COVID-19-Current Therapeutical Approaches and Future Perspectives
Abstract
:1. Introduction
2. Viral Structure, Life Cycle and Therapeutic Targets
3. Drugs Currently Used for COVID-19 Therapy
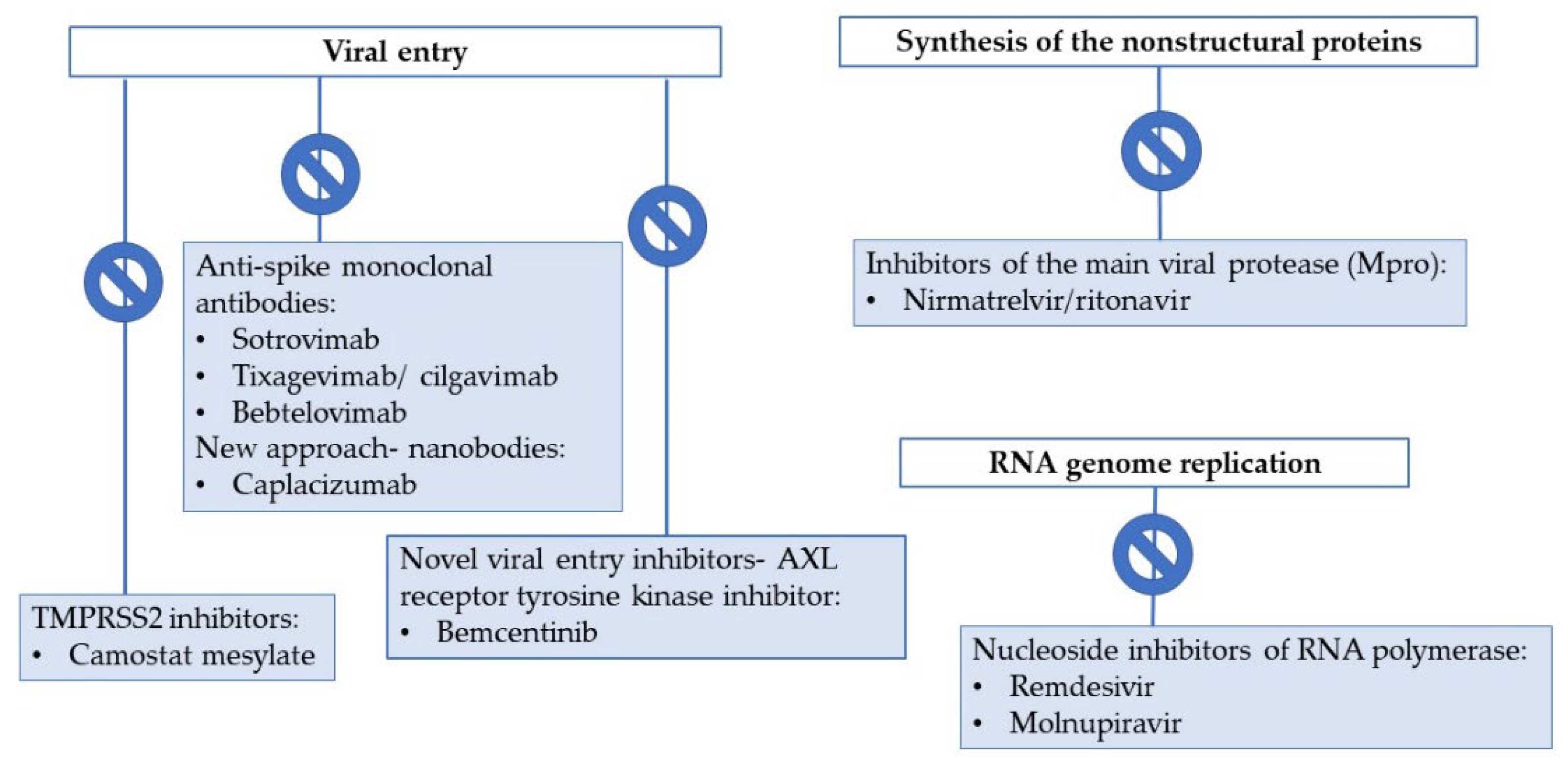
3.1. Entry Inhibitors and Direct-Acting Antiviral Drugs
3.1.1. Entry Inhibitors: Anti-Spike Monoclonal Antibodies
3.1.2. Antivirals Targeting the RNA-Dependent RNA Polymerase (RdRp)
3.1.3. Protease Inhibitors
3.2. Immunomodulators
3.2.1. Corticosteroids
3.2.2. IL-6 Receptor Inhibitors
3.2.3. Interleukin-1 Inhibitors
3.2.4. Janus Kinase Inhibitors
3.2.5. Other Potential Immunomodulatory Drugs
4. New Therapeutical Approaches for COVID-19
4.1. Broadly Neutralizing Antibodies
4.2. Novel Viral Entry Inhibitors
4.3. Inhibitors of Host Transmembrane Surface Protease TMPRSS2
4.4. Interferons
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Our World in Data. Available online: https://ourworldindata.org (accessed on 30 March 2022).
