Abstract
This work is focused on optimising a low-temperature delignification as holocellulose purification pretreatment of Platanus acerifolia leaf waste for second-bioethanol production. Delignification was accomplished by acid-oxidative digestion using green reagents: acetic acid and 30% hydrogen peroxide 1:1. The effect of reaction time (30–90 min), temperature (60–90 °C), and solid loading (5–15 g solid/20 g liquid) on delignification and solid fraction yield were studied. The process parameters were optimised using the Box–Behnken experimental design. The highest attained lignin removal efficiency was larger than 80%. The optimised conditions of delignification, while maximising holocellulose yield, pointed to using the minimum temperature of the examined range. Analysis of variance on the solid fraction yield and the lignin removal suggested a linear model with a negative influence of the temperature on the yield. Furthermore, a negative effect of the solid loading and low effect of temperature and time was found on the degree of delignification. Then the temperature range was extended back to 60 °C, providing 71% holocellulose yield and 70% while improving energy efficiency by working at a lower temperature. Successful lignin removal was confirmed using confocal laser scanning microscopy. As evaluated by scanning electron microscopy, the solid structure presented an increased exposition of the cellulose fibre structure.
1. Introduction
The urgency of accelerating development in a sustainable direction within a context of social demand for practices in compliance with standards of minimum environmental impact is reflected in the prerequisite for intensification of existing processes and reduced risks of scaling up novel cleaner energy technologies based on renewable raw materials [1] while ensuring the continuity of energy supply. In this context, biomass is fundamental to a future energy sector adaptation and effectively mitigates climate change since energy production is the main source of carbon emissions [2]. Biofuels are crucial for reducing fossil-fuel dependency and greenhouse gas emissions. Bioethanol is a promising sustainable candidate for substituting gasoline [3]. Ethanol utilisation in the world is available up to 20% blend in gas fuels without modifying car engines.
Cellulosic ethanol presents an exciting and tangible but underdeveloped economic opportunity for ethanol producers, due to the fuel’s greater greenhouse gas (GHG) reductions [4]. Most of the produced bioethanol is considered a first-generation biofuel because the raw material for the process is starch or glucose, coming mainly from the arable land used for food production [5]. The major negative social impact of first-generation bioethanol production can be sorted by using Lignocellulosic Biomass as a starting material because of effective decoupling from food production. The limitation of the so-called second-generation bioethanol is the nature of the feedstock because of the presence of lignin [6], which hinders yield, and is considered a low-value residue. Many second-generation bioethanol industrial initiatives could fail if the technology gaps are not carefully addressed. A third of the bioethanol world supply was predicted to be produced in 2020, parting from lignocellulosic wastes (LCW) [7], but currently and unfortunately, less than 2% of the market is supplied by this methodology [8]. Most pilot experiences all over the world were unsuccessful at scaling up. Unit integration and system optimisation are the ultimate solutions for making the cellulosic ethanol production process [9].
Conversion of agro-industrial and urban wastes to energy is an innovative approach for waste valorisation and management, simultaneously mitigating environmental pollution. Utilising LCW is an attractive alternative for waste disposal from urban areas [10] or agricultural [11] and forestry sectors [12]. The cellulosic fraction of LCW can be converted into simple sugars that can be fermented into added-value platform chemicals [13]. Particularly, LCW conversion to ethanol has the potential to sustainably provide energy security by addressing the future renewable liquid fuel demand while addressing the issue of food vs. fuel controversy [14]. Garden and street forest waste is an important lignocellulosic feedstock recognised as an energy resource [15]. Besides conventional processing methods like burial, microbial composting [16], and biochar production, biorefinery concepts to produce biogas [17] and bioethanol from kitchen [18] and garden waste [19] have attracted global attention.
Platanus acerifolia is widely planted in major cities since it is a tough, durable tree that can tolerate severe pruning and smog. The plant typically grows up to 25 m and has a good shading capacity for dense leaves. This species is frequently found in many neighbourhoods around Buenos Aires. Thus, LCW waste can reach hundreds of tons per day. Although they are partially treated in a city’s recycling plant [20] and used for compost and pulping, specific information for optimising LCW valorisation is relevant for intensifying and diversifying the processes.
There are four basic processes involved in the biochemical production and obtaining of ethanol by yeasts from cellulosic biomass: pretreatment for cellulose separation from lignin (called delignification), enzymatic hydrolysis of cellulose (called saccharification), fermentation, and distillation. The optimisation of delignification and saccharification are key to improving fermentation efficiency. The pretreatment of LCW is a key stage in disrupting the recalcitrant structure of lignocellulose to increase the holocellulose availability for enzymatic saccharification [14]. LCW pretreatments for ethanol production require physical and chemical processes such as reducing the particle size of the biomass, reducing the crystallinity of cellulose, cleavage of the hemicellulose-lignin complex and separating lignin [14]. Fermentable sugar yield and lignin removal are thus the key indicators for selecting optimal biomass pretreatment [4].
Delignification of LCW strongly facilitates the hydrolysis of holocellulose to fermentable sugars involved in producing biofuels and other bio-based chemicals [21]. Chemical pretreatment using acidic or alkaline solutions, organic solvents, and ionic liquids improves the subsequent enzymatic degradation of cellulose by separating lignin from cellulose and hemicellulose [22], decreasing the crystallinity of the cellulosic component in biomass [14]. The delignification processes traditionally used in the extraction of cellulose for paper production and the methods that use strong acids and alkalis are not adequate to generate a bioavailable substrate for fermentation [23]. Classical dilute acid pretreatments and processes requiring water vapour, such as steam explosion [22], require high temperatures and the remaining lignin still hinders access to the cellulose fibres [17].
Moreover, high temperature and extreme pH favour the formation of aliphatic acids and furans [24], which are inhibitors of saccharification enzymes [25] and toxic to fermenting microorganisms [26,27]. Therefore, developing low-temperature pretreatments is important to reduce energy consumption and avoid the production of inhibitors of enzymatic and fermentative activity [28]. The high oxidative mixture of hydrogen peroxide and acetic acid (PoxAc) has been suggested as a low-temperature pretreatment with high lignin removal efficiency and reducing the holocellulose crystallinity [21] leading to significant recovery of fermentable sugars [29].
The PoxAc delignification process [29] is based on a mixture of hydrogen peroxide in glacial acetic acid, which allows cellulose to be separated and obtained high-quality lignin to be used in a variety of applications, such as Supercritical Water Gasification to produce hydrogen [30] and materials for 3D printing [31]. The PoxAc delignification method uses reagents that decompose into harmless compounds and produces a substrate that allows better productivity of sugars through enzymatic and biological treatments [21,29]. In addition, delignified LCW can be used for other purposes, such as fluid rheology modifiers [32], as reinforcement material in composites and biodegradable polymers, and as strength additives in textile printing and coating products [33,34], among other applications.
This work aims to understand the influence of the key PoxAc LCW pretreatment operational variables towards second-generation bioethanol production. The specific objective of the current article is to optimise conditions to attain delignification of the Platanus acerifolia leaf waste arising from the street sweeping. For this purpose, the low-temperature acid-oxidative hydrolysis of the LCW is accomplished by digestion in a solution of glacial acetic acid and 30% hydrogen peroxide 1:1 (v/v) ratio, which was suggested as the best proportion for delignification [29]. An exploration for the optimum operational conditions was performed by quantifying the influence of the pretreatment on delignification and holocellulose yield at temperatures between 70 and 90 °C, during 30 to 90 min working with a 1 to 4 liquid to solid (L/S) mass ratio. A multivariate model was built from a Box–Behnken Design of Experiment for three independent variables and three levels. Untreated biomass and samples treated with dilute sulphuric acid were used as references. The optimisation was carried out to maximise lignin removal with concomitant maximisation of solid holocellulose yield. Lignin wet chemistry measurements were compared with Confocal Laser Scanning Microscopy (CLSM) images. Biomass structural changes were explored by Scanning Electron Microscopy (SEM).
2. Materials and Methods
2.1. Raw Material Preparation
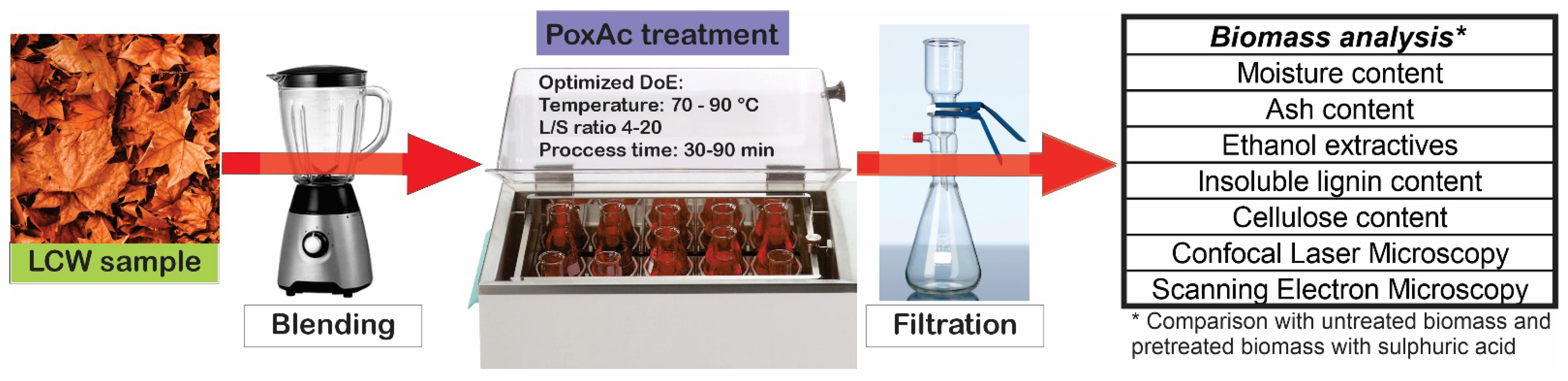
The leaf waste was obtained from the leaves sweeping a street within Buenos Aires city, where all the planted trees were Platanus acerifolia. The material (6 kg) was separated from dust in an industrial sieve, mainly retaining dry leaves and stems. Then, the LCW was humidified, added to the same water mass, and triturated in batches of 1 kg using a 2200 W Turbo blender at 35,000 rpm for 10 min. The resulting LCW was homogenised and kept in a freezer for subsequent characterisation and experiments. Analytical grade hydrogen peroxide, acetic acid, and sulphuric acid were used for the experiments. Figure 1 summarises the workflow of the biomass pretreatment and characterisation methodologies.

