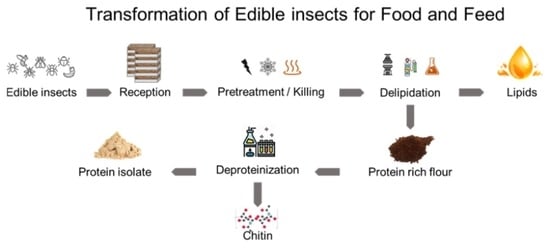
Edible Insects’ Transformation for Feed and Food Uses: An Overview of Current Insights and Future Developments in the Field
Abstract
:1. Introduction
2. Transformation Processes for Edible Insects
2.1. Raw Materials and Sample Preparation
2.2. Extraction Processes
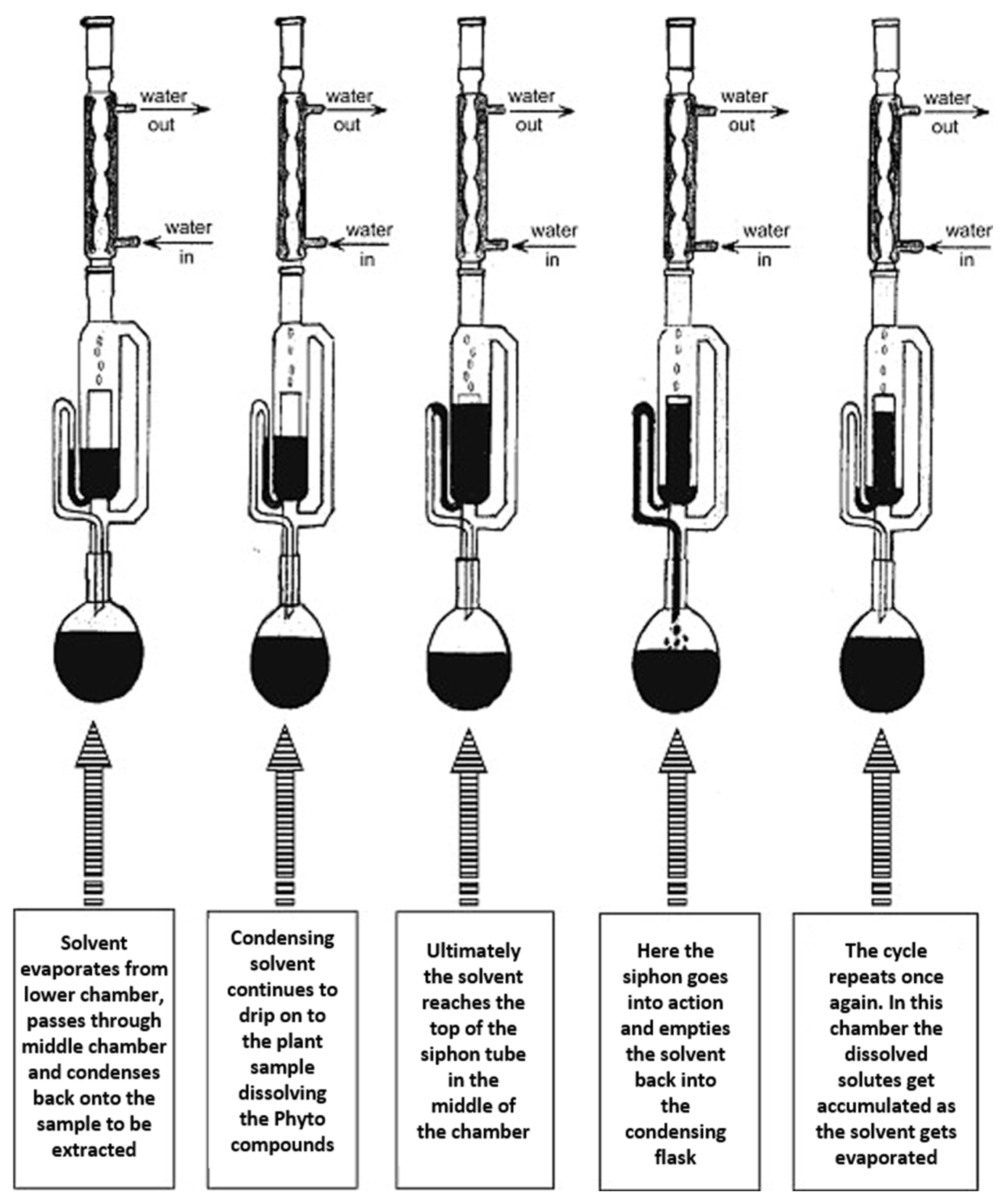
2.2.1. Insect Delipidation
2.2.2. Insect Protein Extraction
2.2.3. Chitin and Chitosan Extraction
3. Future Challenges for the Sector
- Study the integration of the insect rearing step in an ecological and economic space and the valorization of various agricultural wastes and by-products from the agri-food industry (peels, grape pomace, downgraded fruits and vegetables, etc.) by using them as potentially effective raw materials for bioconversion by insects [19];
- Establishing environmental assessments of the two main phases of the insect sector, i.e., the production of insect larvae [58] and the industrial transformation of the larvae into lipid, flour, and protein isolates, by comparing several scenarios of feeding and transformation of insects;
- Water and energy are the main resources used in the transformation of insects. Therefore, a Pinch analysis must be done along with a technical-economic study to reduce the use of these resources through energy integration by using new ways and techniques of extraction. In this context, substantial interest should be paid to the use of emerging technologies to: intensify transfer phenomena, obtain purified extracts, preserve product quality, avoid its oxidation, increase production yield, and reduce both energy and chemical consumption. To do this, low-energy pretreatments such as pulsed electric fields (PEFs) can be applied to insects to permeabilize their cells and facilitate the extraction steps and fractionation downstream. This pretreatment can be used as a killing method at once [35]. Another innovative technology that might be used in this context is the instant controlled pressure drop DIC. It can be used as a pretreatment to intensify the extraction steps, such as DIC-assisted solvent or press extraction of lipids, which have been studied in various cases of pulses and oleaginous grains and seeds [59,60], or to intensify the drying process and preserve the quality of products [61]. DIC-autovaporisation can also be studied as a highly effective desolventation way;
