Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bueno, J.M.; Ramos-Escudero, F.; Saez-Plaza, P.; Muñoz, A.M.; Jose Navas, M.; Asuero, A.G. Analysis and antioxidant capacity of anthocyanin pigments. Part I: General considerations concerning polyphenols and flavonoids. Crit. Rev. Anal. Chem. 2012, 42, 102–125. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. A critical review on polyphenols and health benefits of black soybeans. Nutrients 2017, 9, 455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mourtzinos, I.; Goula, A. Polyphenols in agricultural byproducts and food waste. In Polyphenols in Plants; Ross Watson, R., Ed.; Academic Press: London, UK, 2019; pp. 23–44. [Google Scholar]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Ambriz-Pérez, D.L.; Leyva-López, N.; Castilo-López, R.I.; Heredia, J.B. Bioavailability of dietary phenolic compounds. Rev. Española Nutr. Hum. Y Dietética 2016, 20, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Faria, A.; Calhau, C.; de Freitas, V.; Mateus, N. Procyanidins as antioxidants and tumor cell growth modulators. J. Agric. Food Chem. 2006, 54, 2392–2397. [Google Scholar] [CrossRef] [PubMed]
- Praud, D.; Parpinel, M.; Guercio, V.; Bosetti, C.; Serraino, D.; Facchini, G.; Montella, M.; La Vecchia, C.; Rossi, M. Proanthocyanidins and the risk of prostate cancer in Italy. Cancer Causes Control. 2018, 29, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Dávila, I.; Robles, E.; Egüés, I.; Labidi, J.; Gullón, P. The biorefinery concept for the industrial valorization of grape processing by-products. In Handbook of Grape Processing by-Products; Academic Press: London, UK, 2017; pp. 29–53. [Google Scholar]
- Argon, Z.U.; Celenk, V.U.; Gumus, Z.P. Cold pressed grape (Vitis vinifera) seed oil. In Cold Pressed Oils; Academic Press: London, UK, 2020; pp. 39–52. [Google Scholar]
- Maamoun, M.A.I. An Insight into the Brilliant Benefits of Grape Waste. In Mediterranean Fruits Bio-Wastes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 433–465. [Google Scholar]
- Hogervorst, J.C.; Miljić, U.; Puškaš, V. Extraction of bioactive compounds from grape processing by-products: Sustainable solutions. In Handbook of Grape Processing By-Products; Academic Press: London, UK, 2017; pp. 105–135. [Google Scholar]
- Goula, A.M.; Thymiatis, K.; Kaderides, K. Valorization of grape pomace: Drying behavior and ultrasound extraction of phenolics. Food Bioprod. Process 2016, 100, 132–144. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, J. Antioxidant and antiproliferative properties of a tocotrienol-rich fraction from grape seeds. Food Chem. 2009, 114, 1386–1390. [Google Scholar] [CrossRef]
- Maroun, R.G.; Rajha, H.N.; Vorobiev, E.; Louka, N. Emerging technologies for the recovery of valuable compounds from grape processing by-products. In Handbook of Grape Processing by-Products; Academic Press: London, UK, 2017; pp. 155–181. [Google Scholar]
- Spigno, G.; Marinoni, L.; Garrido, G.D. State of the art in grape processing by-products. In Handbook of Grape Processing By-Products; Academic Press: London, UK, 2017; pp. 1–27. [Google Scholar]
- Pinelo, M.; Arnous, A.; Meyer, A.S. Upgrading of grape skins: Significance of plant cell-wall structural components and extraction techniques for phenol release. Trends Food Sci. Technol. 2006, 17, 579–590. [Google Scholar] [CrossRef]
- Gómez-Brandón, M.; Lores, M.; Insam, H.; Domínguez, J. Strategies for recycling and valorization of grape marc. Crit. Rev. Biotechnol. 2019, 39, 437–450. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.; Avramescu, S.M.; Sieniawska, E. Recovery of natural antioxidants from agro-industrial side streams through advanced extraction techniques. Molecules 2019, 24, 4212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedonia Group. World Nutraceutical Ingredients Industry Study with Forecasts for 2017 & 2022. Industry Market Research Brochure. 2013. Available online: http://www.freedoniagroup.com/brochure/30xx/3079smwe.pdf (accessed on 24 January 2022).