Figure 1.
LCW pretreatment and comparative analysis.
2.2. PoxAc Pretreatment
The oxidative hydrolysis was carried out in batch mode in 100 mL Erlenmeyer, with orbital shaking. A solution (20 mL) containing equivalent volumes of glacial acetic acid and 30% hydrogen peroxide was contacted with different masses of LCW (5 to 15 g). The orbital shaker allowed for regulating temperature and time. The temperature was modified within the 60–90 °C range, while digestion was varied between 30 and 90 min. For reference, one sample was treated with a solution of sulphuric acid 1 M under the conditions (90 min, 90 °C, 5 g) that were found as most severe within the explored operational window.
2.3. Biomass Proximal Composition
The moisture of the samples was determined using a Precisa XM50 moisture analyser (Precisa Gravimetrics AG, Dietikon, Switzerland). Standard methods from the Technical Association of the Pulp and Paper Industry (TAPPI) were implemented to determine the ash content (TAPPI T 211, [35]) and solvent extractives (TAPPI T 204, [36]).
Lignin acid-insoluble contents of the untreated and treated samples were determined by the TAPPI T222 test method [37]. A sample portion of 0.3 g was mixed with 4 mL of 72% v/v sulphuric acid and left to react for 3 h at room temperature (28 °C). Then, the solution was diluted to 5% v/v (1 M) with distilled water and heated to 100 °C for 2.5 h. The suspension was filtrated, dried, and weighted. The lignin content was obtained as the final dry mass, referred to as the initial solid dry mass.
The cellulose content of the untreated sample was determined by dissolving 0.3 g of the substrate into 4 mL of 72% v/v sulphuric acid. Immediately afterwards, the solution was diluted to 5% v/v (1 M) with distilled water, and the hydrolysis proceeded by heating to 100 °C for 2.5 h. The suspension was filtrated, dried, and weighted [38,39]. The cellulose content was determined as the difference between final and initial dry masses.
The treated samples were filtered from the acid-oxidant liquor with a cheesecloth. Moisture and lignin content of the obtained solid samples were determined to get the solid fraction yield and lignin removal efficiency, as expressed in Equations (1) and (2), where and are the initial and final mass of solid samples on a dry and ash-free basis, and %Lignin indicates the fraction of insoluble lignin on a dry and ash-free basis.
For data analysis, the so-called severity factor [40], Ro, defined as in Equation (3), was considered, where “t” represents the digestion time in minutes, “T” is the treatment temperature in °C, and “Tref” is 100 °C, a reference temperature [10,25].
2.4. Confocal Laser Scanning Microscopy (CLSM)
Variations of the lignin content between treated and untreated samples were assessed by CLSM, discriminating the leaves and stems portions of the LCW. Under the conditions used to observe the samples, lignin exhibits autofluorescence and can be easily distinguished by this technique from other components without contrast reagents [41,42]. The images were obtained using an Olympus FV300/BX61 confocal microscope (Olympus, Japan).
The samples were first dried, fixed on glass slides, and covered with a thin glass lid. They were first observed under an optical microscope (UPLFL 10X). After establishing the proper focus, multiple stacks of 1024p × 1024p pictures were taken with laser excitation at 405 nm and fluorescence emission detection at 550 nm, characteristic of lignin, in scanning mode [41]. Magnification was at 10× and an objective lens (UPLFL 10X). Images were analysed using the ImageJ open-source software (https://imagej.nih.gov/, accessed on 10 March 2022). The stack was averaged over the Z-axis and converted to a 0–255 grayscale (8-bit). Mean intensity was calculated from these images by averaging all pixels of the best-focused image, as suggested by Hernández-Hernández et al. [42,43].
2.5. Scanning Electron Microscopy (SEM)
Changes in surface morphology of untreated and delignified samples were examined under a scanning electron microscope Carl Zeiss NTS-Supra 40 (Carl Zeiss AG, Oberkochen, Germany). Before analysis, the samples were fixed on the sample plates using carbon tape as a non-conducting adhesive. Then, the samples were subjected to gold sputtering to increase their conductivity before taking the micrographs.
3. Experimental Design
The acid-oxidative hydrolysis of the LCW is a multivariable process in which the variables interact with one another. Therefore, experimental design techniques present a more balanced alternative to the ceteris paribus (i.e., one variable at a time) approach to obtain information and improve the conditions for the delignification stage while optimising holocellulose yield [44,45]. The Statistical Design of Experiments (DoE) combined with the Response Surface Methodology (RSM) analysis covers only a fraction of the experimental space. The DoE-RSM method allows for drawing effective conclusions while estimating the interactions between the different variables. Methods for experimental design can be broadly divided into two categories:
- (i)
- One includes designs used to explore a potentially large number of input variables to discover statistically significant ones and estimate their magnitude. Plackett–Burman (PBSD) and Factorial Fractional designs are examples of these methods [46].
- (ii)
- The other category includes methods for optimising a process given a reduced number of variables selected previously. A typical scenario is Central Composite (CC) or Box–Behnken Design (BBD) and an RSM analysis on linear or quadratic models. Moreover, RSM analysis allows fine-tuning of any process variables, an issue to be considered when an industrial scale is aimed [46]. The objectives of the RSM are to confirm observed or assumed effects in the variable selection stage and quantify the most appropriate values for the parameters under study to optimise the response. Finally, the predicted optimum must be verified after model building and optimisation.
Box–Behnken Design of Experiment
Box–Behnken DoE aims to find the effects of factors, estimate the curvature or quadratic effects, and determine the most appropriate values for the parameters under study to find the response surface and minimise the required set of experiments. The influences of solid loading, acid-oxidative hydrolysis temperature, and time on delignification efficiency and solid fraction yield were evaluated using full factorial design in Minitab 17 software. Mathematical matrices were constructed within the ranges of the chosen variables. The three independent variables have three levels (−1, 0, and +1; Table 1) and three central points, leading to 15 combinations (Table 2). The severity factor calculated from the time and temperature conditions is included in Table 2. Apart from the combinations arising from the Box and Behnken Design, the hydrolysis conditions with sulphuric acid were compared. The experiments carried out to extend the analysis of the temperature influence are indicated in Table 2.

Table 1.
Levels of factors tested in the Box and Behnken Design of Experiments for optimising LCW delignification.

Table 2.
Factor combinations considered for the Box and Behnken Design.
4. Results and Discussion
4.1. Effect of the Acid-Oxidative Hydrolysis on the LCW Characteristics
The proximal composition of the Platanus acerifolia leaf waste was studied before and after pretreatment to evaluate their potential for biofuel or biochemical production. LCW moisture was evaluated every time it was used for experiments to assess the exact solid dry mass. The dry leaf waste contained a minor amount of ash (3.2% w/w) and ethanol extractives (4% w/w), significant insoluble lignin (44% w/w), cellulose (12% w/w), and hemicellulose (36% w/w). After hydrolysis, moisture and lignin content were determined to get the solid fraction yield and delignification efficiency. Figure 2 illustrates a photograph of the untreated sample and samples treated with dilute sulphuric acid (Run #S1 of Table 2) The severity factor calculated from the time and temperature conditions is included in Table 2 in a logarithmic form. Apart from the combinations arising from the Box and Behnken Design, the hydrolysis conditions with sulphuric acid were compared. The experiments carried out to extend the analysis of the temperature influence are indicated in Table 2 and the acid-oxidative hydrolysis (Runs #6, #7, #11, and #E1). Apart from the combinations arising from the Box and Behnken Design, the hydrolysis conditions with sulphuric acid were compared. There is a significant difference in the colour of the sample, which correlates with the lignin content.

Figure 2.
Photograph of the untreated LCW, the LCW pretreated with a dilute sulphuric acid solution (Run #S1-Table 2), and the LCW pretreated by the acid-oxidative hydrolysis at conditions of Run #6, #7, #11, and E1.