- Atzrodt, C.L.; Maknojia, I.; McCarthy, D.P.; Oldfield, T.M.; Jonathan, P.; Kenny, T.L.; Stepp, H.E.; Clements, T. A Guide to COVID-19: A global pandemic caused by the novel coronavirus SARS-CoV-2. FEBS J. 2020, 287, 3633–3650. [Google Scholar] [CrossRef] [PubMed]
- Lythgoe, K.A.; Hall, M.D. Shared SARS-CoV-2 diversity suggests localized transmission of minority variants. Science 2021, 372, 1–10. [Google Scholar] [CrossRef]
- Ramanathan, M.; Ferguson, I.D.; Miao, W.; Khavari, P.A. SARS-CoV-2 B.1.1.7 and B.1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect. Dis. 2021, 21, 1070. [Google Scholar] [CrossRef]
- Davies, N.G.; Jarvis, C.I.; Edmunds, W.J.; Jewell, N.P.; Diaz-Ordaz, K.; Keogh, R.H. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature 2021, 593, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy, and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef] [PubMed]
- Therapeutics and COVID-19: Living Guideline-World Health Organization (WHO). Available online: https://apps.who.int/iris/bitstream/handle/10665/351006/WHO-2019-nCoV-therapeutics-2022.1-eng.pdf (accessed on 26 February 2022).
- Emergency Use Authorization (EUA) of COVID-19 Convalescent Plasma for Treatment of Coronavirus Disease 2019 (COVID-19). Available online: https://www.fda.gov/media/141478/download (accessed on 27 February 2022).
- Abd-Elsalam, S.; Noor, R.A.; Badawi, R.; Khalaf, M.; Esmail, E.S.; Soliman, S.; Abd El Ghafar, M.S.; Elbahnasawy, M.; Moustafa, E.F.; Hassany, S.M.; et al. Clinical study evaluating the efficacy of ivermectin in COVID-19 treatment: A randomized controlled study. J. Med. Virol. 2021, 93, 5833–5838. [Google Scholar] [CrossRef]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of favipiravir in the treatment of COVID-19. Int. J. Infect. Dis. 2021, 102, 501–508. [Google Scholar] [CrossRef]
- Buonfrate, D.; Chesini, F.; Martini, D.; Roncaglioni, M.C.; Ojeda, M.L.; Alvisi, M.F.; Ruli, E. High-dose ivermectin for early treatment of COVID-19 (COVER study): A randomized, double-blind, multicentre, phase II, dose-finding, proof-of-concept clinical trial. Int. J. Antimicrob. Agents 2022, 59, 106516. [Google Scholar] [CrossRef]
- Paludan, S.R.; Mogensen, T.H. Innate immunological pathways in COVID-19 pathogenesis. Sci. Immunol. 2022, 7, eabm5505. [Google Scholar] [CrossRef]
- Majumder, J.; Minko, T. Recent Developments on Therapeutic and Diagnostic Approaches for COVID-19. AAPS J. 2021, 23, 14. [Google Scholar] [CrossRef] [PubMed]
- Mititelu, M.; Stanciu, T.I.; Udeanu, D.I.; Popa, D.E. The Impact of COVID-19 lockdown on the lifestyle and dietary patterns among romanian population. Farmacia 2021, 69, 1. [Google Scholar] [CrossRef]
- Arsene, A.L.; Dumitrescu, B.I.; Udeanu, D.I.; Dragoi, C.M. A new era for the therapeutic management of the ongoing COVID-19 pandemic. Farmacia 2020, 68, 2. [Google Scholar] [CrossRef]
- Van Der Hoek, L.; Pyrc, K.; Jebbink, M.F.; Vermeulen-Oost, W.; Berkhout, R.J.; Wolthers, K.C.; Wertheim-van Dillen, P.M.; Kaandorp, J.; Spaargaren, J.; Berkhout, B. Identification of a new human coronavirus. Nat. Med. 2004, 10, 368–373. [Google Scholar] [CrossRef]
- Astuti, I.; Ysrafil. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): An overview of viral structure and host response. Diabetes Metab. Syndr. 2020, 14, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Dubey, A.; Choudhary, S.; Kumar, P.; Tomar, S. Emerging SARS-CoV-2 Variants: Genetic Variability and Clinical Implications. Curr. Microbiol. 2021, 79, 20. [Google Scholar] [CrossRef]
- Alexandersen, S.; Chamings, A.; Bhatta, T.R. SARS-CoV-2 genomic and subgenomic RNAs in diagnostic samples are not an indicator of active replication. Nat. Commun. 2020, 11, 6059. [Google Scholar] [CrossRef]
- JHMI Clinical Recommendations for Pharmacologic Treatment of COVID-19. Available online: https://www.hopkinsguides.com/hopkins/ub?cmd=repview&type=479-1225&name=30_538747_PDF (accessed on 23 February 2022).