- Innovative and less energy-consuming dehydration techniques should be adopted, such as concentration by the superheated steam while preserving the quality of proteins [62];
- Improving the efficiency of the SC-CO2 extraction process, especially with the use of co-solvents [41].
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azagoh, C.; Ducept, F.; Garcia, R.; Rakotozafy, L.; Cuvelier, M.E.; Keller, S.; Lewandowski, R.; Mezdour, S. Extraction and Physicochemical Characterization of Tenebrio molitor Proteins. Food Res. Int. 2016, 88, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Waterhouse, G.I.N.; You, L.; Zhang, J.; Liu, Y.; Ma, L.; Gao, J.; Dong, Y. Transforming Insect Biomass into Consumer Wellness Foods: A Review. Food Res. Int. 2016, 89, 129–151. [Google Scholar] [CrossRef] [PubMed]
- Leni, G.; Caligiani, A.; Sforza, S. Killing Method Affects the Browning and the Quality of the Protein Fraction of Black Soldier Fly (Hermetia illucens) Prepupae: A Metabolomics and Proteomic Insight. Food Res. Int. 2019, 115, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Hilborn, R.; Banobi, J.; Hall, S.J.; Pucylowski, T.; Walsworth, T.E. The Environmental Cost of Animal Source Foods. Front. Ecol. Environ. 2018, 16, 329–335. [Google Scholar] [CrossRef] [Green Version]
- Green, A.; Blatmann, C.; Chen, C.; Mathys, A. The Role of Alternative Proteins and Future Foods in Sustainable and Contextually-Adapted Flexitarian Diets. Trends Food Sci. Technol. 2022, 124, 250–258. [Google Scholar] [CrossRef]
- Rawiwan, P.; Peng, Y.; Paramayuda, I.G.P.B.; Quek, S.Y. Red Seaweed: A Promising Alternative Protein Source for Global Food Sustainability. Trends Food Sci. Technol. 2022, 123, 37–56. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Lonkila, A.; Yang, B. Alternative Proteins and EU Food Law. Food Control 2021, 130, 108336. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat Alternatives: Life Cycle Assessment of Most Known Meat Substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Jongema, Y. List of Edible Insects of the World (1 April 2017)—WUR. Available online: https://www.wur.nl/en/Research-Results/Chair-groups/Plant-Sciences/Laboratory-of-Entomology/Edible-insects/Worldwide-species-list.htm (accessed on 9 February 2022).