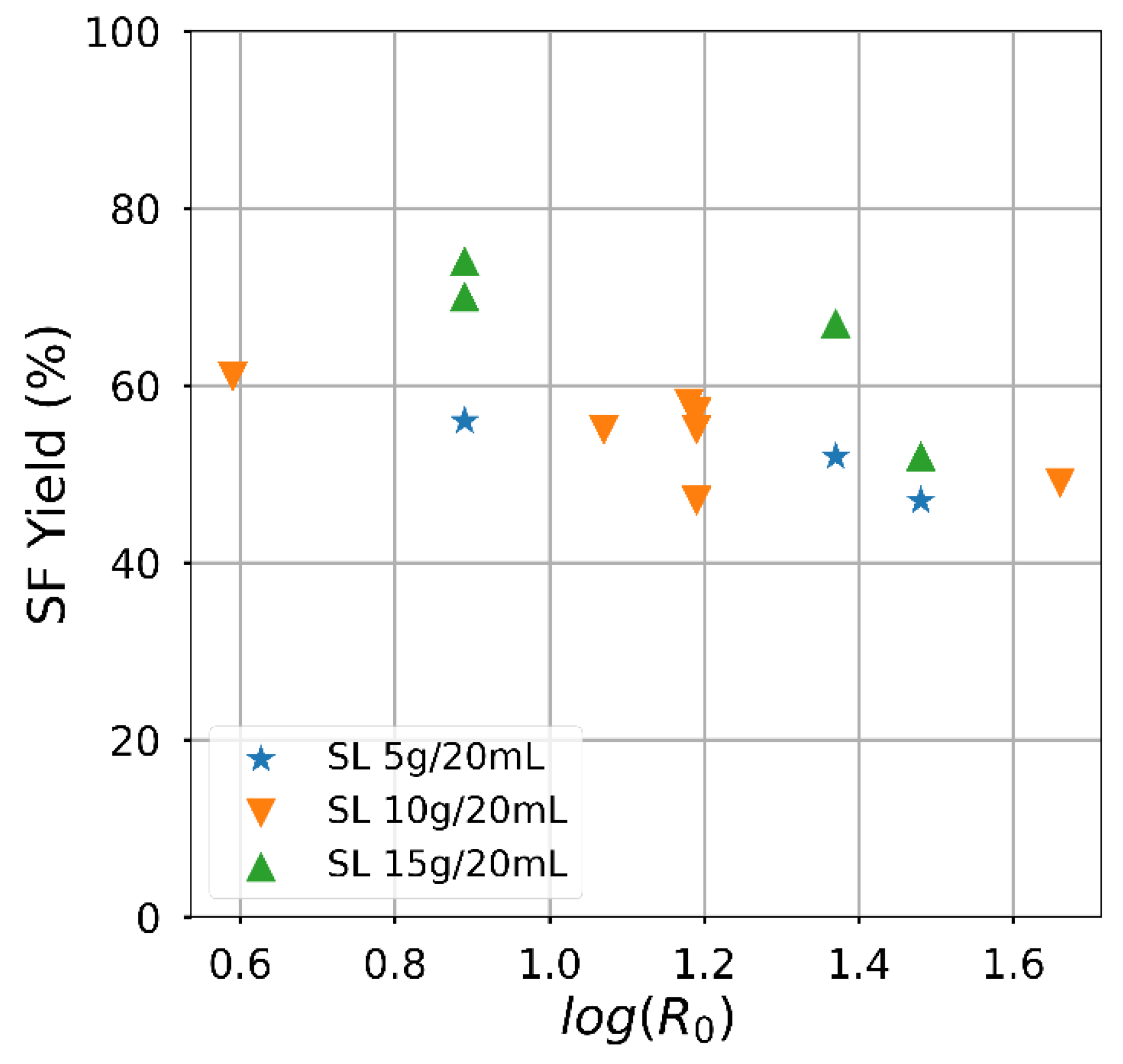
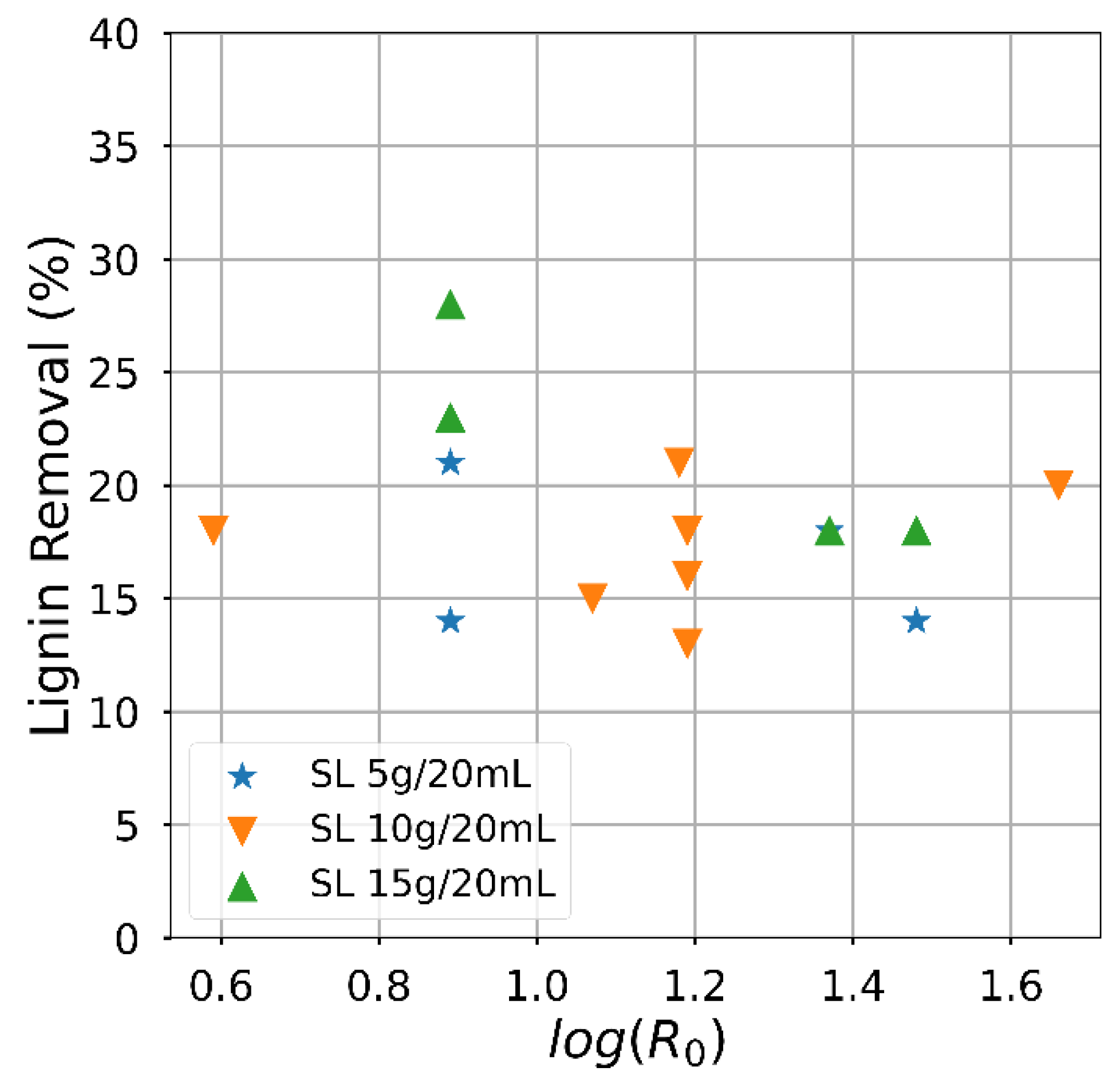
Attaining a high solid fraction with the acid-oxidative hydrolysis pretreatment since the carbohydrates usable for bioprocess remain in the solid. The solid fraction decreased as the severity increased (Figure 3), mainly related to the temperature effect. The yield had an almost linear negative dependence on the severity factor, particularly for the lowest solid loading. On the contrary, the effect of the severity factor on the delignification efficiency (Figure 4) was slightly positive, and an influence of the solid loading was perceived. Despite significant variations, the attained delignification was always larger than 60% except for one condition corresponding to the highest solid loading examined. The highest delignification degree was attained for the lowest examined solid loading, reaching a value of 85.2% for Run #4 of Table 2. The severity factor calculated from the time and temperature conditions is included in Table 2. Apart from the combinations arising from the Box and Behnken Design, the hydrolysis conditions with sulphuric acid were compared. The experiments carried out to extend the analysis of the temperature influence are indicated in Table 2, although solid yield for this condition was less than 50%.
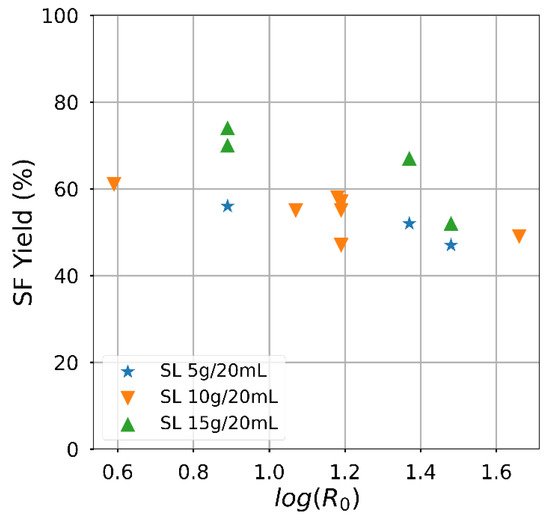
Figure 3.
Semi-log plot showing the effect of the severity factor on the solid fraction (SF) yield.
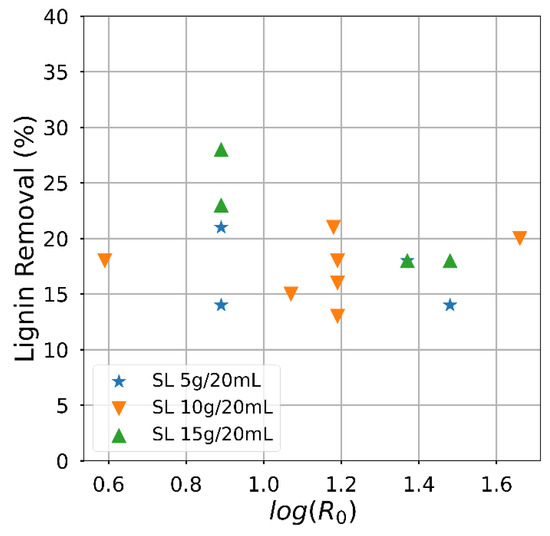
Figure 4.
Semi-log plot showing the effect of the severity factor on the delignification efficiency, represented by the proportion of lignin removal.