- Bhimraj, A.; Morgan, R.; Shumaker, A.H.; Lavergne, V.; Baden, L.; Cheng, V. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19, IDSA Guidelines on COVID-19. Available online: https://www.idsociety.org/COVID19guidelines (accessed on 26 February 2022).
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef]
- O’Brien, M.P.; Forleo-Neto, E.; Sarkar, N. Effect of Subcutaneous Casirivimab and Imdevimab Antibody Combination vs Placebo on Development of Symptomatic COVID-19 in Early Asymptomatic SARS-CoV-2 Infection. JAMA 2022, 327, 432. [Google Scholar] [CrossRef]
- COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 27 February 2022).
- Dougan, M.; Nirula, A.; Azizad, M.; Mocherla, B.; Gottlieb, R.L.; Chen, P.; Hebert, C.; Perry, R.; Boscia, J.; Heller, B.; et al. Bamlanivimab plus Etesevimab in Mild or Moderate COVID-19. N. Engl. J. Med. 2021, 385, 1382–1392. [Google Scholar] [CrossRef] [PubMed]
- Bamlanivimab and Etesevimab, Monoclonal Antibodies. Available online: https://www.precisionvaccinations.com/vaccines/bamlanivimab-and-etesevimab-monoclonal-antibodies (accessed on 27 February 2022).
- Heo, Y.A. Sotrovimab: First Approval. Drugs 2022, 82, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Tortorici, M.A.; Czudnochowski, N.; Starr, T.N.; Marzi, R.; Walls, A.C.; Zatta, F.; Bowen, J.E.; Jaconi, S.; Di Iulio, J.; Wang, Z.; et al. Broad sarbecovirus neutralization by a human monoclonal antibody. Nature 2021, 597, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Gonzalez-Rojas, Y.; Juarez, E.; Crespo Casal, M.; Moya, J.; Falci, D.R.; Sarkis, E.; Solis, J.; Zheng, H.; Scott, N.; et al. COMET-ICE Investigators. Early Treatment for COVID-19 with SARS-CoV-2 Neutralizing Antibody Sotrovimab. N. Engl. J. Med. 2021, 385, 1941–1950. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for Evusheldtm (Tixagevimab Co-Packaged with Cilgavimab). Available online: https://www.fda.gov/media/154701/download (accessed on 27 February 2022).
- Levin, M.J.; Ustianowski, A.; De Wit, S. LB5. PROVENT: Phase 3 Study of Efficacy and Safety of AZD7442 (Tixagevimab/Cilgavimab) for Pre-exposure Prophylaxis of COVID-19 in Adults. Open Forum. Infect. Dis. 2021, 8 (Suppl. S1), S810. [Google Scholar] [CrossRef]
- Evusheld Long-Acting Antibody Combination Retains Neutralizing Activity Against Omicron Variant in Independent FDA study. Available online: https://www.astrazeneca.com/media-centre/press-releases/2021/evusheld-long-acting-antibody-combination-retains-neutralising-activity-against-omicron-variant-in-independent-fda-study.html (accessed on 30 March 2022).