- Pinelo, M.; Rubilar, M.; Jerez, M.; Sineiro, J.; Nuñez, M.J. Effect of solvent, temperature, and solvent-to-solid ratio on the total phenolic content and antiradical activity of extracts from different components of grape pomace. J. Agric. Food Chem. 2005, 53, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity—A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Parisi, O.I.; Puoci, F.; Restuccia, D.; Iemma, F.; Picci, N. Polyphenols and Their Formulations: Different Strategies to Overcome the Drawbacks Associated with Their Poor Stability and Bioavailability. Polyphen. Hum. Health Dis. 2014, 4, 29–45. [Google Scholar]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound assisted extraction for the recovery of phenolic compounds from vegetable sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Markouli, E.; Gekas, V. Recovery and fractionation of different phenolic classes from winery sludge using ultrafiltration. Sep. Purif. Technol. 2013, 107, 245–251. [Google Scholar] [CrossRef]
- Sciortino, M.; Avellone, G.; Scurria, A.; Bertoli, L.; Carnaroglio, D.; Bongiorno, D.; Pagliaro, M.; Ciriminna, R. Green and Quick Extraction of Stable Biophenol-Rich Red Extracts from Grape Processing Waste. ACS Food Sci. Technol. 2021, 1, 937–942. [Google Scholar] [CrossRef]
- Nawaz, H.; Shi, J.; Mittal, G.S.; Kakuda, Y. Extraction of polyphenols from grape seeds and concentration by ultrafiltration. Sep. Purif. Technol. 2006, 48, 176–181. [Google Scholar] [CrossRef]
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process. Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Perra, M.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Segura-Carretero, A.; Pedraz, J.L.; Bacchetta, G.; Muntoni, A.; De Gioannis, G.; Manca, M.L.; Manconi, M. Extraction of the antioxidant phytocomplex from wine-making by-products and sustainable loading in phospholipid vesicles specifically tailored for skin protection. Biomed Pharm. 2021, 142, 111959. [Google Scholar] [CrossRef]
- Syed, U.T.; Brazinha, C.; Crespo, J.G.; Ricardo-da-Silva, J.M. Valorisation of grape pomace: Fractionation of bioactive flavan-3-ols by membrane processing. Sep. Purif. Technol. 2017, 172, 404–414. [Google Scholar] [CrossRef]
- Crespo, J.G.; Brazinha, C. Membrane processing: Natural antioxidants from winemaking by-products. Filtr. Sep. 2010, 47, 32–35. [Google Scholar] [CrossRef]
- Oliveira, J.; Alhinho da Silva, M.; Teixeira, N.; De Freitas, V.; Salas, E. Screening of anthocyanins and anthocyanin-derived pigments in red wine grape pomace using LC-DAD/MS and MALDI-TOF techniques. J. Agric Food Chem. 2015, 63, 7636–7644. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Galanakis, C.M. Introduction in functional components for membrane separations. In Separation of Functional Molecules in Food by Membrane Technology; Academic Press: London, UK, 2019; pp. 31–77. [Google Scholar]
- Camel, V. Recent extraction techniques for solid matrices—Supercritical fluid extraction, pressurized fluid extraction and microwave-assisted extraction: Their potential and pitfalls. Analyst 2001, 126, 1182–1193. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Jain, V.; Pandey, R.; Vyas, A.; Shukla, S.S. Microwave assisted extraction for phytoconstituents–An overview. Asian J. Res. Chem. 2009, 2, 19–25. [Google Scholar]
- Kaufmann, B.; Christen, P. Recent extraction techniques for natural products: Microwave-assisted extraction and pressurised solvent extraction. Phytochem. Anal. 2002, 13, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of polyphenols from agri-food by-products: The olive oil and winery industries cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef]
- Puri, M.; Sharma, D.; Barrow, C.J. Enzyme-assisted extraction of bioactives from plants. Trends Biotechnol. 2012, 30, 37–44. [Google Scholar] [CrossRef]