4.2. CLSM Autofluorescence Imaging
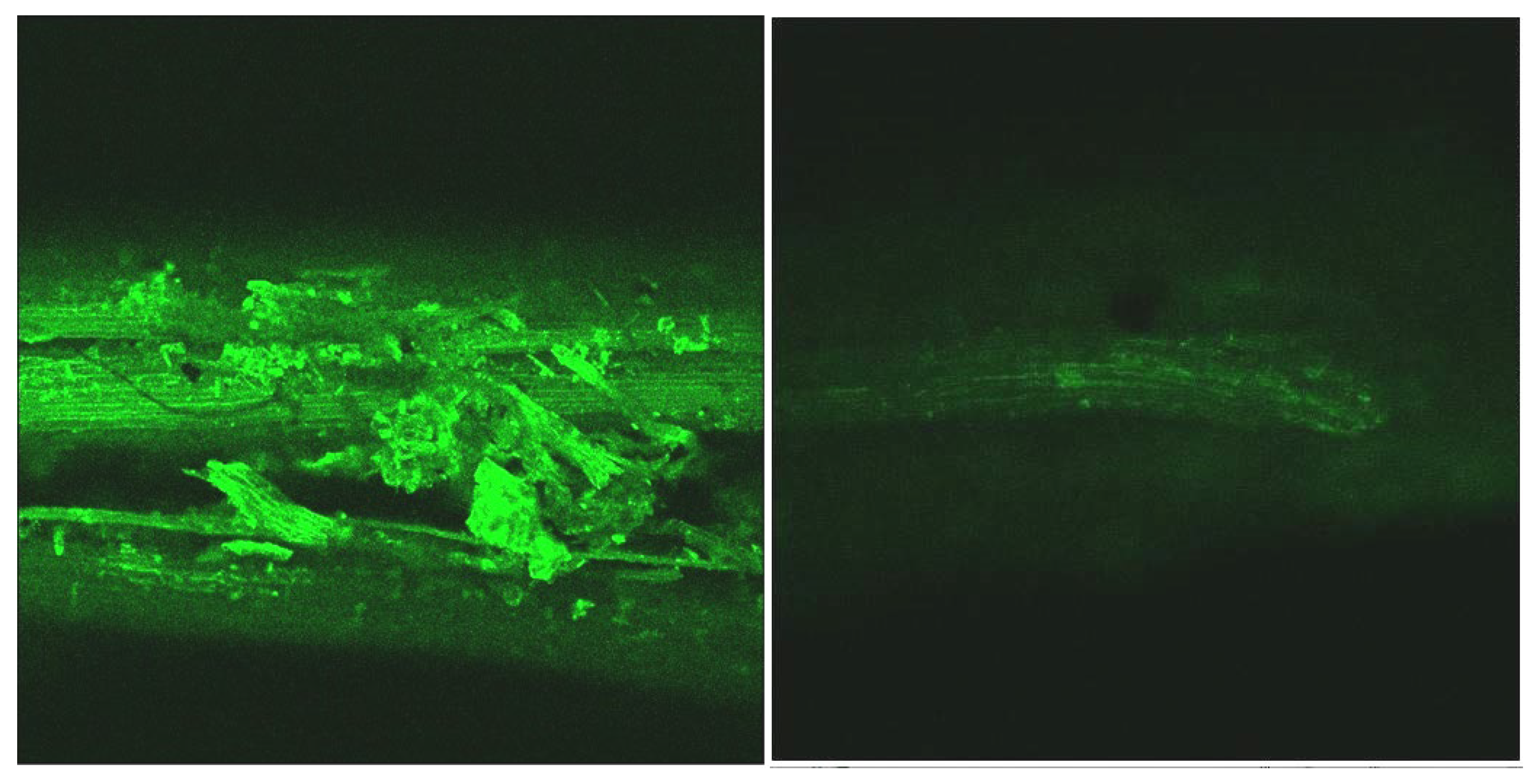
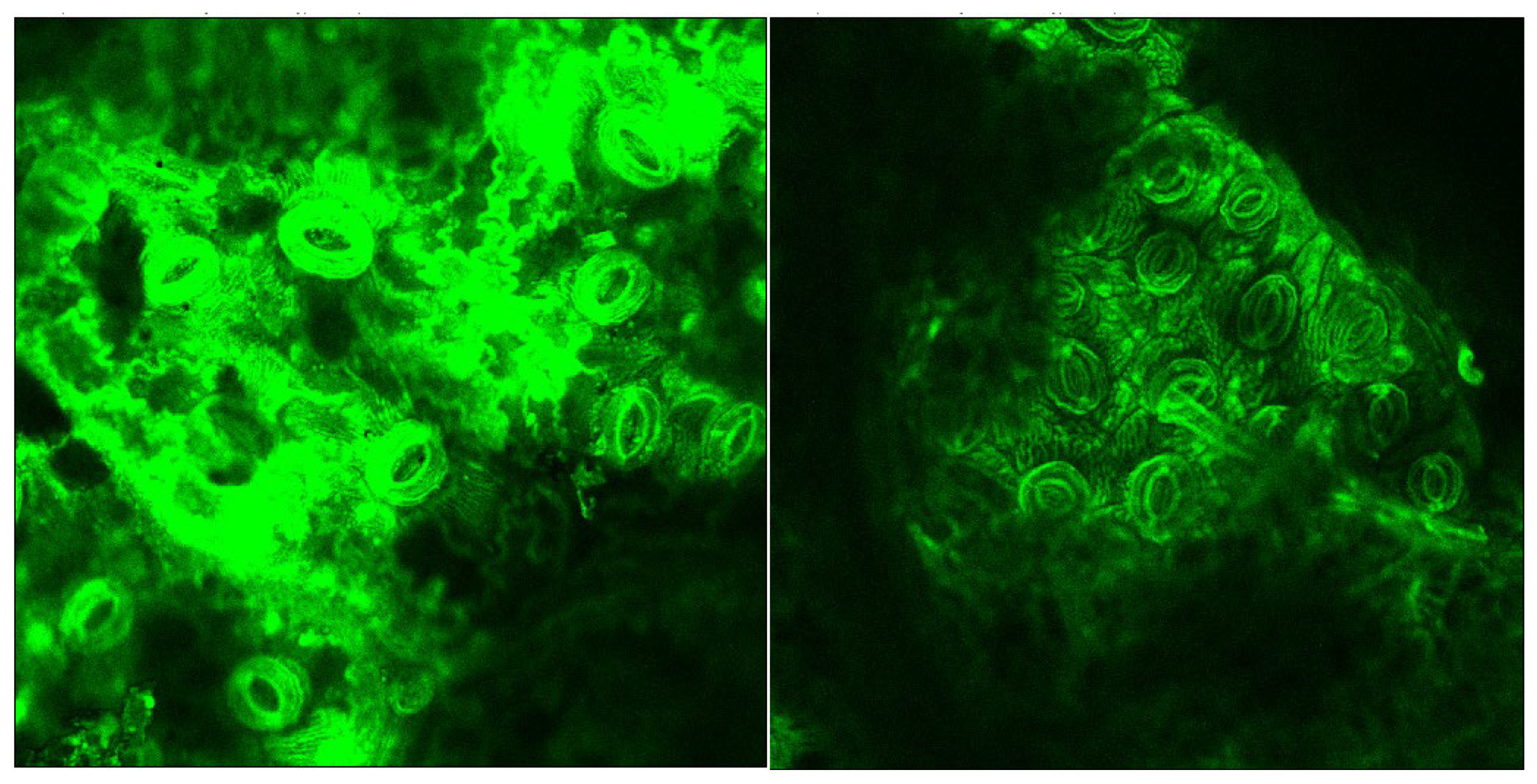
Figure 5 and Figure 6 show micrographs highlighting the lignin autofluorescence (in green) of the samples before and after the acid-oxidative hydrolysis, keeping the same laser excitation intensity, optical augmentation, and scan rate. Images were taken for the untreated sample and obtained after runs #4, #6, and #S1.
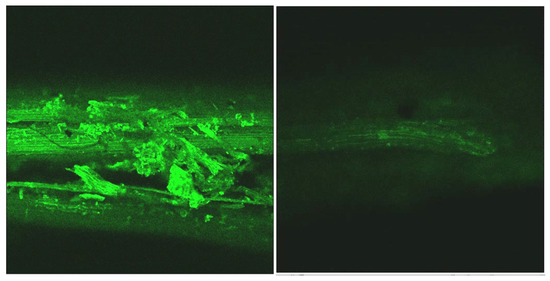
Figure 5.
CLSM taken from the portion containing stems of the untreated sample (left) and the sample after the oxidative hydrolysis under Run #4 (right) conditions.
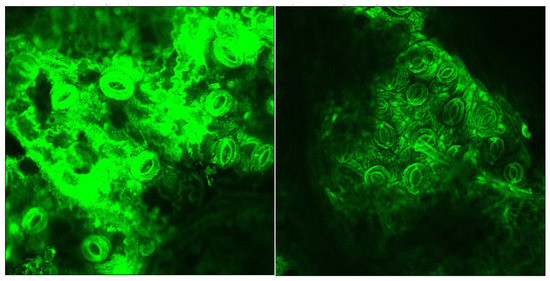
Figure 6.
CLSM taken from a portion of the untreated leaf sample (left) and the sample after the oxidative hydrolysis under Run #4 (right) conditions.
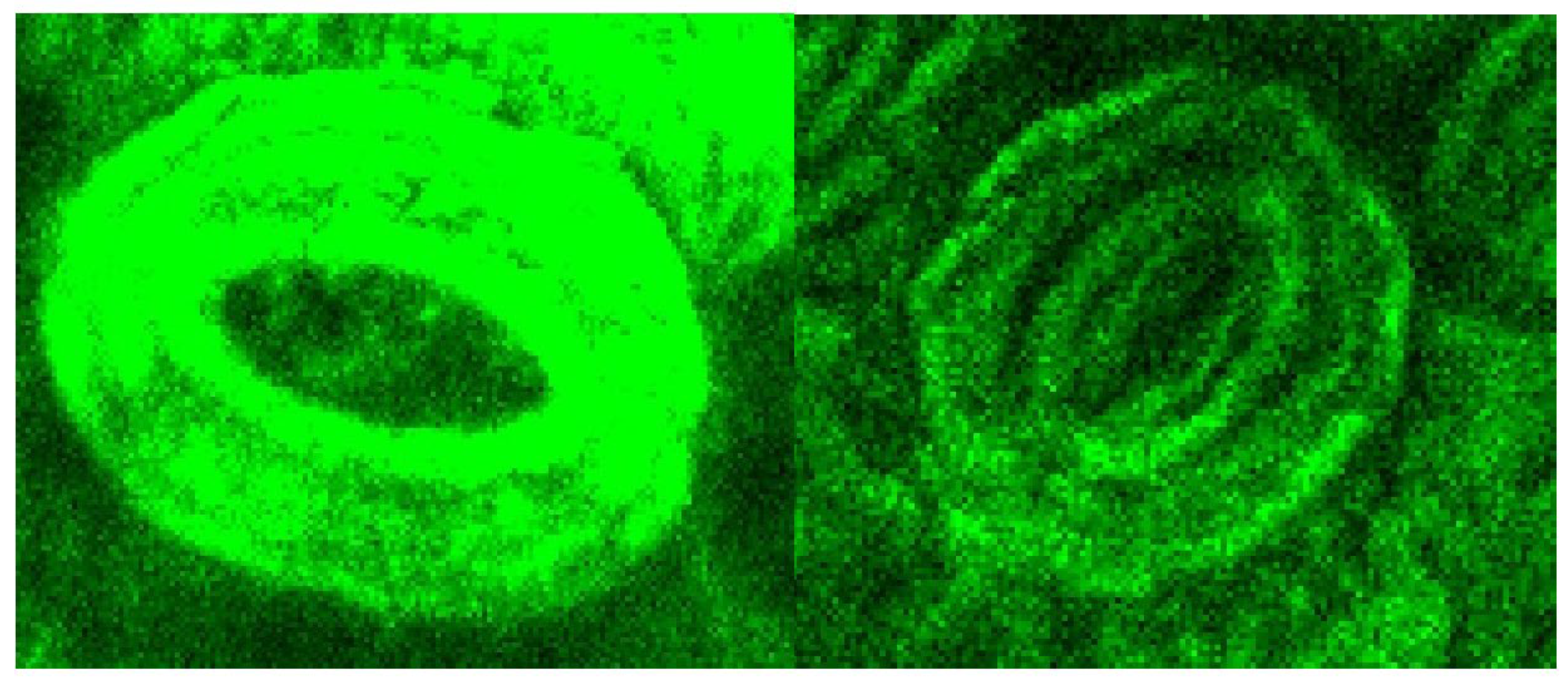
A significant reduction of the lignin autofluorescence intensity was observed in the treated samples compared to the original, both in the leaves and stems portions of the LCW. Figure 7 shows details of a stoma from the nerve side of the leaves before and after treatment illustrating that the lignin content was high in the stomata region and largely removed by the treatment.