- Westendorf, K.; Žentelis, S.; Wang, L.; Foster, D.; Vaillancourt, P.; Wiggin, M.; Lovett, E.; van der Lee, R.; Hendle, J.; Pustilnik, A.; et al. LY-CoV1404 (bebtelovimab) potently neutralizes SARS-CoV-2 variants. BioRxiv 2022, preprint. [Google Scholar] [CrossRef]
- Humeniuk, R.; Mathias, A.; Cao, H.; Osinusi, A.; Shen, G.; Chng, E.; Ling, J.; Vu, A.; German, P. Safety, Tolerability, and Pharmacokinetics of Remdesivir, An Antiviral for Treatment of COVID-19, in Healthy Subjects. Clin. Transl. Sci. 2020, 13, 896–906. [Google Scholar] [CrossRef]
- EMA Veklury RCP. Available online: https://www.ema.europa.eu/en/documents/product-information/veklury-epar-product-information_ro.pdf (accessed on 15 March 2022).
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef]
- Ader, F.; Bouscambert-Duchamp, M.; Hites, M.; Peiffer-Smadja, N.; Pissy, J.; Belhadi, D. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): A phase 3, randomized, controlled, open-label trial. Lancet Infect. Dis. 2022, 22, 209–221. [Google Scholar] [CrossRef]
- Vermillion, M.S.; Murakami, E.; Ma, B.; Pitts, J.; Tomkinson, A.; Rautiola, D.; Babusis, D.; Irshad, H.; Siegel, D.; Kim, C.; et al. Inhaled remdesivir reduces viral burden in a nonhuman primate model of SARS-CoV-2 infection. Sci. Transl. Med. 2021, 14, eabl828. [Google Scholar] [CrossRef]
- Schäfer, A.; Martinez, D.A.; Won, J.; Meganck, R.; Moreira, F.; Brown, A.; Gully, K.; Zweigart, M.; Conrad, W. Therapeutic treatment with an oral prodrug of the remdesivir parental nucleoside is protective against SARS-CoV-2 pathogenesis in mice. Sci. Transl. Med. 2022, 14, eabm3410. [Google Scholar] [CrossRef] [PubMed]
- Mount Sinai Health System Treatment Guidance for SARS-CoV-2 Infection (COVID-19), Updated in the setting of Omicron Variant of Concern. Available online: https://www.mountsinai.org/files/MSHealth/Assets/HS/About/Coronavirus/Mount-Sinai-Health-System-Treatment-Guidelines-for-COVID-Updated.pdf (accessed on 27 February 2022).
- PK and Safety of Remdesivir for Treatment of COVID-19 in Pregnant and Non-Pregnant Women in the US. Available online: https://clinicaltrials.gov/ct2/show/record/NCT04582266 (accessed on 26 February 2022).
- Goldman, D.L.; Aldrich, M.L.; Hagmann, S.H.; Bamford, A.; Camacho-Gonzalez, A.; Lapadula, G.; Lee, P.; Bonfanti, P.; Carter, C.C.; Zhao, Y. Compassionate Use of Remdesivir in Children with Severe COVID-19. Pediatrics 2021, 147, e2020047803. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus (COVID-19) Update: FDA Approves First COVID-19 Treatment for Young Children. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-approves-first-covid-19-treatment-young-children (accessed on 1 May 2022).
- Cox, R.M.; Wolf, J.D.; Plemper, R.K. Therapeutically administered ribonucleoside analog MK-4482/EIDD-2801 blocks SARS-CoV-2 transmission in ferrets. Nat. Microbiol. 2021, 6, 11–18. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Manabe, T.; Kambayashi, D.; Akatsu, H.; Kudo, K. Favipiravir for the treatment of patients with COVID-19: A systematic review and meta-analysis. BMC Infect. Dis. 2021, 21, 489. [Google Scholar] [CrossRef] [PubMed]
- Özlüşen, B.; Kozan, Ş.; Akcan, R.E.; Kalender, M.; Yaprak, D.; Peltek, İ.B.; Ergönül, Ö. Effectiveness of favipiravir in COVID-19: A live systematic review. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2021, 40, 2575–2583. [Google Scholar] [CrossRef] [PubMed]
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for Paxlovid. Available online: https://www.fda.gov/media/155050/download (accessed on 26 February 2022).
- Eng, H.; DAntonio, A.; Kadar, E.; Obach, S.; Di, L.; Lin, J.; Patel, N.; Boras, B.; Walker, G.; Novak, J.; et al. Disposition of PF-07321332 (Nirmatrelvir), an Orally Bioavailable Inhibitor of SARS-CoV-2 3CL Protease, across Animals and Humans. Available online: https://dmd.aspetjournals.org/content/dmd/early/2022/02/13/dmd.121.000801.full.pdf (accessed on 27 February 2022).