- Vernès, L.; Vian, M.; Chemat, F. Ultrasound and microwave as green tools for solid-liquid extraction. In Liquid-Phase Extraction, Handbooks in Separation Science; Poole, C.F., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 355–374. [Google Scholar]
- Cassano, A.; De Luca, G.; Conidi, C.; Drioli, E. Effect of polyphenols-membrane interactions on the performance of membrane-based processes. A review. Coord Chem. Rev. 2017, 351, 45–75. [Google Scholar] [CrossRef]
- Conidi, C.; Drioli, E.; Cassano, A. Membrane-based agro-food production processes for polyphenol separation, purification and concentration. Curr. Opin. Food Sci. 2018, 23, 149–164. [Google Scholar] [CrossRef]
- Giacobbo, A.; Moura Bernardes, A.; de Pinho, M.N. Sequential pressure-driven membrane operations to recover and fractionate polyphenols and polysaccharides from second racking wine. Sep. Purif. Technol. 2017, 173, 49–54. [Google Scholar] [CrossRef]
- Negrão Murakami, A.N.; Dias de Mello Castanho Amboni, R.; Schwinden Prudêncio, E.; Amante, E.R.; de Moraes Zanotta, L.; Maraschin, M.; Cunha Petrus, J.C.; Teófilo, R.F. Concentration of phenolic compounds in aqueous mate (Ilex paraguariensis A. St. Hill) extract through nanofiltration. LWT Food Sci. Technol. 2011, 44, 2211–2216. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. A membrane-bassed study for the recovery of polyphenols from bergamote juice. J. Membr. Sci. 2011, 375, 182–190. [Google Scholar] [CrossRef]
- Pan, B.; Yan, P.; Li, X. Concentration of coffee extract using nanofiltration membranes. Desalination 2013, 317, 127–131. [Google Scholar] [CrossRef]
- Cissé, M.; Vaillant, F.; Pallet, D.; Dornier, M. Selecting ultrafiltration and nanofiltration membranes to concentrate anthocyanins from roselle extract (Hibiscus sabdariffa L.). Food Res. Int. 2011, 44, 2607–2614. [Google Scholar] [CrossRef]
- Santamaria, B.; Salazar, G.; Beltrán, S.; Cabezas, J. Membrane sequences for fractionation of polyphenolic extracts from defatted milled grape seeds. Desalination 2002, 148, 103–109. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Yáñez-Fernández, J.; Fíla, V. Phenolic compounds recovered from agro-food by-products using membrane technologies: An overview. Food Chem. 2016, 213, 753–762. [Google Scholar] [CrossRef]
- Galanakis, C.M. Separation of functional macromolecules and micromolecules: From ultrafiltration to the border of nanofiltration. Trends Food Sci. Technol. 2015, 42, 44–63. [Google Scholar] [CrossRef]
- Loginov, M.; Boussetta, N.; Lebovka, N.; Vorobiev, E. Separation of polyphenols and proteins from flaxseed hull extracts by coagulation and ultrafiltration. J. Membr. Sci. 2013, 442, 177–186. [Google Scholar] [CrossRef]
- Díaz-Reinoso, B.; Moure, A.; Domínguez, H.; Parajó, J.C. Ultra-and nanofiltration of aqueous extracts from distilled fermented grape pomace. J. Food Eng. 2009, 91, 587–593. [Google Scholar] [CrossRef]
- Rouquié, C.; Dahdouh, L.; Delalonde, M.; Wisniewski, C. An innovative lab-scale strategy for the evaluation of Grape Processing Residues (GPR) filterability: Application to GPR valorization by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2017, 41, 314–322. [Google Scholar] [CrossRef]
- Conidi, C.; Destani, F.; Cassano, A. Performance of hollow fiber ultrafiltration membranes in the clarification of blood orange juice. Beverages 2015, 1, 341–353. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Salas, E.; Fulcrand, H.; Poncet-Legrand, C.; Meudec, E.; Köhler, N.; Winterhalter, P.; Cheynier, V. Isolation of flavanol-anthocyanin adducts by countercurrent chromatography. J. Chromatogr. Sci. 2005, 43, 488–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1985, 25, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Hagerman, A.E. Tannin Handbook; Miami University; Ann Hagerman Lab: Oxford, OH, USA, 2002; Available online: www.users.muohio.edu/hagermae/ (accessed on 27 December 2021).