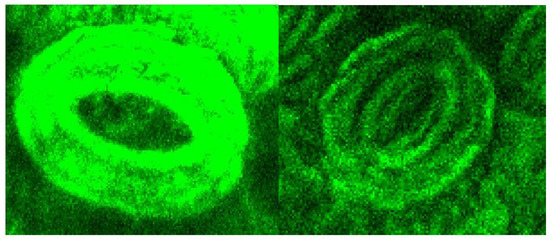
Figure 7.
CLSM of a stoma from a portion of the untreated leaf sample (left) and the sample after the oxidative hydrolysis under Run #4 (right) conditions.
Mean fluorescence intensity was estimated from the images using the ImageJ software by averaging over the Z-axis converted to a 0–255 grayscale (8-bit). It was found that the mean intensity correlated well with the amount of lignin in the sample (Table 3). The fluorescence intensity of the samples decreased after the acid-oxidative hydrolysis, proportional to the severity factor. However, despite having the highest severity factor, the sample treated with sulphuric acid showed the highest lignin content and fluorescence intensity, indicating the low degree of delignification and partial dissolution of cellulose.

Table 3.
Autofluorescence intensity as a function of the treatment severity.
4.3. SEM Imaging
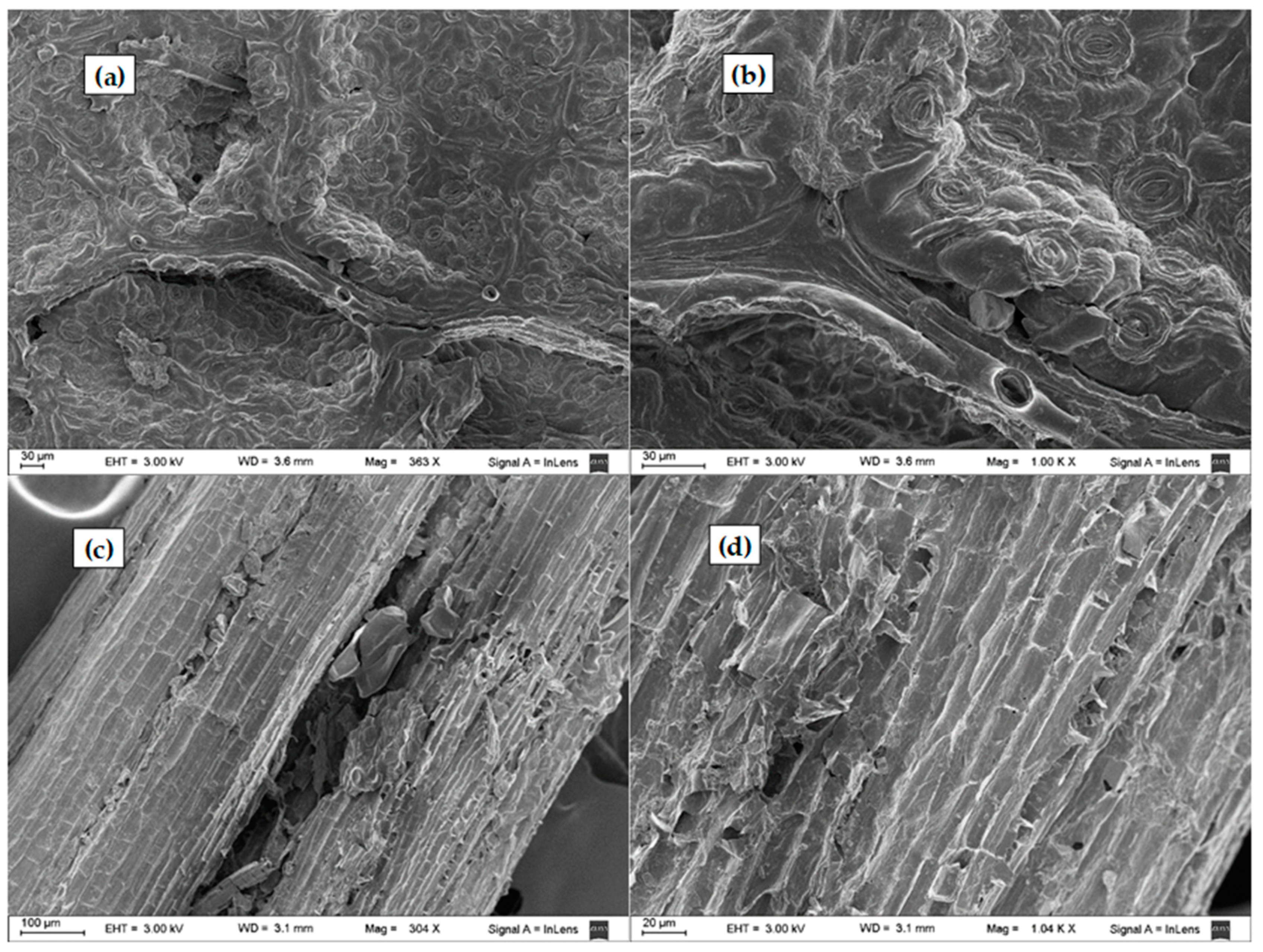
The surface morphological characteristics of the untreated and treated LCW samples were analysed by SEM, and representative results are presented in Figure 8 and Figure 9. Leaves and stem structures were observed in the SEM of the untreated sample (Figure 8). Stomata with guard cells were observed in the leaves (Figure 8a,b), as previously reported (Pourkhabbaz et al., 2010). The untreated leaf samples exhibited a compact, non-porous, uniform appearance of surface structure. In contrast, the stems presented porous longitudinal arrangement of fibrils (Figure 8c,d), as observed for other LCW (Hernández-Hernández et al., 2014).
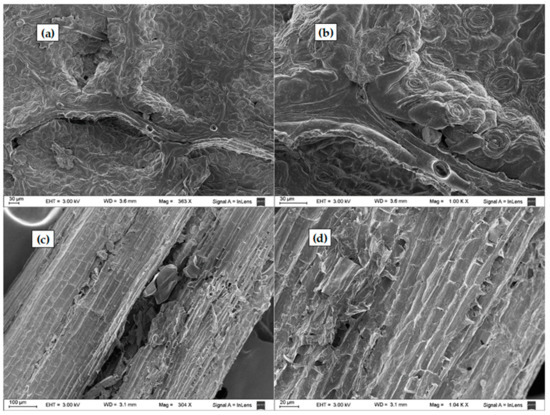
Figure 8.
SEM photographs of (a,b) untreated leaves stomata and (c,d) untreated stems.