- Owen, D.R.; Allerton, C.; Anderson, A.; Avery, M.; Berritt, S.; Cardin, R.; Zhu, Y. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, NEJMoa2118542. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Lee, J.-C.; Chiu, C.-W.; Lee, C.-C.; Tsai, P.-J.; Hsu, I.-L.; Ko, W.-C. Oral Nirmatrelvir/Ritonavir Therapy for COVID-19: The Dawn in the Dark? Antibiotics 2022, 11, 220. [Google Scholar] [CrossRef]
- PAXLOVID (Nirmatrelvir; Ritonavir) Product Monograph. Available online: https://covid-vaccine.canada.ca/info/pdf/paxlovid-pm-en.pdf (accessed on 26 February 2022).
- Assessing a Patient for Paxlovid (Nirmatrelvir/Ritonavir). Available online: https://www.covid19-druginteractions.org/prescribing-resources (accessed on 26 February 2022).
- Abdelnabi, R.; Foo, C.S.; Jochmans, D.; Vangeel, L.; De Jonghe, S.; Augustijns, P.; Mols, R.; Weynand, B.; Wattanakul, T.; Hoglund, R.M.; et al. The oral protease inhibitor (PF-07321332) protects Syrian hamsters against infection with SARS-CoV-2 variants of concern. Nat. Commun. 2022, 13, 719. [Google Scholar] [CrossRef]
- Ullrich, S.; Ekanayake, K.B.; Otting, G.; Nitsche, C. Main protease mutants of SARS-CoV-2 variants remain susceptible to nirmatrelvir. Bioorg. Med. Chem. Lett. 2022, 62, 128629. [Google Scholar] [CrossRef] [PubMed]
- Finney, L.J.; Glanville, N.; Farne, H.; Aniscenko, J.; Fenwick, P.; Kemp, S.V.; Trujillo-Torralbo, M.-B.; Loo, S.L.; Calderazzo, M.A.; Wedzicha, J.A.; et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J. Allergy Clin. Immunol. 2021, 147, 510–519.e5. [Google Scholar] [CrossRef] [PubMed]
- EMA. Insufficient Data on Use of Inhaled Corticosteroids to Treat COVID-19. Available online: https://www.ema.europa.eu/en/news/insufficient-data-use-inhaled-corticosteroids-treat-covid-19 (accessed on 15 March 2022).
- Gordon, A.C.; Mouncey, P.R.; Al-Beidh, F.; Rowan, K.M.; Nichol, A.D.; Arabi, Y.M. The REMAP-CAP Investigators, Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 384, 1491–1502. [Google Scholar] [CrossRef]
- NIH Guidelines. Available online: https://osp.od.nih.gov/biotechnology/nih-guidelines/ (accessed on 17 March 2022).
- Fact Sheet for Healthcare Providers: Emergency Use Authorization for Actemra® (Tocilizumab). Available online: https://www.gene.com/download/pdf/actemra_eua_hcp_fact_sheet.pdf (accessed on 27 February 2022).
- Recovery Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. Lancet 2021, 397, 1637–1645. [Google Scholar] [CrossRef]
- Davidescu, E.I.; Odajiu, I.; Ilie, M.D.; Bunea, T.; Sandu, G.; Stratan, L.; Iftode, N.; Arama, V.; Popescu, B.O. Influence of Tocilizumab on the Outcome of Patients with COVID-19. Retrospective observational study. Farmacia 2020, 68, 5. [Google Scholar] [CrossRef]
- Meira, F.; Albiach, L.; Carbonell, C.; Martín-Oterino, J.Á.; Martín-Ordiales, M.; Linares, L.; Macaya, I.; Agüero, D.; Ambrosioni, J.; Bodro, M.; et al. Experience with the use of siltuximab in patients with SARS-CoV-2 infection. Rev. Española Quimioter. 2021, 34, 337. [Google Scholar] [CrossRef]
- EMA Recommends Approval for Use of Kineret in Adults with COVID-19. Available online: https://www.ema.europa.eu/en/news/ema-recommends-approval-use-kineret-adults-covid-19 (accessed on 26 February 2022).