- Bußler, S.; Rumpold, B.A.; Jander, E.; Rawel, H.M.; Schlüter, O.K. Recovery and Techno-Functionality of Flours and Proteins from Two Edible Insect Species: Meal Worm (Tenebrio molitor) and Black Soldier Fly (Hermetia illucens) Larvae. Heliyon 2016, 2, e00218. [Google Scholar] [CrossRef]
- Otero, P.; Gutierrez-Docio, A.; Navarro del Hierro, J.; Reglero, G.; Martin, D. Extracts from the Edible Insects Acheta Domesticus and Tenebrio Molitor with Improved Fatty Acid Profile Due to Ultrasound Assisted or Pressurized Liquid Extraction. Food Chem. 2020, 314, 126200. [Google Scholar] [CrossRef]
- van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Food and Agriculture Organization of the United Nations. Edible Insects. In Future Prospects for Food and Feed Security; FAO: Rome, Italy, 2013; Volume 171, ISBN 9789251075951. [Google Scholar]
- Dagevos, H. A Literature Review of Consumer Research on Edible Insects: Recent Evidence and New Vistas from 2019 Studies. J. Insects Food Feed. 2021, 7, 249–259. [Google Scholar] [CrossRef]
- Bisconsin-Júnior, A.; Rodrigues, H.; Behrens, J.H.; da Silva, M.A.A.P.; Mariutti, L.R.B. “Food Made with Edible Insects”: Exploring the Social Representation of Entomophagy Where It Is Unfamiliar. Appetite 2022, 173, 106001. [Google Scholar] [CrossRef] [PubMed]
- Tzompa-Sosa, D.A.; Yi, L.; van Valenberg, H.J.F.; van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect Lipid Profile: Aqueous versus Organic Solvent-Based Extraction Methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Tuccillo, F.; Marino, M.G.; Torri, L. Italian Consumers’ Attitudes towards Entomophagy: Influence of Human Factors and Properties of Insects and Insect-Based Food. Food Res. Int. 2020, 137, 109619. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Sogari, G.; Diaz, S.E.; Menozzi, D.; Paci, G.; Moruzzo, R. Exploring the Future of Edible Insects in Europe. Foods 2022, 11, 455. [Google Scholar] [CrossRef]
- Gasco, L.; Biancarosa, I.; Liland, N.S. From Waste to Feed: A Review of Recent Knowledge on Insects as Producers of Protein and Fat for Animal Feeds. Curr. Opin. Green Sustain. Chem. 2020, 23, 67–79. [Google Scholar] [CrossRef]
- Ojha, S.; Bußler, S.; Schlüter, O.K. Food Waste Valorisation and Circular Economy Concepts in Insect Production and Processing. Waste Manag. 2020, 118, 600–609. [Google Scholar] [CrossRef]
- Moruzzo, R.; Riccioli, F.; Espinosa Diaz, S.; Secci, C.; Poli, G.; Mancini, S. Mealworm (Tenebrio molitor): Potential and Challenges to Promote Circular Economy. Animals 2021, 11, 2568. [Google Scholar] [CrossRef]
- FAO. Regulation (EC) No 1069/2009 of the European Parliament and of the Council. Off. J. Eur. Union 2009, L300, 1–33. [Google Scholar]