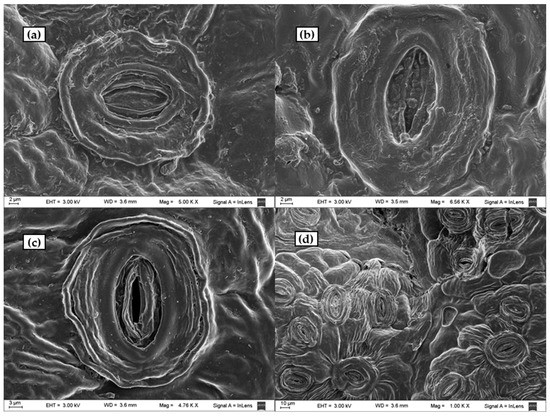
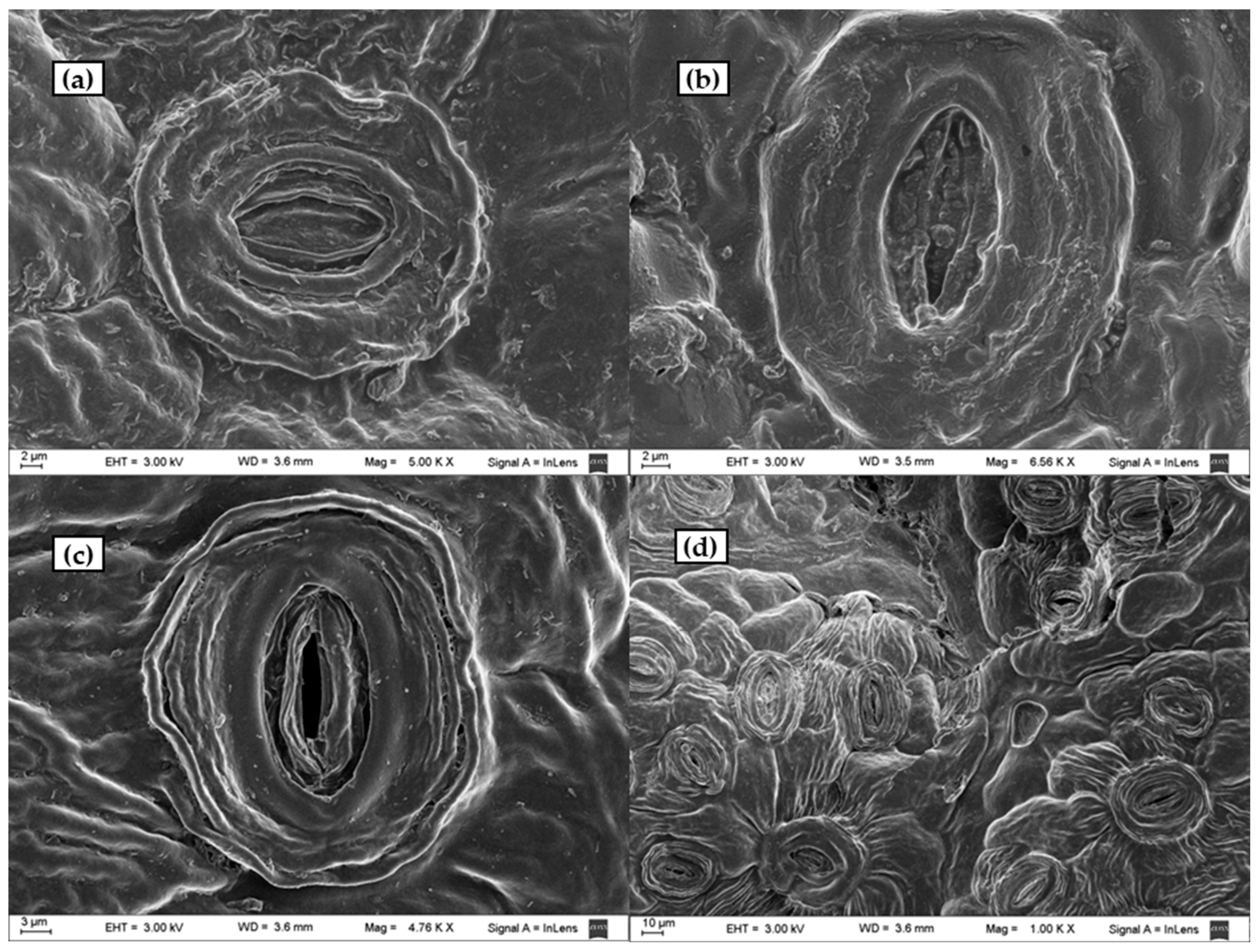
Figure 9.
SEM photographs of: (a) untreated leaf stoma; (b) leaf stoma of a sample digested with a dilute solution of sulphuric acid at T = 90°, t = 90 min using 5 g solid/20 mL liquid ratio; (c,d) leaf stomata of a sample digested at T = 90°, t = 90 min using 10 g solid/20 mL liquid ratio.
Enlargements of stomata untreated and treated with the acid-oxidative solution and with dilute sulphuric acid are illustrated in Figure 9 for comparison. The stomata structures were preserved after the treatments. However, the untreated surface (Figure 9a) was apparently filled and covered by lignin, as confirmed by CLSM. The sample treated with sulphuric acid (Figure 9b), with negligible delignification, appeared very similar to the untreated sample. Physical alterations of surface morphology observed in the samples treated with the acid-oxidative method for which lines were more defined and less uniform (Figure 9c,d) could be due to lignin removal. SEM images revealed that the treatment further exposed cellulose fibres by dissolving the covering lignin, turning the substrates rougher. Even though the cellulose fibre structure was exposed, the polymer structure was kept, suggesting that the hydrolysis to develop reducing sugars may require tough conditions.
4.4. Pretreatment Performance Response Surface Regression
The results obtained from the pretreatment DoE were analysed based on RSM. A polynomial quadratic regression equation was obtained, representing the effect of independent factors and their interactions towards the output (solid fraction yield or % delignification). The interactive effects of parameters were analysed based on 3D response surface plots. Each response was tested for a suitable best-fitting model. Analysis of variance (ANOVA) was done for the model terms (Table 4). The measured responses will be subjected to multiple least squares regression analysis. The Student’s t-test is used to evaluate statistical significance. Fischer’s F-test weights the adequacy of the mathematical regression model.

Table 4.
ANOVA of the RSM analysis for the solid fraction yield.
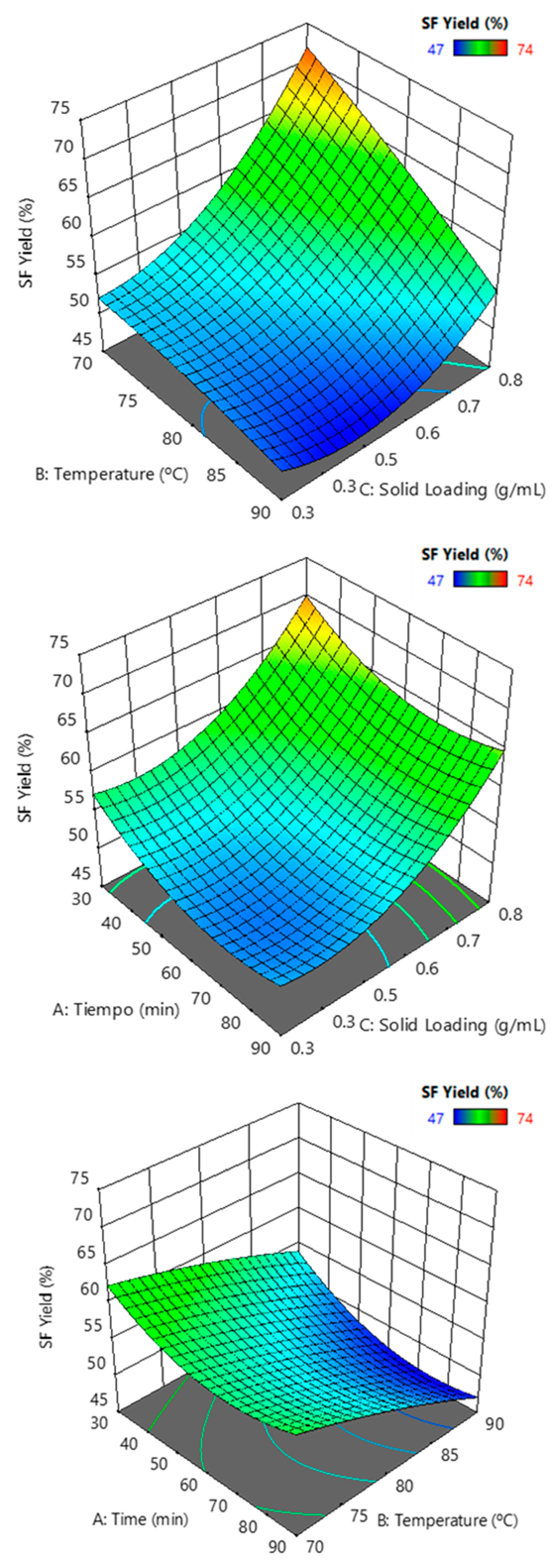
Interpretation of the parametric interaction of hemicellulose yield and lignin removal was evaluated as a combined effect of the three factors: time, temperature, and solid loading. Analysis of variance indicated a satisfactory linear model fit the solid fraction yield, with a significant influence on the temperature and solid loading (Table 4). Maximum yields were attained for lower temperatures and the highest solid loadings (Figure 10).
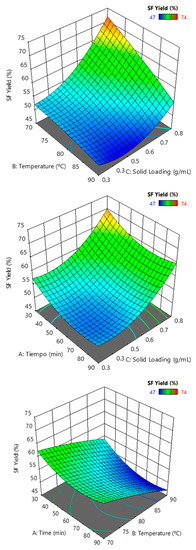
Figure 10.
Solid fraction yield (SF Yield) response surfaces against binary combinations of solid loading, temperature, and time.