- Kennedy, J.A.; Jones, G.P. Analysis of proanthocyanidin cleavage products following acid-catalysis in the presence of excess phloroglucinol. J. Agric. Food Chem. 2001, 49, 1740–1746. [Google Scholar] [CrossRef]
- Berké, B.; Chèze, C.; Vercauteren, J.; Deffieux, G. Bisulfite addition to anthocyanins: Revisited structures of colourless adducts. Tetrahedron Lett. 1998, 39, 5771–5774. [Google Scholar] [CrossRef]
- Salas, E.; Dueñas, M.; Schwarz, M.; Winterhalter, P.; Cheynier, V.; Fulcrand, H. Characterization of pigments from different high speed countercurrent chromatography wine fractions. J. Agric. Food Chem. 2005, 53, 4536–4546. [Google Scholar] [CrossRef]
- Dueñas, M.; Salas, E.; Cheynier, V.; Dangles, O.; Fulcrand, H. UV−visible spectroscopic investigation of the 8, 8-methylmethine catechin-malvidin 3-glucoside pigments in aqueous solution: Structural transformations and molecular complexation with chlorogenic acid. J. Agric Food Chem. 2006, 54, 189–196. [Google Scholar] [CrossRef]
- Vatai, T.; Skerget, M.; Knez, Z. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.O.; Lee, C.Y. Extraction and isolation of polyphenolics. Curr. Protoc. Food Anal. Chem. 2002, 6, I1–I2. [Google Scholar] [CrossRef]