- Rumpold, B.A.; Schlüter, O.K. Nutritional Composition and Safety Aspects of Edible Insects. Mol. Nutr. Food Res. 2013, 57, 802–823. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Unlocking the Biological Potential of Proteins from Edible Insects through Enzymatic Hydrolysis: A Review. Innov. Food Sci. Emerg. Technol. 2017, 43, 239–252. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.D.; Wong, N.A.K.; Auh, J.H. Defatting and Sonication Enhances Protein Extraction from Edible Insects. Korean J. Food Sci. Anim. Resour. 2017, 37, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.; Vincken, J.-P.; van den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible Insects: Tenebrio molitor, Alphitobius diaperinus, and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.C.; Lima, R.C.; Maia, M.R.G.; Almeida, A.A.; Fonseca, A.J.M.; Cabrita, A.R.J.; Cunha, L.M. Impact of Defatting Freeze-Dried Edible Crickets (Acheta domesticus and Gryllodes sigillatus) on the Nutritive Value, Overall Liking and Sensory Profile of Cereal Bars. LWT—Food Sci. Technol. 2019, 113, 108335. [Google Scholar] [CrossRef]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of Functional Properties of Edible Insects and Protein Preparations Thereof. LWT—Food Sci. Technol. 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; Boekel, M.A.J.S.V. Extraction and Characterisation of Protein Fractions from Five Insect Species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Mishyna, M.; Martinez, J.J.I.; Chen, J.; Benjamin, O. Extraction, Characterization and Functional Properties of Soluble Proteins from Edible Grasshopper (Schistocerca gregaria) and Honey Bee (Apis mellifera). Food Res. Int. 2019, 116, 697–706. [Google Scholar] [CrossRef]
- Xia, Z.; Wu, S.; Pan, S.; Kim, J.M. Nutritional Evaluation of Protein from Clanis bilineata (Lepidoptera), an Edible Insect. J. Sci. Food Agric. 2012, 92, 1479–1482. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef] [Green Version]
- Alles, M.C.; Smetana, S.; Parniakov, O.; Shorstkii, I.; Toepfl, S.; Aganovic, K.; Heinz, V. Bio-Refinery of Insects with Pulsed Electric Field Pre-Treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 102403. [Google Scholar] [CrossRef]
- Zhao, X.; Vázquez-Gutiérrez, J.L.; Johansson, D.P.; Landberg, R.; Langton, M. Yellow Mealworm Protein for Food Purposes—Extraction and Functional Properties. PLoS ONE 2016, 11, e0147791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Psarianos, M.; Dimopoulos, G.; Ojha, S.; Cavini, A.C.M.; Bußler, S.; Taoukis, P.; Schlüter, O.K. Effect of Pulsed Electric Fields on Cricket (Acheta domesticus) Flour: Extraction Yield (Protein, Fat and Chitin) and Techno-Functional Properties. Innov. Food Sci. Emerg. Technol. 2022, 76, 102908. [Google Scholar] [CrossRef]
- Smetana, S.; Mhemdi, H.; Mezdour, S.; Heinz, V. Chapter 11—PEF Treated Insects and Algae as Future Food Ingredients. In Pulsed Electric Fields to Obtain Healthier and Sustainable Food for Tomorrow; Elsevier Science: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Amarender, R.V.; Bhargava, K.; Dossey, A.T.; Gamagedara, S. Lipid and Protein Extraction from Edible Insects—Crickets (Gryllidae). LWT—Food Sci. Technol. 2020, 125, 109222. [Google Scholar] [CrossRef]