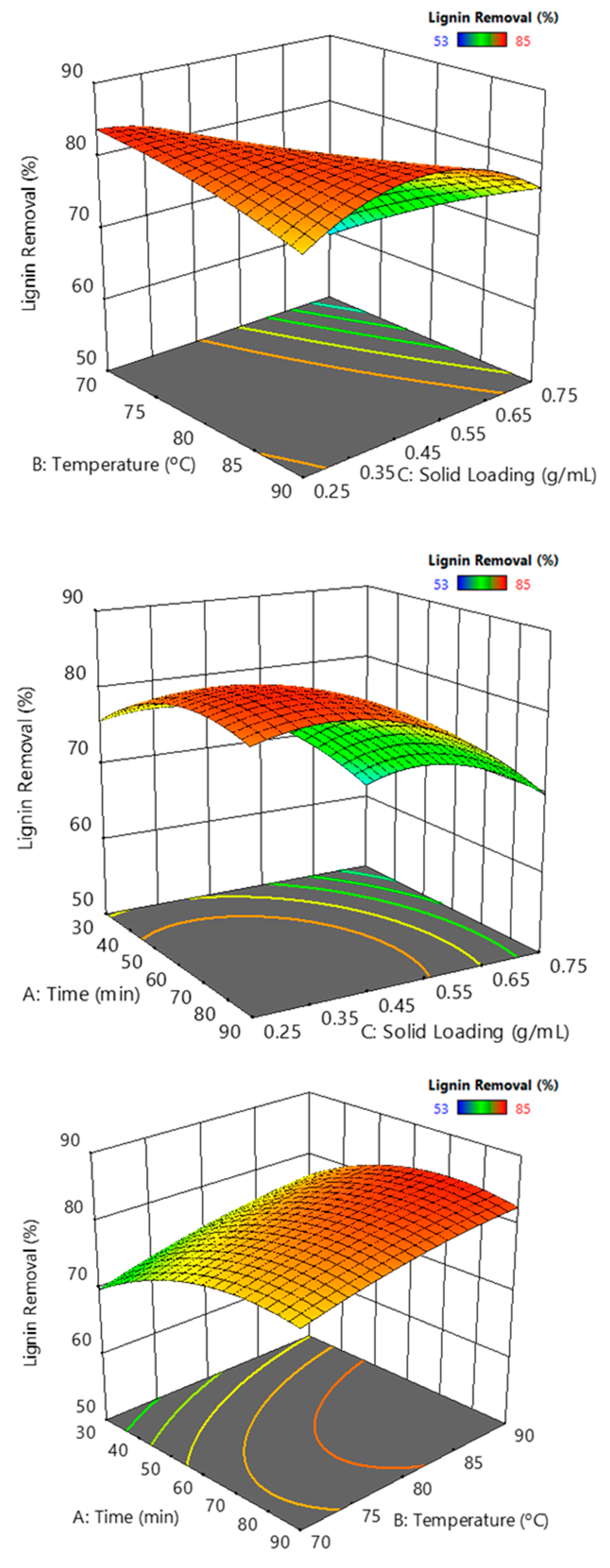
Delignification was not well predicted by a linear or a single quadratic model; the best fit with a full quadratic model led to an R square of 76%. The analysis of variance indicated a significant influence of the solid loading and the temperature-solid loading interaction. As observed in Figure 11, the effect of solid loading on delignification is negative, interpretable mainly by the stoichiometry of the reaction between the oxidant hydrogen peroxide and the lignin, which must be largely deconstructed to be extracted. However, the solid loading variable has a significant positive influence on the solid yield, which should be considered since the solid contains fermentable sugars. Temperature influence depends on the solid loading; it slightly affects the lowest solid loading. The optimum temperature found within the operation range can be interpreted as a compromise relationship between the severity factor and the thermal decomposition reaction of hydrogen peroxide, which competes with lignin oxidation. The hydrolysis time did not significantly modify the results; however, the surface response suggested that 60 min hydrolysis would provide better delignification.
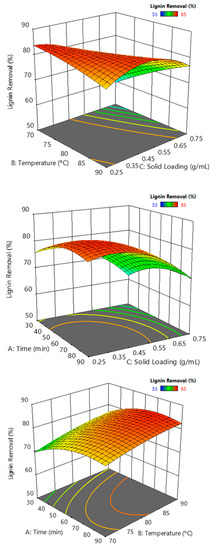
Figure 11.
Lignin removal response surfaces against binary combinations of solid loading, temperature, and time.
4.5. Impact of LCW Pretreatment Optimisation on Bioethanol Productivity
The few commercial scale-up experiences of second-generation bioethanol production have largely failed due to not being able to overcome energy efficiency requirements and waste disposal issues [9]. In line with recent literature, we identified LCW pretreatment as the main roadblock affecting second-generation bioethanol competitiveness [4,14]. PoxAc pretreatment has a very promising prospect from the point of view of sustainability both due to the use of green reagents [21,47] while uniquely avoiding enzyme inhibitors and toxic molecules for fermenting microorganisms. LCW pretreated by PoxAc constitutes a highly suitable substrate for enzymatic saccharification with enzyme complexes containing cellulase and xylanase activities to produce fermentable substrate towards bioethanol production.
The outcome of this work indicates an inverse effect of the pretreatment severity factor on delignification efficiency and the fermentable solid yield. Optimising the pretreatment process is thus essential to reach both high fermentable solid yield and substrate availability for saccharification and fermentation.
Since temperature influence on lignin removal is modest while being significantly detrimental to holocellulose yield, a different experiment was carried out at 60 °C, which provided 70% holocellulose yield and a 71% degree of delignification. Under these conditions, the decrease in solid yield was occasioned mostly by lignin removal, optimal for recovering the fermentable sugars from the solid. It is observed that holocellulose recovery and delignification efficiency obtained for the optimised conditions are very similar to that found by Budiyono et al. (2022) [48] and Meng et al. (2022) [49], applying the same pretreatment at 85 °C to fruit peel waste and bamboo residues respectively. Moreover, since the temperature of the PoxAc optimised conditions are up to 15 °C lower while matching the performance of the reported literature involving the use of the PoxAc mixture, this work proves that there is a substantial upstream improvement opportunity for second-generation bioethanol in terms of energy efficiency.
5. Conclusions
The effect of low-temperature acid-oxidative digestion on the delignification of urban forest leaf waste typical of parks and streets of Buenos Aires city was investigated. Optimal PoxAc delignification conditions were found experimentally using the Surface Response Methodology to interpret the parametric interaction among the examined factors (time, temperature, and solid loading). Box and Behnken design of experiments successfully provided an acceptable surface model that could predict the behaviour of further experiments, even outside the initial parameter range. No systematic studies on the optimal conditions for the pretreatment have been shown before in the literature, but it is important to address this issue for process efficiency purposes.
The degree of delignification had a significant negative influence on the solid loading. The highest attained lignin removal was larger than 80% within the examined conditions. Even if the temperature positively influences lignin removal, it negatively affects the solid fraction yield, mainly containing the remaining cellulose and hemicellulose fractions, eventually leading to a decrease of available fermentable sugars for subsequent bioprocesses. Decreasing the hydrolysis temperature to 60 °C led to 71% delignification and 70% solid yield, which are not far from those predicted as optimal by the models considered in this work. Confocal laser scanning microscopy confirmed the delignification of the samples by a significant decrease in the characteristic autofluorescence of lignin.
Scanning electron microscopy analysis of the samples indicated better exposition of the cellulose fibre structure. The polymer structure was mainly conserved, suggesting that the hydrolysis to develop reducing sugars may require tough conditions. However, the optimised developed PoxAc pretreatment was highly effective for removing lignin (more than 82%) from lignocellulosic cell walls, reducing the cellulose’s crystallinity for potential enhanced enzymatic accessibility of the substrate and more efficient holocellulose hydrolysis, maximising utilisation of lignocellulosic biomass.
Moreover, the PoxAc pretreatment produces fewer metabolic inhibitory compounds of the ethanologenic microorganism after PoxAc pretreatment, enabling more efficient bioethanol production or other value-added metabolites from a cellulosic refinery.
Author Contributions
G.K. and M.d.P.B.: Investigation, experimental data acquisition, data curation; G.S.: data curation, formal analysis, writing and visualisation; M.C.: Conceptualisation, experimental design, writing original draft, supervision, funding acquisition; C.D.B.: Consultation, review, and editing; M.G.: Conceptualisation, experimental design, consultation, funding acquisition and editing. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge the financial support from Universidad de Buenos Aires (UBACyT 20020170100604BA), UNSAM-CONICET (PIO 1562015-0100018CO), CONICET (PIP 1122015-0100902CO), and Suomen Kulttuurirahasto (00210970).
Data Availability Statement
All data have been exposed in the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Publications Office of the European Union. Masterplan for a Competitive Transformation of EU Energy-Intensive Industries Enabling a Climate-Neutral, Circular Economy by 2050. Available online: http://op.europa.eu/en/publication-detail/-/publication/be308ba7-14da-11ea-8c1f-01aa75ed71a1 (accessed on 21 September 2020).
- International Energy Agency. Renewables 2018: Analysis and Forecasts to 2023; International Energy Agency: Paris, France, 2018; ISBN 978-92-64-30684-4. [Google Scholar]
- Vargas, F.; Domínguez, E.; Vila, C.; Rodríguez, A.; Garrote, G. Agricultural Residue Valorization Using a Hydrothermal Process for Second Generation Bioethanol and Oligosaccharides Production. Bioresour. Technol. 2015, 191, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An Overview on Bioethanol Production from Lignocellulosic Feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Gallone, B.; Mertens, S.; Gordon, J.L.; Maere, S.; Verstrepen, K.J.; Steensels, J. Origins, Evolution, Domestication and Diversity of Saccharomyces Beer Yeasts. Curr. Opin. Biotechnol. 2018, 49, 148–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takada, M.; Chandra, R.; Wu, J.; Saddler, J.N. The Influence of Lignin on the Effectiveness of Using a Chemithermomechanical Pulping Based Process to Pretreat Softwood Chips and Pellets Prior to Enzymatic Hydrolysis. Bioresour. Technol. 2020, 302, 122895. [Google Scholar] [CrossRef]
- Pimentel, D. (Ed.) Biofuels, Solar and Wind as Renewable Energy Systems: Benefits and Risks; Springer: Dordrecht, The Netherlands, 2008; ISBN 978-1-4020-8653-3. [Google Scholar]
- McCaherty, J.; Wilson, C.; Cooper, G. 2019 Ethanol Industry Outlook; Renewable Fuels Association: Washington, DC, USA, 2019. [Google Scholar]
- Liu, C.-G.; Xiao, Y.; Xia, X.-X.; Zhao, X.-Q.; Peng, L.; Srinophakun, P.; Bai, F.-W. Cellulosic Ethanol Production: Progress, Challenges and Strategies for Solutions. Biotechnol. Adv. 2019, 37, 491–504. [Google Scholar] [CrossRef]
- Ahmed, I.N.; Yang, X.-L.; Dubale, A.A.; Shao, R.; Guan, R.-F.; Meng, X.; Xie, M.-H. Zirconium Based Metal-Organic Framework in-Situ Assisted Hydrothermal Pretreatment and Enzymatic Hydrolysis of Platanus X Acerifolia Exfoliating Bark for Bioethanol Production. Bioresour. Technol. 2019, 280, 213–221. [Google Scholar] [CrossRef]
- Alves-Ferreira, J.; Lourenço, A.; Morgado, F.; Duarte, L.C.; Roseiro, L.B.; Fernandes, M.C.; Pereira, H.; Carvalheiro, F. Delignification of Cistus Ladanifer Biomass by Organosolv and Alkali Processes. Energies 2021, 14, 1127. [Google Scholar] [CrossRef]
- Lee, C.S.; Conradie, A.V.; Lester, E. Review of Supercritical Water Gasification with Lignocellulosic Real Biomass as the Feedstocks: Process Parameters, Biomass Composition, Catalyst Development, Reactor Design and Its Challenges. Chem. Eng. J. 2021, 415, 128837. [Google Scholar] [CrossRef]
- López-Gómez, J.P.; Pérez-Rivero, C.; Venus, J. Valorisation of Solid Biowastes: The Lactic Acid Alternative. Process Biochem. 2020, 99, 222–235. [Google Scholar] [CrossRef]
- Vu, H.P.; Nguyen, L.N.; Vu, M.T.; Johir, M.A.H.; McLaughlan, R.; Nghiem, L.D. A Comprehensive Review on the Framework to Valorise Lignocellulosic Biomass as Biorefinery Feedstocks. Sci. Total Environ. 2020, 743, 140630. [Google Scholar] [CrossRef]
- Eades, P.; Kusch-Brandt, S.; Heaven, S.; Banks, C.J. Estimating the Generation of Garden Waste in England and the Differences between Rural and Urban Areas. Resources 2020, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Dessie, W.; Luo, X.; Tang, J.; Tang, W.; Wang, M.; Qin, Z.; Tan, Y. Towards Full Utilisation of Biomass Resources: A Case Study on Industrial Hemp Residue and Spent Mushroom Substrate. Processes 2021, 9, 1200. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Liu, S. Process Optimization for the Anaerobic Digestion of Poplar (Populus L.) Leaves. Bioengineered 2020, 11, 439–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, S.; Karimi, K. Efficient Ethanol Production from Kitchen and Garden Wastes and Biogas from the Residues. J. Clean. Prod. 2018, 187, 37–45. [Google Scholar] [CrossRef]
- Yu, Q.; Qin, L.; Liu, Y.; Sun, Y.; Xu, H.; Wang, Z.; Yuan, Z. In Situ Deep Eutectic Solvent Pretreatment to Improve Lignin Removal from Garden Wastes and Enhance Production of Bio-Methane and Microbial Lipids. Bioresour. Technol. 2019, 271, 210–217. [Google Scholar] [CrossRef]
- Ciudad Autonoma de Buenos Aires Planta de Residuos Forestales. Available online: https://www.buenosaires.gob.ar/ciudadverde/centro-de-reciclaje/planta-de-residuos-forestales (accessed on 1 March 2022).