- Muñiz-Márquez, D.B.; Martínez-Ávila, G.C.; Wong-Paz, J.E.; Belmares-Cerda, R.; Rodríguez-Herrera, R.; Aguilar, C.N. Ultrasound-assisted extraction of phenolic compounds from Laurus nobilis L. and their antioxidant activity. Ultrason. Sonochem. 2013, 20, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- d’Alessandro, L.G.; Kriaa, K.; Nikov, I.; Dimitrov, K. Ultrasound assisted extraction of polyphenols from black chokeberry. Sep. Purif. Technol. 2012, 93, 42–47. [Google Scholar] [CrossRef]
- Garcia-Castello, E.M.; Rodriguez-Lopez, A.D.; Mayor, L.; Ballesteros, R.; Conidi, C.; Cassano, A. Optimization of conventional and ultrasound assisted extraction of flavonoids from grapefruit (Citrus paradisi L.) solid wastes. LWT Food Sci. Technol. 2015, 64, 1114–1122. [Google Scholar] [CrossRef]
- Galanakis, C.M. (Ed.) Handbook of Grape Processing by-Products: Sustainable Solutions; Academic Press: London, UK, 2017. [Google Scholar]
- McMurrough, I.; Madigan, D.; Smyth, M.R. Semipreparative chromatographic procedure for the isolation of dimeric and trimeric proanthocyanidins from barley. J. Agric Food Chem. 1996, 44, 1731–1735. [Google Scholar] [CrossRef]
- Labarbe, B.; Cheynier, V.; Brossaud, F.; Souquet, J.M.; Moutounet, M. Quantitative fractionation of grape proanthocyanidins according to their degree of polymerization. J. Agric Food Chem. 1999, 47, 2719–2723. [Google Scholar] [CrossRef]