- Kharazmi, A.B.; Moradi, O.; Haghighi, M.; Kouchek, M.; Manafi-Rasi, A.; Raoufi, M.; Shoaei, S.D.; Hadavand, F.; Nabavi, M.; Miri, M.M.; et al. A randomized controlled clinical trial on efficacy and safety of anakinra in patients with severe COVID-19. Immun. Inflamm. Dis. 2022, 10, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Katia, F.; Myriam, D.P.; Ucciferri, C. Efficacy of canakinumab in mild or severe COVID-19 pneumonia. Immun. Inflamm. Dis. 2021, 9, 399–405. [Google Scholar] [CrossRef]
- Generali, D.; Bosio, G.; Malberti, F.; Cuzzoli, A.; Testa, S.; Romanini, L.; Fioravanti, A.; Morandini, A.; Pianta, L.; Giannotti, G.; et al. Canakinumab as treatment for COVID-19-related pneumonia: A prospective case-control study. Int. J. Infect. Dis. 2021, 104, 433–440. [Google Scholar] [CrossRef]
- Caricchio, R.; Abbate, A.; Gordeev, I. Effect of Canakinumab vs. Placebo on Survival Without Invasive Mechanical Ventilation in Patients Hospitalized with Severe COVID-19: A Randomized Clinical Trial. JAMA 2021, 326, 230–239. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Qiao, W.; Zhang, J.; Qi, Z. Baricitinib, a drug with potential effect to prevent SARS-CoV-2 from entering target cells and control cytokine storm induced by COVID-19. Int. Immunopharmacol. 2020, 86, 106749. [Google Scholar] [CrossRef] [PubMed]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.; Tomashek, K.; Wolfe, C.; Marconi, V.; Kline, S.; Tapson, V. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Marconi, V.C.; Ramanan, A.V.; de Bono, S.; Kartman, C.E.; Krishnan, V.; Liao, R.; Piruzeli, M.L.B.; Goldman, J.D.; Alatorre-Alexander, J.; de Cassia Pellegrini, R.; et al. Efficacy and safety of baricitinib for the treatment of hospitalized adults with COVID-19 (COV-BARRIER): A randomized, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir. Med. 2021, 9, 1407–1418. [Google Scholar] [CrossRef]
- Fact Sheet for Healthcare Providers Emergency Use Authorization (EUA) of Baricitinib. Available online: https://www.fda.gov/media/143823/download (accessed on 26 February 2022).
- Le Corre, P.; Loas, G. Difficulty in Repurposing Selective Serotonin Reuptake Inhibitors and Other Antidepressants with Functional Inhibition of Acid Sphingomyelinase in COVID-19 Infection. Front. Pharmacol. 2022, 13, 849095. [Google Scholar] [CrossRef] [PubMed]
- Reis, G.; dos Santos Moreira-Silva, E.A.; Silva, D.C.M.; Thabane, L.; Milagres, A.C.; Ferreira, T.S.; Dos Santos, C.V.Q.; de Souza Campos, V.H.; Nogueira, A.M.R.; de Almeida, A.P.F.G.; et al. Effect of early treatment with fluvoxamine on risk of emergency care and hospitalization among patients with COVID-19: The together randomised, platform clinical trial. Lancet Glob Health 2022, 10, e42–e51. [Google Scholar] [CrossRef]
- Cameroni, E.; Bowen, J.E.; Rosen, L.E. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 2022, 602, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Vanshylla, K.; Fan, C.; Wunsch, M.; Poopalasingam, N.; Meijers, M.; Kreer, C.; Kleipass, F.; Ruchnewitz, D.; Ercanoglu, M.S.; Gruell, H.; et al. Discovery of ultrapotent broadly neutralizing antibodies from SARS-CoV-2 elite neutralizers. Cell Host Microbe 2022, 30, 69–82.e10. [Google Scholar] [CrossRef]
- Renn, A.; Fu, Y.; Hu, X.; Hall, M.D.; Simeonov, A. Fruitful neutralizing antibody pipeline brings hope to defeat SARS-CoV-2. Trends Pharmacol. Sci. 2020, 41, 815–829. [Google Scholar] [CrossRef]
- Kumar, M.; Kuroda, K.; Dhangar, K.; Mazumder, P.; Sonne, C.; Rinklebe, J.; Kitajima, M. Potential emergence of antiviral-resistant pandemic viruses via environmental drug exposure of animal reservoirs. Environ. Sci. Technol. 2020, 54, 8503–8505. [Google Scholar] [CrossRef]