- Mota, T.R.; Oliveira, D.M.; Morais, G.R.; Marchiosi, R.; Buckeridge, M.S.; Ferrarese-Filho, O.; dos Santos, W.D. Hydrogen Peroxide-Acetic Acid Pretreatment Increases the Saccharification and Enzyme Adsorption on Lignocellulose. Ind. Crops Prod. 2019, 140, 111657. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Emerging Technologies for the Pretreatment of Lignocellulosic Biomass. Bioresour. Technol. 2018, 262, 310–318. [Google Scholar] [CrossRef] [Green Version]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second Generation Bioethanol Production: A Critical Review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Jimenez-Gutierrez, J.M.; Verlinden, R.A.J.; van der Meer, P.C.; van der Wielen, L.A.M.; Straathof, A.J.J. Liquid Hot Water Pretreatment of Lignocellulosic Biomass at Lab and Pilot Scale. Processes 2021, 9, 1518. [Google Scholar] [CrossRef]
- Sheng, Y.; Tan, X.; Gu, Y.; Zhou, X.; Tu, M.; Xu, Y. Effect of Ascorbic Acid Assisted Dilute Acid Pretreatment on Lignin Removal and Enzyme Digestibility of Agricultural Residues. Renew. Energy 2021, 163, 732–739. [Google Scholar] [CrossRef]
- Muaaz-Us-Salam, S.; Cleall, P.J.; Harbottle, M.J. Application of Enzymatic and Bacterial Biodelignification Systems for Enhanced Breakdown of Model Lignocellulosic Wastes. Sci. Total Environ. 2020, 728, 138741. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Y.; Cho, E.J.; Park, C.S.; Oh, C.H.; Park, B.-J.; Bae, H.-J. A Strategy for Sequential Fermentation by Saccharomyces Cerevisiae and Pichia Stipitis in Bioethanol Production from Hardwoods. Renew. Energy 2019, 139, 1281–1289. [Google Scholar] [CrossRef]
- Wi, S.G.; Cho, E.J.; Lee, D.-S.; Lee, S.J.; Lee, Y.J.; Bae, H.-J. Lignocellulose Conversion for Biofuel: A New Pretreatment Greatly Improves Downstream Biocatalytic Hydrolysis of Various Lignocellulosic Materials. Biotechnol. Biofuels 2015, 8, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of Operational Issues in Hydrothermal Liquefaction and Supercritical Water Gasification Processes: A Review. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Roman, J.; Neri, W.; Fierro, V.; Celzard, A.; Bentaleb, A.; Ly, I.; Zhong, J.; Derré, A.; Poulin, P. Lignin-Graphene Oxide Inks for 3D Printing of Graphitic Materials with Tunable Density. Nano Today 2020, 33, 100881. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Mei, C.; Chen, W.; Han, J.; Yue, Y.; Ren, S.; French, A.D.; Aita, G.M.; Eggleston, G.; et al. Cellulose Nanofibers from Rapidly Microwave-Delignified Energy Cane Bagasse and Their Application in Drilling Fluids as Rheology and Filtration Modifiers. Ind. Crops Prod. 2020, 150, 112378. [Google Scholar] [CrossRef]
- Louis, A.C.F.; Venkatachalam, S. Energy Efficient Process for Valorization of Corn Cob as a Source for Nanocrystalline Cellulose and Hemicellulose Production. Int. J. Biol. Macromol. 2020, 163, 260–269. [Google Scholar] [CrossRef]
- Taşar, Ş.; Özer, A. A Comparative Study of Hemicellulose Isolation with Hot Water, Alkaline, and Delignification Methods from Tea Leaf Brewing Waste. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Technical Association of the Pulp and Paper Industry (TAPPI). Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C, Test Method T 211 Om-16. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T211.aspx (accessed on 2 May 2022).
- Technical Association of the Pulp and Paper Industry (TAPPI). Solvent Extractives of Wood and Pulp, Test Method T 204 Cm-17. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T204.aspx (accessed on 2 May 2022).
- Technical Association of the Pulp and Paper Industry (TAPPI). Acid-Insoluble Lignin in Wood and Pulp, Test Method T 222 Om-21. Available online: https://imisrise.tappi.org/TAPPI/Products/01/T/0104T222.aspx (accessed on 2 May 2022).
- Otalora, C.M.; Bonifazi, E.; Fissore, E.N.; Basanta, F.; Gerschenson, L.N. Thermal Stability of Betalains in By-Products of the Blanching and Cutting of Beta Vulgaris L. Var Conditiva. Pol. J. Food Nutr. Sci. 2020, 70, 15–24. [Google Scholar] [CrossRef]
- Hernández, Y.R.; García Serrano, L.A.; Maruri, D.T.; Jiménez Aparicio, A.R.; Camacho Díaz, B.H.; Arenas Ocampo, M.L. Optimization of the Microwave-Assisted Ethanosolv Extraction of Lignocellulosic Compounds from the Bagasse of Agave Angustifolia Haw Using the Response Methodology. J. Agric. Food Chem. 2018, 66, 3533–3540. [Google Scholar] [CrossRef] [PubMed]
- Novelli Poisson, G.F.; Juárez, Á.B.; Noseda, D.G.; Ríos de Molina, M.C.; Galvagno, M.A. Adaptive Evolution Strategy to Enhance the Performance of Scheffersomyces Stipitis for Industrial Cellulosic Ethanol Production. Ind. Biotechnol. 2020, 16, 281–289. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Chanona-Pérez, J.J.; Terrés, E.; Vega, A.; Ligero, P.; Farrera-Rebollo, R.R.; Villanueva, S. Microscopy and Spectroscopy Tools for the Description of Delignification. Cellul. Chem. Technol. 2019, 53, 87–97. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Chanona-Pérez, J.J.; Vega, A.; Ligero, P.; Mendoza-Pérez, J.A.; Calderón-Domínguez, G.; Terrés, E.; Farrera-Rebollo, R.R. Acetosolv Treatment of Fibers from Waste Agave Leaves: Influence of Process Variables and Microstructural Study. Ind. Crops Prod. 2016, 86, 163–172. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.M.; Chanona-Pérez, J.J.; Calderón-Domínguez, G.; Perea-Flores, M.J.; Mendoza-Pérez, J.A.; Vega, A.; Ligero, P.; Palacios-González, E.; Farrera-Rebollo, R.R. Evaluation of Agave Fiber Delignification by Means of Microscopy Techniques and Image Analysis. Microsc. Microanal. 2014, 20, 1436–1446. [Google Scholar] [CrossRef]
- Savic, I.M.; Savic Gajic, I.M. Optimization Study on Extraction of Antioxidants from Plum Seeds (Prunus Domestica L.). Optim. Eng. 2021, 22, 141–158. [Google Scholar] [CrossRef]
- Mukherjee, A.; Banerjee, S.; Halder, G. Parametric Optimization of Delignification of Rice Straw through Central Composite Design Approach towards Application in Grafting. J. Adv. Res. 2018, 14, 11–23. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; ISBN 978-1-118-14692-7. [Google Scholar]
- Song, Y.; Lee, Y.G.; Cho, E.J.; Bae, H.-J. Production of Xylose, Xylulose, Xylitol, and Bioethanol from Waste Bamboo Using Hydrogen Peroxide-Acetic Acid Pretreatment. Fuel 2020, 278, 118247. [Google Scholar] [CrossRef]
- Budiyono; Agustiani, V.; Khoiriyah, L.; Hawali Abdul Matin, H.; Rachmawati, S. Effect of Hydrogen Peroxide Acetic Acid Pretreatment on Kapok (Ceiba Pentandra) Fruit Peel Waste for Bioethanol Production Using Separated Hydrolysis and Fermentation Methods. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Meng, F.; Li, N.; Yang, H.; Shi, Z.; Zhao, P.; Yang, J. Investigation of Hydrogen Peroxide-Acetic Acid Pretreatment to Enhance the Enzymatic Digestibility of Bamboo Residues. Bioresour. Technol. 2022, 344, 126162. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).