- Bolat, B.; Ugur, A.E.; Oztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure Assisted Degreasing on the Technological Properties of Insect Powders Obtained from Acheta domesticus & Tenebrio molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.I.; Kim, Y.-B.; Jung, S.; Kim, H.-W.; Choi, Y.-S. Effects of Organic Solvent on Functional Properties of Defatted Proteins Extracted from Protaetia brevitarsis Larvae. Food Chem. 2021, 336, 127679. [Google Scholar] [CrossRef]
- Mandal, S.C.; Mandal, V.; Das, A.K. Classification of Extraction Methods. In Essentials of Botanical Extraction; Elsevier: Amsterdam, The Netherlands, 2015; pp. 83–136. [Google Scholar] [CrossRef]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated Solvent Extraction: A Technique for Sample Preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar] [CrossRef]
- Laroche, M.; Perreault, V.; Marciniak, A.; Gravel, A.; Chamberland, J.; Doyen, A. Comparison of Conventional and Sustainable Lipid Extraction Methods for the Production of Oil and Protein Isolate from Edible Insect Meal. Foods 2019, 8, 572. [Google Scholar] [CrossRef] [Green Version]
- Eggers, L.F.; Schwudke, D. Liquid Extraction: Folch. In Encyclopedia of Lipidomics; Wenk, M.R., Ed.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–6. [Google Scholar]
- Sahena, F.; Zaidul, I.S.M.; Jinap, S.; Karim, A.A.; Abbas, K.A.; Norulaini, N.A.N.; Omar, A.K.M. Application of Supercritical CO2 in Lipid Extraction—A Review. J. Food Eng. 2009, 95, 240–253. [Google Scholar] [CrossRef]
- Wu, S. Supercritical Carbon Dioxide Extraction of Oil from Clanis bilineata (Lepidoptera), an Edible Insect. Afr. J. Biotechnol. 2012, 11, 4607–4610. [Google Scholar] [CrossRef]
- Baigts-Allende, D.; Doost, A.S.; Ramírez-Rodrigues, M.; Dewettinck, K.; van der Meeren, P.; de Meulenaer, B.; Tzompa-Sosa, D. Insect Protein Concentrates from Mexican Edible Insects: Structural and Functional Characterization. LWT—Food Sci. Technol. 2021, 152, 112267. [Google Scholar] [CrossRef]
- Yoon, S.; Wong, N.A.K.; Chae, M.; Auh, J.H. Comparative Characterization of Protein Hydrolysates from Three Edible Insects: Mealworm larvae, Adult crickets, and Silkworm pupae. Foods 2019, 8, 563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingwascharapong, P.; Chaijan, M.; Karnjanapratum, S. Ultrasound-Assisted Extraction of Protein from Bombay Locusts and Its Impact on Functional and Antioxidative Properties. Sci. Rep. 2021, 11, 17320. [Google Scholar] [CrossRef] [PubMed]
- Brogan, E.N.; Park, Y.-L.; Matak, K.E.; Jaczynski, J. Characterization of Protein in Cricket (Acheta domesticus), Locust (Locusta migratoria), and Silk Worm Pupae (Bombyx mori) Insect Powders. LWT—Food Sci. Technol. 2021, 152, 112314. [Google Scholar] [CrossRef]
- Kim, T.K.; Yong, H.I.; Chun, H.H.; Lee, M.A.; Kim, Y.B.; Choi, Y.S. Changes of Amino Acid Composition and Protein Technical Functionality of Edible Insects by Extracting Steps. J. Asia-Pac. Entomol. 2020, 23, 298–305. [Google Scholar] [CrossRef]