- Fragoso, S.; Guasch, J.; Aceña, L.; Mestres, M.; Busto, O. Prediction of red wine colour and phenolic parameters from the analysis of its grape extract. Int. J. Food Sci. Technol. 2011, 46, 2569–2575. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordević, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2022, 9, 1627. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]

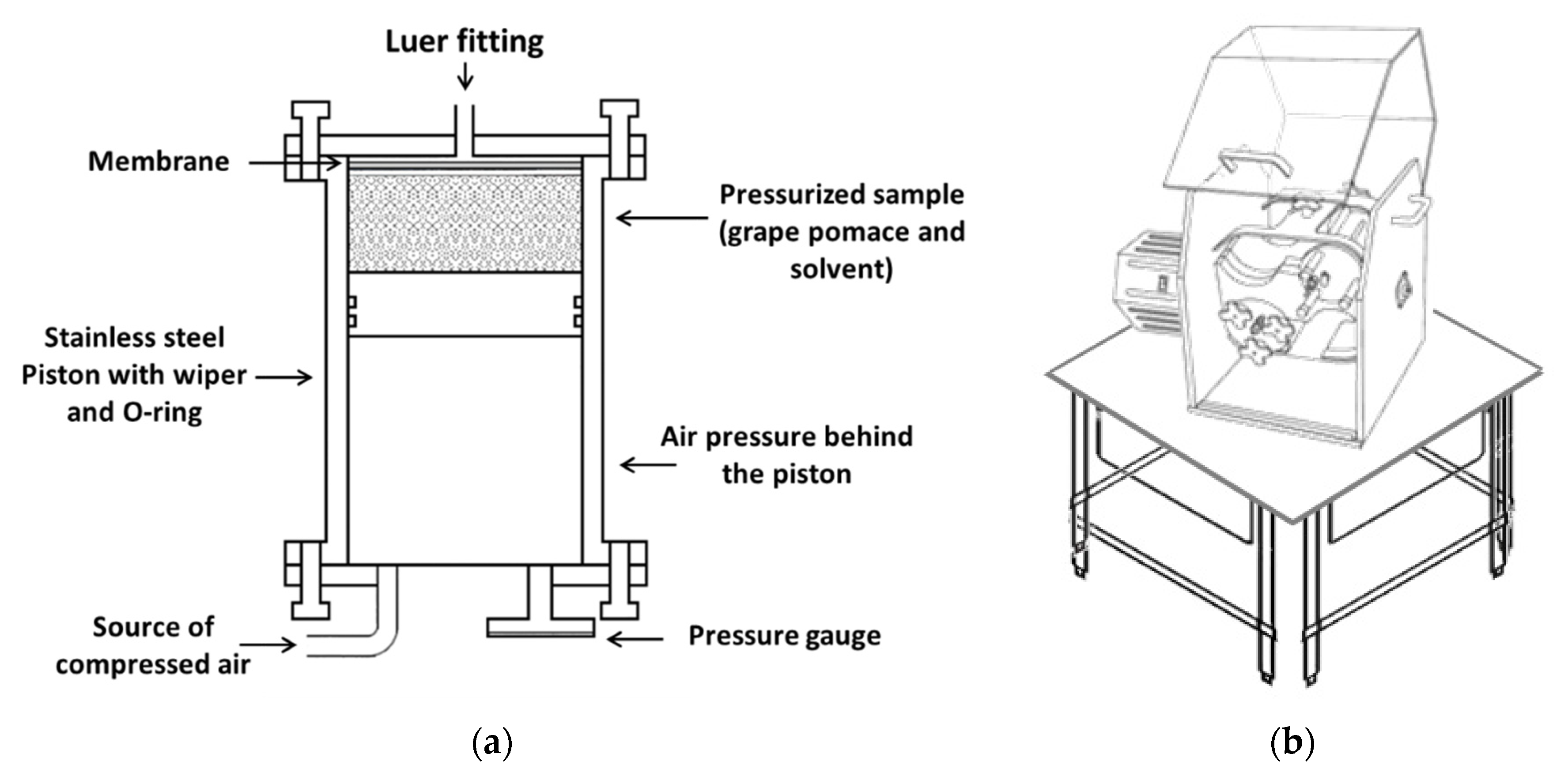

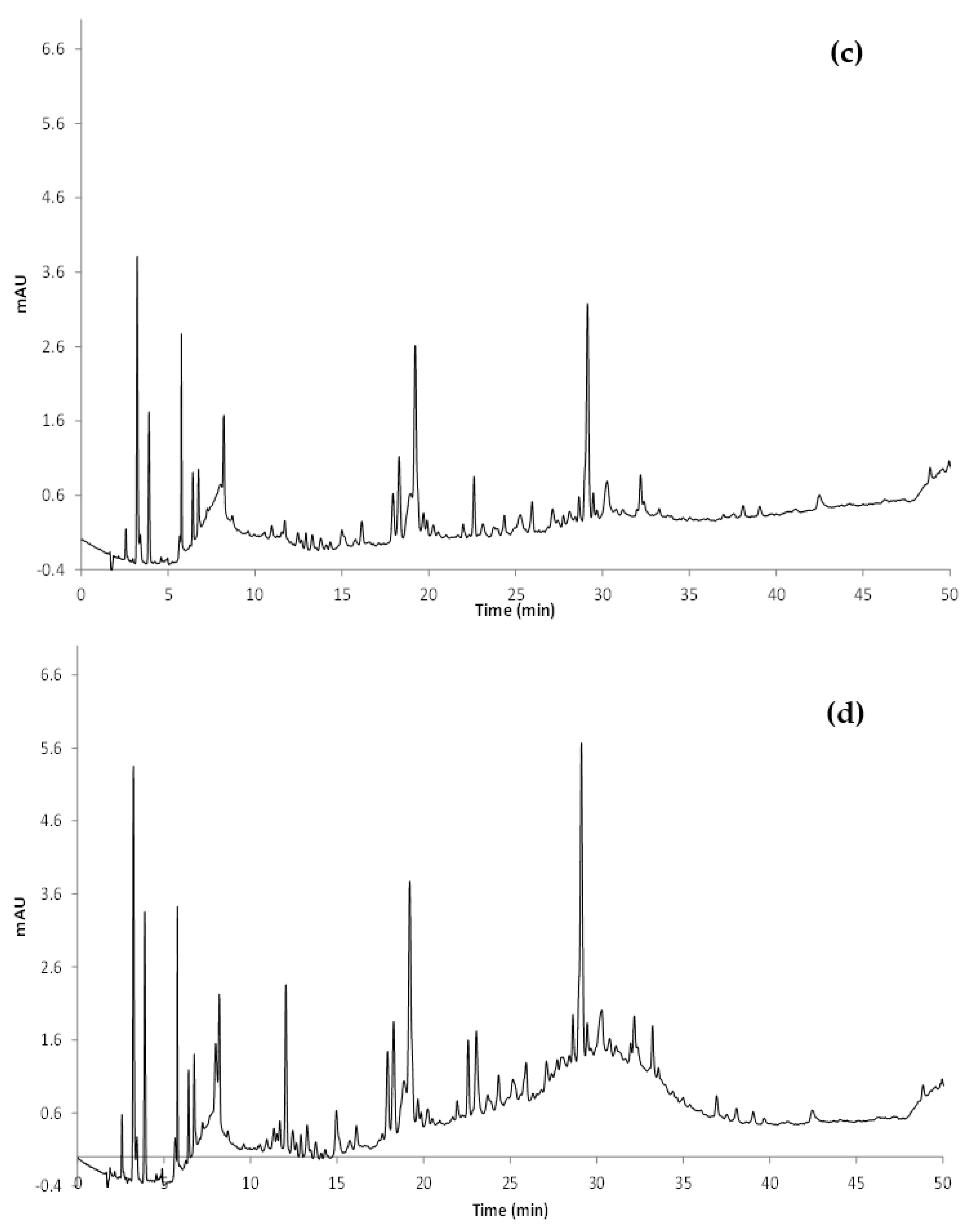

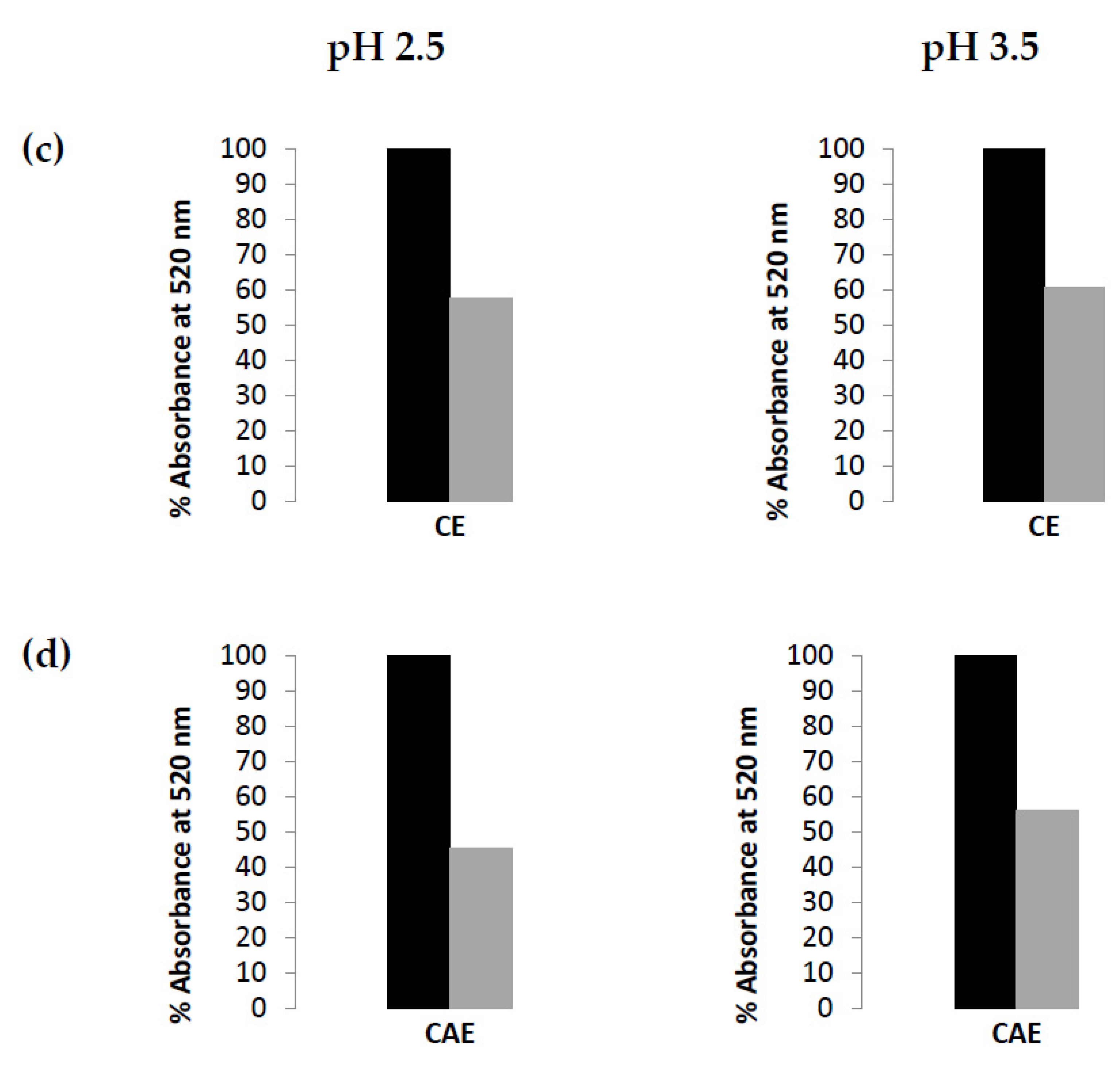
| Sample | TPC HPLC | Phenolic Acids HPLC | Flavonols HPLC | Total Anthocyanins HPLC |
|---|---|---|---|---|
| EtOH | 1.61 ± 0.09 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.31 ± 0.05 |
| MeOH | 2.35 ± 0.18 | 0.09 ± 0.02 | 0.15 ± 0.03 | 0.50 ± 0.08 |
| Ethyl acetate | 1.18 ± 0.12 | 0.01 ± 0.01 | 0.08 ± 0.01 | 0.15 ± 0.02 |
| Sample | S1 | S2 | S3 |
|---|---|---|---|
| 1 | positive | positive | positive |
| 2 | positive | positive | positive |
| 3 | positive | positive | positive |
| Sample | AE | ME | CE | CAE |
|---|---|---|---|---|
| 1 | negative | positive | positive | positive |
| 2 | negative | positive | positive | positive |
| 3 | negative | positive | positive | positive |
| Sample | TPC HPLC |
|---|---|
| AE | 1231.07 ± 206.56 a |
| ME | 751.33 ± 274.07 ab |
| CE | 280.19 ± 96.04 b |