- Stanfield, R.L.; Dooley, H.; Flajnik, M.F.; Wilson, I.A. Crystal structure of a shark single-domain antibody V region in complex with lysozyme. Science 2004, 305, 1770–1773. [Google Scholar] [CrossRef]
- Bannas, P.; Hambach, J.; Koch-Nolte, F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies as Antitumor Therapeutics. Front. Immunol. 2017, 8, 1603. [Google Scholar] [CrossRef] [PubMed]
- Jovcevska, I.; Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessalah, S.; Jebahi, S.; Mejri, N.; Salhi, I.; Khorchani, T.; Hammadi, M. Perspective on therapeutic and diagnostic potential of camel nanobodies for coronavirus disease-19 (COVID-19). 3 Biotech 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Wrapp, D.; de Vlieger, D.; Corbett, K.S.; Torres, G.M.; Wang, N.; Van Breedam, W.; Roose, K.; van Schie, L.; Hoffmann, M.; Pöhlmann, S.; et al. Structural basis for potent neutralization of betacoronaviruses by single-domain camelid antibodies. Cell 2020, 181, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Esparza, T.J.; Martin, N.P.; Anderson, G.P. High affinity nanobodies block SARS-CoV-2 spike receptor binding domain interaction with human angiotensin converting enzyme. Sci. Rep. 2020, 10, 22370. [Google Scholar] [CrossRef]
- Koenig, P.A.; Das, H.; Liu, H.; Kümmerer, B.M.; Gohr, F.N.; Jenster, L.M.; Schiffelers, L.D.; Tesfamariam, Y.M.; Uchima, M.; Wuerth, J.D.; et al. Structure-guided multivalent nanobodies block SARS-CoV-2 infection and suppress mutational escape. Science 2021, 371, eabe6230. [Google Scholar] [CrossRef]
- Pymm, P.; Adair, A.; Chan, L.J.; Cooney, J.P.; Mordant, F.L.; Allison, C.C.; Lopez, E.; Haycroft, E.R.; O’Neill, M.T.; Tan, L.L.; et al. Nanobody cocktails potently neutralize SARS-CoV-2 D614G N501Y variant and protect mice. Proc. Natl. Acad. Sci. USA 2021, 118, e2101918118. [Google Scholar] [CrossRef]
- Wen, W.; Su, W.; Tang, H.; Le, W.; Zhang, X.; Zheng, Y.; Liu, X.; Xie, L.; Li, J.; Ye, J.; et al. Immune cell profiling of COVID-19 patients in the recovery stage by single-cell sequencing. Cell Discov. 2020, 6, 31. [Google Scholar] [CrossRef]
- Nosenko, M.A.; Atretkhany, K.N.; Mokhonov, V.V.; Efimov, G.A.; Kruglov, A.A.; Tillib, S.V.; Drutskaya, M.S.; Nedospasov, S.A. VHH-Based Bispecific Antibodies Targeting Cytokine Production. Front. Immunol. 2017, 8, 1073. [Google Scholar] [CrossRef] [Green Version]
- Coppieters, K.; Dreier, T.; Silence, K.; Lauwereys, M.; Casteels, P.; Beirnaert, E. Formatted anti-tumor necrosis factor alpha VHH proteins derived from camelids show superior potency and targeting to inflamed joints in a murine model of collagen-induced arthritis. Arthritis Rheum. 2006, 54, 1856–1866. [Google Scholar] [CrossRef]
- BerGenBio, Oslo University Hospital to Test Bemcentinib in Hospitalised COVID-19 Patients. Available online: https://www.clinicaltrialsarena.com/news/bergenbio-trial-bemcentinib-covid/ (accessed on 19 March 2022).
- Hoffmann, M.; Hofmann-Winkler, H.; Smith, J.C. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine 2021, 65, 103255. [Google Scholar] [CrossRef] [PubMed]
- Shapira, T.; Monreal, I.A.; Dion, S.P.; Buchholz, D.W.; Imbiakha, B.; Olmstead, A.D.; Jager, M.; Désilets, A.; Gao, G.; Martins, M.; et al. A TMPRSS2 inhibitor acts as a pan-SARS-CoV-2 prophylactic and therapeutic. Nature 2022, 605, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Meng, B.; Abdullahi, A.; Ferreira, I.A.; Goonawardane, N.; Saito, A.; Kimura, I.; Yamasoba, D.; Gerber, P.P.; Fatihi, S.; Rathore, S.; et al. Altered TMPRSS2 usage by SARS-CoV-2 Omicron impacts infectivity and fusogenicity. Nature 2022, 603, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bastard, P.; Liu, Z.; Le Pen, J.; Moncada-Velez, M.; Chen, J. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science 2020, 370, eabd4570. [Google Scholar] [CrossRef] [PubMed]
