Abstract
Microalgae have a lot of potential as a source of several compounds of interest to various industries. However, developing a sustainable and efficient harvesting process on a large scale is still a major challenge. This is particularly a problem when the production of low-value products is intended. Chemical flocculation, followed by sedimentation, is seen as an alternative method to improve the energetic and economic balance of the harvesting step. In this study, inorganic (aluminum sulfate, ferric sulfate, ferric chloride) and organic (Zetag 8185, chitosan, Tanfloc SG) flocculants were tested to harvest Chlorella vulgaris in batch mode. Preliminary assays were conducted to determine the minimum dosages of each flocculant that generates primary flocs at different pH. Except for chitosan, the organic flocculants required small dosages to initiate floc formation. Additional studies were performed for the flocculants with a better performance in the preliminary assays. Zetag 8185 had the best results, reaching 98.8% and 97.9% efficiencies with dosages of 50 and 100 mg L−1, respectively. Lastly, a 24 full factorial design experiment was performed to determine the effects of the flocculant dosage, settling time, and mixing time on the Zetag 8185 harvesting efficiency. The harvesting efficiency of C. vulgaris was optimal at a dosage of 100 mg L−1 and 3 min of rapid mixing.
1. Introduction
Microalgae are photosynthetic microorganisms that have a wide variety of environmental applications: (i) the bioremediation of wastewater in terms of nutrient removal/recovery; (ii) carbon dioxide (CO2) sequestration from biogas and industrial flue gases; and (iii) the bioenergy production of fuels such as biodiesel, bioethanol, biohydrogen, and others [1,2]. Besides their high content in polysaccharides, proteins, and lipids, microalgae also accumulate high-value products such as antioxidants, pigments, and vitamins that have already been commercially applied in supplementary food products, pharmaceuticals, and cosmetics [3]. Chlorella vulgaris is a green microalgal widely cultivated on a large scale and commercially produced because of its economic potential. It has a high photosynthetic rate and the ability to grow in different environmental conditions [4]. Maximum oxygen production rates of approximately 135 g O2 kg cell−1 h−1 were estimated at different C. vulgaris cell concentrations [5]. Zheng et al. [6] reported carbon dioxide fixation rates of 454 mg CO2 L−1 d−1 at a 5% CO2 concentration. Furthermore, C. vulgaris is a carbohydrate-rich microalga with the potential to produce bioethanol [7]. Despite their numerous applications, microalgae are only commercially produced when they are intended for use in synthesizing high-value products due to the costly harvesting processes involved [8]. This step represents 20–30% of the total biomass production costs and may represent 90% of the equipment costs [9]. For low-value products, such as biofuels, the production costs must be significantly reduced for the process to be economically viable and able to compete with actual fossil fuel prices.
Microalgae have a microscopic size (2–30 μm), and the cultures present low biomass concentrations with densities close to water, making sedimentation difficult [8,10]. Usually, the biomass concentration ranges from 0.2 to 6 g L−1, being about 0.5 g L−1 in a typical 20 cm deep raceway pond [8,11]. Furthermore, microalgae present carboxylic and amine groups on the cell surface, having a negative surface charge at the common pH values (above 4–5) of the cultures since the groups tend to be deprotonated. Microalgal cells also carry algogenic organic matter (AOM), which includes extracellular organic matter (EOM), a product of algal metabolism released by diffusion, and intracellular organic matter, released during cell lysis [12]. During microalgal growth, AOM characteristics and quantity are considerably influenced by EOM [13]. Gonzalez-Camejo et al. [14] studied the impact of different stressing factors on EOM production in a Chlorella-dominated culture, concluding that nutrient limitation and sudden temperature changes should be avoided, as they seem to increase EOM production. AOM and the negative surface charge of the cultures do not allow microalgae to aggregate easily, remaining in stable suspensions [1,8]. These factors make the harvesting process challenging and consequently, expensive. Decreasing harvesting costs is thus seen as a critical factor in developing a long-term, full-scale production of microalgal biomass. At this moment, microalgal harvesting is typically performed by mechanical (gravity sedimentation, centrifugation, filtration, flotation), biological (bioflocculation), chemical (chemical flocculation), or physical methods (electroflocculation, magnetic separation). Although these methods can be employed individually, microalgal harvesting is usually a two-step concentration process: there is a thickening and a dewatering step. First, the diluted cell suspension is concentrated in a slurry of about 2–7% of the total suspended solids (TSS). Then, the slurry is concentrated by centrifugation or filtration, resulting in a cake with 15–25% TSS [1]. This allows for a more efficient and economical process, as the thickening step can be performed by a cost-effective process, such as flocculation, prior to the energy-consuming step of dewatering that requires expensive equipment [9].