- Kaya, M.; Sofi, K.; Sargin, I.; Mujtaba, M. Changes in Physicochemical Properties of Chitin at Developmental Stages (Larvae, Pupa and Adult) of Vespa crabro (Wasp). Carbohydr. Polym. 2016, 145, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, J.; Yu, L.; Zhang, C.; Bi, J.; Zhu, F.; Qu, M.; Jiang, C.; Yang, Q. Extraction and Characterization of Chitin from the Beetle Holotrichia parallela Motschulsky. Molecules 2012, 17, 4604–4611. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging Chitin and Chitosan Nanofibrous Materials for Biomedical Applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef]
- Song, Y.S.; Kim, M.W.; Moon, C.; Seo, D.J.; Han, Y.S.; Jo, Y.H.; Noh, M.Y.; Park, Y.K.; Kim, S.A.; Kim, Y.W.; et al. Extraction of Chitin and Chitosan from Larval Exuvium and Whole Body of Edible Mealworm, Tenebrio Molitor. Entomol. Res. 2018, 48, 227–233. [Google Scholar] [CrossRef]
- González, C.M.; Garzón, R.; Rosell, C.M. Insects as Ingredients for Bakery Goods. A Comparison Study of H. illucens, A. domestica and T. molitor Flours. Innov. Food Sci. Emerg. Technol. 2019, 51, 205–210. [Google Scholar] [CrossRef]
- Kaya, M.; Erdogan, S.; Mol, A.; Baran, T. Comparison of Chitin Structures Isolated from Seven Orthoptera Species. Int. J. Biol. Macromol. 2015, 72, 797–805. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods—A Review. Food Technol. Biotechnol. 2012, 51, 12–25. [Google Scholar]
- Kaya, M.; Baran, T.; Erdoğan, S.; Menteş, A.; Aşan Özüsağlam, M.; Çakmak, Y.S. Physicochemical Comparison of Chitin and Chitosan Obtained from Larvae and Adult Colorado Potato Beetle (Leptinotarsa decemlineata). Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A. Edible Insects: An Overview on Nutritional Characteristics, Safety, Farming, Production Technologies, Regulatory Framework, and Socio-Economic and Ethical Implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Bamerni, F. Plant-Based (Camelina sativa) Biodiesel Manufacturing Using the Technology of Instant Controlled Pressure Drop(DIC): Process Performance and Biofuel Quality, Chemical and Process Engineering. Ph.D. Thesis, Université de La Rochelle, La Rochelle, France, 2018. [Google Scholar]
- Mkaouar, S.; Bahloul, N.; Gelicus, A.; Allaf, K.; Kechaou, N. Instant Controlled Pressure Drop Texturing for Intensifying Ethanol Solvent Extraction of Olive (Olea europaea) Leaf Polyphenols. Sep. Purif. Technol. 2015, 145, 139–146. [Google Scholar] [CrossRef]
- Mounir, S.; Allaf, T.; Mujumdar, A.S.; Allaf, K. Swell Drying: Coupling Instant Controlled Pressure Drop DIC to Standard Convection Drying Processes to Intensify Transfer Phenomena and Improve Quality—An Overview. Dry. Technol. 2012, 30, 1508–1531. [Google Scholar] [CrossRef]
- Sehrawat, R.; Nema, P.K.; Kaur, B.P. Effect of Superheated Steam Drying on Properties of Foodstuffs and Kinetic Modeling. Innov. Food Sci. Emerg. Technol. 2016, 34, 285–301. [Google Scholar] [CrossRef]










| Order | Family | Genus | Species | Common Name | Stage | References |
|---|---|---|---|---|---|---|
| Coleoptera | Tenebrionidae | Tenebrio | molitor | Yellow mealworm | Larvae | [1,10,24,25,26,27] |
| Tenebrionidae | Alphitobius | diaperinus | Lesser mealworm | Larvae | [15,25,28] | |
| Tenebrionidae | Zophobas | morio | Superworm | Larvae | [28] | |
| Orthoptera | Gryllidae | Gryllodes | sigillatus | Tropical house cricket | Adult | [26,27] |
