectivities for para-, ortho-, and meta-xylenes production were determined to be dependent on the Pt content, particularly when supported on HZSM-5-zeolite; however, the selectivities were not dependent on the Pt content with mordenite; catalyst activity was reported to increase with 1–3% of HF, because of the increase in the number of acid sites and strength. | [74,75] | ||
| Boron and silicalite-1 coating | CB = 8–22.5%; SPX = ~98% | Boron modification led to a decrease in strong acidity but increased the total acid sites; meanwhile, with silicalite-1, there was a decrease in both strong acidity and total acidity. | [76] |
| Production Methods | Raw Material | Catalyst | Trimethylbenzene Yield | Advantages/Disadvantages | Ref. |
|---|---|---|---|---|---|
| Alkylation method | Mix-xylene, CH3Cl | AlCl3 | The content of trimethylbenzene reached about 14%. | Raw materials cheap and easy to obtain, higher product content. However, production of halogenated hydrocarbon pollution, serious corrosion of equipment, product production costs. | [83,88] |
| Olefin, mesitylene | Solid acid catalyst | The purity of 99% mesitylene products. | Low price, moderate alkylation reaction conditions. | ||
| Isomerization method | Pseudocumene | Mo, Ni/Mordenite, PtAl2(OH), HF, BF3, etc. | The conversion rate of pseudocumene was 45%, the selectivity of mesitylene was up to 98%. | Catalysts are expensive and yield was low. | |
| Combined isomerization and alkylation method | Trimethylbenzene | Solid acid catalyst | The purity of mesitylene was 98.5%, the total conversion rate of pseudocumene was over 90%. | The production cost of both trimethylbenzene and tetramethylbenzene was greatly reduced. Inhibits the accumulation of methyl-ethylbenzene. Increases the content of mesitylene. Reduces production cost. Improves conversion rate and product purity. | |
| Distillation method | Mixed oil | / | Small production. | Reserve investment was large, and production was small. | [83] |
| Sulfonated extraction method | Produces a large amount of waste acid that needs to be treated, the technical and economic indicators were poor, it had been basically eliminated. | ||||
| Cryogenic crystallization method | / | / | Small production. | Large investment, high energy consumption. | |
| Other methods | Acetone | H2SO4 | The yield was only 0.010~0.015. | The reaction device was simple, the raw material was easy to obtain, but the reaction cycle was too long, the yield was low. | [88] |
| HCl | The yield was only 0.010~0.015. | The reaction rate was assumed to be faster. |
| Reaction Type | Catalysts | Kinetic Model | Activation Energy/kJ/mol | k (m6/kgcat s) (×102) | Ref. |
|---|---|---|---|---|---|
| Benzene methylation | H-ZSM-5 | Diffusion limitations | 58 ± 3 | 6.8 | [89] |
| H-SPP | 58 ± 2 | 8.3 | |||
| H-ZSM-5 | Unimolecular/Bimolecular | 58 | 9.9 | [15] | |
| H-beta | 56 | 2.6 | |||
| Toluene methylation | ZSM-5 | Extended cluster | 147 | / | [90] |
| H-ZSM-5 | Diffusion limitations | 52 ± 4 | 48.1 | [89] | |
| H-SPP | 47 ± 3 | 31.4 | |||
| HZSM-8 | Langmuir–Hinshelwood–Hougen–Watson | 60.52 | 3.91 | [91] | |
| ZSM-5 | Time on stream | 67.79 | 4.83 | [92] | |
| ZSM-5 | Langmuir–Hinshelwood | 79.83 | 4.52 | ||
| MgZSM-5 | Power law and Eley–Rideal | 60.4 | / | [93] | |
| HZSM-5 | Power law | 57.1 | 5.29 | [43] | |
| Mordenite/ZSM-5 | 23.3 | 12.83 | |||
| ZM13 | 17.1 | 15.20 | |||
| USY | Time on stream | 43.18 | 0.0186 | [94] | |
| Zeolite | Power law | 76.66 | 8.46 | [81] | |
| Solid acid | Discrete particle model | 45.7 ± 0.4 | / | [95] | |
| Xylene methylation | ZSM-5 | Time on stream | 24.49 | 4.86 | [92] |
| HZSM-5 | Power law | 70.0 | 1.99 | [43] | |
| Mordenite/ZSM-5 | 15.3 | 18.29 | |||
| ZM13 | 17.8 | 10.29 | |||
| HZSM-8 | Langmuir–Hinshelwood–Hougen–Watson | 35.83 | 1.25 | [91] | |
| H-ZSM-5 | Diffusion limitations | 62 ± 3 | 13.4 | [90] | |
| H-SPP | 62 ± 3 | 10.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, P.; Shao, T.; Zhao, Y.; Ji, D.; Yang, Y.; Zhao, X.; Li, H.; Tian, J.; Wang, D.; Li, G. Progress of Methylation of C6-8~Arene with Methanol: Mechanism, Catalysts, Kinetic/Thermodynamics and Perspectives. Processes 2022, 10, 881. https://doi.org/10.3390/pr10050881
Dong P, Shao T, Zhao Y, Ji D, Yang Y, Zhao X, Li H, Tian J, Wang D, Li G. Progress of Methylation of C6-8~Arene with Methanol: Mechanism, Catalysts, Kinetic/Thermodynamics and Perspectives. Processes. 2022; 10(5):881. https://doi.org/10.3390/pr10050881
Chicago/Turabian StyleDong, Peng, Tingna Shao, Yu Zhao, Dong Ji, Yong Yang, Xinhong Zhao, Hongwei Li, Junying Tian, Dongliang Wang, and Guixian Li. 2022. "Progress of Methylation of C6-8~Arene with Methanol: Mechanism, Catalysts, Kinetic/Thermodynamics and Perspectives" Processes 10, no. 5: 881. https://doi.org/10.3390/pr10050881