- Bastard, P.; Rosen, L.B.; Zhang, Q.; Michailidis, E.; Hoffmann, H.-H.; Zhang, Y.; Dorgham, K. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020, 370, eabd4585. [Google Scholar] [CrossRef] [PubMed]
- Hung, I.F.; Lung, K.C.; Tso, E.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: An open-label, randomised, Phase 2 trial. Lancet 2020, 395, 1695–1704. [Google Scholar] [CrossRef]
- WHO Solidarity Trial Consortium; Pan, H.; Peto, R. Repurposed antiviral drugs for COVID-19-interim WHO Solidarity Trial results. N. Engl. J. Med. 2021, 384, 497–511. [Google Scholar]
- Prokunina-Olsson, L.; Alphonse, N.; Dickenson, R.E.; Durbin, J.E.; Glenn, J.S.; Hartmann, R.; Kotenko, S.V. COVID-19 and emerging viral infections: The case for interferon lambda. J. Exp. Med. 2020, 217, e20200653. [Google Scholar] [CrossRef] [Green Version]
- Jagannathan, P.; Andrews, J.R.; Bonilla, H.; Hedlin, H.; Jacobson, K.B.; Balasubramanian, V.; Purington, N. Peginterferon Lambda-1a for treatment of outpatients with uncomplicated COVID-19: A randomized placebo-controlled trial. Nat. Commun. 2021, 12, 1967. [Google Scholar] [CrossRef]
- Feld, J.J.; Kandel, C.; Biondi, M.J.; Kozak, R.A.; Zahoor, M.A.; Lemieux, C.; Borgia, S.M. Peginterferon lambda for the treatment of outpatients with COVID-19: A phase 2, placebo-controlled randomized trial. Lancet. Respir. Med. 2021, 9, 498–510. [Google Scholar] [CrossRef]
| Therapeutic Agent | Pharmacological Classification | Mechanism of Action | Outpatient | Inpatient |
|---|---|---|---|---|
| Sotrovimab | Entry inhibitors-Anti-Spike Monoclonal Antibodies | Bind to the spike protein of SARS-CoV-2, inhibiting attachment to the ACE2 receptor | ✔ | ✔ ✔ |
| Tixagevimab/cilgavimab | ✔ | ✔ | ||
| Bebtelovimab Casirivimab/imdevimab * Bamlanivimab/etesevimab * | ✔ | ✔ | ||
| Remdesivir | Antivirals targeting the RNA-dependent RNA polymerase (RdRp) | Inhibition of viral genome replication | ✔ | ✔ |
| Molnupiravir | ✔ | |||
| Nirmatrelvir/Ritonavir | Protease inhibitors | Inhibit polyprotein cleavage and the formation of non-structural proteins essential for viral replication | ✔ | |
| Corticosteroids | Immunomodulators | Decrease inflammatory response | ✔ | ✔ |
| Tocilizumab | Anti-Interleukin-6 Receptor Monoclonal Antibodies | Interleukin (IL)-6 receptor antagonists, inhibition of IL-6 signaling pathway | ✔ | |
| Sarilumab | ✔ | |||
| Siltuximab | ✔ | |||
| Baricitinib | Janus kinase inhibitors | Selective JAK1/JAK2 inhibitors | ✔ | |
| Ruxolitinib | ✔ | |||
| Anakinra | Monoclonal antibody anti IL1 beta receptor | ✔ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lupașcu, R.E.; Ilie, M.I.; Velescu, B.Ș.; Udeanu, D.I.; Sultana, C.; Ruță, S.; Arsene, A.L. COVID-19-Current Therapeutical Approaches and Future Perspectives. Processes 2022, 10, 1053. https://doi.org/10.3390/pr10061053
Lupașcu RE, Ilie MI, Velescu BȘ, Udeanu DI, Sultana C, Ruță S, Arsene AL. COVID-19-Current Therapeutical Approaches and Future Perspectives. Processes. 2022; 10(6):1053. https://doi.org/10.3390/pr10061053
Chicago/Turabian StyleLupașcu (Moisi), Raluca Elisabeta, Marina Ionela Ilie, Bruno Ștefan Velescu, Denisa Ioana Udeanu, Camelia Sultana, Simona Ruță, and Andreea Letiția Arsene. 2022. "COVID-19-Current Therapeutical Approaches and Future Perspectives" Processes 10, no. 6: 1053. https://doi.org/10.3390/pr10061053
APA StyleLupașcu, R. E., Ilie, M. I., Velescu, B. Ș., Udeanu, D. I., Sultana, C., Ruță, S., & Arsene, A. L. (2022). COVID-19-Current Therapeutical Approaches and Future Perspectives. Processes, 10(6), 1053. https://doi.org/10.3390/pr10061053