| CAE | 834.12 ± 321.27 ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orozco-Flores, L.A.; Salas, E.; González-Sánchez, G.; Chávez-Flores, D.; Ramírez-García, R.A.; Rocha-Gutiérrez, B.A.; Peralta-Pérez, M.D.R.; Ballinas-Casarrubias, M.D.L. Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace. Processes 2022, 10, 1112. https://doi.org/10.3390/pr10061112
Orozco-Flores LA, Salas E, González-Sánchez G, Chávez-Flores D, Ramírez-García RA, Rocha-Gutiérrez BA, Peralta-Pérez MDR, Ballinas-Casarrubias MDL. Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace. Processes. 2022; 10(6):1112. https://doi.org/10.3390/pr10061112
Chicago/Turabian StyleOrozco-Flores, Laura A., Erika Salas, Guillermo González-Sánchez, David Chávez-Flores, Raúl A. Ramírez-García, Beatriz A. Rocha-Gutiérrez, María Del R. Peralta-Pérez, and María De L. Ballinas-Casarrubias. 2022. "Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace" Processes 10, no. 6: 1112. https://doi.org/10.3390/pr10061112
APA StyleOrozco-Flores, L. A., Salas, E., González-Sánchez, G., Chávez-Flores, D., Ramírez-García, R. A., Rocha-Gutiérrez, B. A., Peralta-Pérez, M. D. R., & Ballinas-Casarrubias, M. D. L. (2022). Novel Zero Headspace Solid-Liquid Extraction for the Recovery of Polyphenolic Fractions from Grape Pomace. Processes, 10(6), 1112. https://doi.org/10.3390/pr10061112