| Acrididae | Schistocerca | gregaria | Desert locust | Adult | [27,29] | |
| Gryllidae | Acheta | domesticus | House cricket | Adult | [15,26,28] | |
| Gryllidae | Gryllus | bimaculatus | Two-spotted cricket | Adult | [24] | |
| Blattodea | Blaberidae | Blaptica | dubia | Dubia cockroach | Adult | [15,28] |
| Lepidoptera | Sphingidae | Clanis | bilineata | Two-lined Velvet hawkmoth | Larvae | [30] |
| Bombycidae | Bombyx | mori | Silkworm moth | Pupae | [24] | |
| Diptera | Stratiomyidae | Hermetia | illucens | Black soldier fly | Larvae and prepupae | [3,10,25] |
| Hymenoptera | Apidae | Apis | melifera | Honey bee | Larvae and pupae | [29] |
| Solvent | A. domesticus | G. sigillatus | ||
|---|---|---|---|---|
| Whole | Defatted | Whole | Defatted | |
| Acetone | 24.6 ± 1.02 b | 4.7 ± 1.99 ab | 23.5 ± 0.27 b | 5.8 ± 1.93 ab |
| Diethyl ether | 20.8 ± 1.17 c | 4.4 ± 0.55 ab | 20.8 ± 0.96 c | 6.5 ± 2.28 b |
| Ethanol | 28.2 ± 1.63 a | 1.4 ± 0.74 a | 28.4 ± 1.10 a | 2.3 ± 0.65 a |
| Ether petroleum | 21.3 ± 0.31 c | 4.1 ± 0.66 ab | 20.2 ± 0.36 c | 6.9 ± 0.50 b |
| Hexane | 18.9 ± 1.65 c | 5.5 ± 2.47 b | 20.8 ± 0.97 c | 4.6 ± 1.02 ab |
| Insect Species | Extracted Lipid (g/100 g Fresh Insects) | Yield (%) (ALE/FLE) | Yield (%) (SLE/FLE) | ||
|---|---|---|---|---|---|
| Aqueous | Soxhlet | Folch | |||
| T. molitor | 7.8 ± 0.4 A | 12.7 ± 2.4 B | 12.9 ± 0.2 B | 60.3 ± 0.4 | 98.4 ± 2.4 |
| A. diaperinus | 5.5 ± 1.0 A | 10.7 ± 0.5 B | 9.4 ± 1.0 B | 58.3 ± 1.4 | 113.5 ± 1.1 |
| A. domesticus | 1.6 ± 0.1 A | 6.0 ± 0.3 B | 8.0 ± 1.1 C | 19.2 ± 1.1 | 74.8 ± 1.1 |
| B. dubia | 3.1 ± 0.3 A | 7.6 ± 0.2 B | 7.5 ± 0.3 B | 40.9 ± 0.4 | 100.5 ± 0.4 |
| Sample | Extraction Yield (%) of Defatted Cricket Powder | True Protein (%) | Extraction Rate of Protein (%) |
|---|---|---|---|
| Defatted protein extract (ascorbic acid) | 87.75 ± 1.53 a | 69.69 | 82.95 |
| Defatted protein extract (NaOH) | 80.78 ± 0.17 b | 61.75 | 67.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Hajj, R.; Mhemdi, H.; Besombes, C.; Allaf, K.; Lefrançois, V.; Vorobiev, E. Edible Insects’ Transformation for Feed and Food Uses: An Overview of Current Insights and Future Developments in the Field. Processes 2022, 10, 970. https://doi.org/10.3390/pr10050970
El Hajj R, Mhemdi H, Besombes C, Allaf K, Lefrançois V, Vorobiev E. Edible Insects’ Transformation for Feed and Food Uses: An Overview of Current Insights and Future Developments in the Field. Processes. 2022; 10(5):970. https://doi.org/10.3390/pr10050970
Chicago/Turabian StyleEl Hajj, Rachelle, Houcine Mhemdi, Colette Besombes, Karim Allaf, Victor Lefrançois, and Eugène Vorobiev. 2022. "Edible Insects’ Transformation for Feed and Food Uses: An Overview of Current Insights and Future Developments in the Field" Processes 10, no. 5: 970. https://doi.org/10.3390/pr10050970
APA StyleEl Hajj, R., Mhemdi, H., Besombes, C., Allaf, K., Lefrançois, V., & Vorobiev, E. (2022). Edible Insects’ Transformation for Feed and Food Uses: An Overview of Current Insights and Future Developments in the Field. Processes, 10(5), 970. https://doi.org/10.3390/pr10050970