Chemical flocculation, followed by sedimentation, is considered a low-cost, alternative method for improving the energetic and economic balance of microalgal harvesting [9,15]. Although some authors may differentiate flocculation and coagulation, most do not distinguish between these processes when reporting on microalgae harvesting. Thus, this differentiation was not made in this work either; we chose to use only the term flocculation. In this process, inorganic or organic compounds with flocculant activity allow floc formation by adsorption and charge neutralization, adsorption and interparticle bridging, and enmeshment in a precipitate (sweep flocculation) [16]. Flocculation usually includes a rapid mixing (RM) stage performed at 100–300 rpm for 1–5 min, where suspension destabilization occurs, and a slow mixing (SM) stage to allow the formation of larger agglomerates, performed at 20–50 rpm for 9–20 min [17,18,19,20]. Typically, inorganic flocculants based on iron or aluminum salts such as ferric chloride, ferric sulfate, and aluminum sulfate are used [18,21]. However, these flocculants are non-biodegradable; their use promotes secondary pollution and produces toxic sludge, which is expensive and complex to treat [22]. A study showed that the bioaccumulation of ferric ions is superior to that of ferrous ions in different microalgae, with Chlorella being the most resistant [23]. Other authors studied the adverse effects of ferric chloride and aluminum sulfate in microalgae, concluding that more than 95% of metals were transferred to the biomass [24]. Some studies also point to the harm associated with human exposure to aluminum, linking it to neurodegenerative diseases such as Alzheimer’s disease [25,26]. On the other hand, organic flocculants are generally synthetic polymers that require lower dosages, reducing the production of contaminated sludge [27]. Nevertheless, they are more expensive than the inorganic ones. Zetag 8185 is a synthetic polyacrylamide-based polymer that proves to be efficient in the harvesting of the microalga Neochloris oleoabundans [28]. Organic flocculants can also be natural-based. There has been a growing interest in applying chitosan [20,29] and tannin-based flocculants [15] to harvest microalgae.
Although organic polymers have been reported in prior investigations, there is limited information about their use in harvesting C. vulgaris. Further, there have been almost no studies comparing the utilization of metallic salts with both natural and synthetic organic polymers, and none perform process optimization. For this purpose, this study aims to optimize chemical flocculation. Aluminum sulfate, ferric chloride, and ferric sulfate (inorganic flocculants), Zetag 8185 (a synthetic organic polymer), Tanfloc SG (a tannin-based flocculant derived from Acacia mearnsii), and medium molecular weight chitosan were tested for the harvesting of C. vulgaris. Preliminary assays were conducted to determine the minimum dosages of each flocculant that generated primary flocs at different pH levels. Additional studies were conducted for the flocculants that had a better performance in the preliminary assays. Lastly, a 24 full factorial design experiment was performed to determine the effects of flocculant dosage, settling time, and RM and SM time on the harvesting efficiency of the best flocculant. In general, organic flocculants had a better performance, with Zetag 8185 being the most efficient flocculant.
2. Materials and Methods
2.1. Materials
2.1.1. Microalgal Culture
The microalgal C. vulgaris CCAP 211/11B used in this study was obtained from the Culture Collection of Algae and Protozoa (CCAP, Oban, UK). The microalgal was cultivated in 5 L bottles, stirred by air injection, and maintained under continuous artificial light with an intensity of 140 µmol m−2 s−1. The biomass concentration was monitored by an optical density at 680 nm (OD680). The relation between OD680 and the biomass dry weight (DW) concentration was established through a calibration curve (Equation (1)). The cultures were collected at concentrations above 340 mgDW L−1.
OD680 = 3.7837 × Biomass concentration (gDW L−1) − 0.0016
(R2 = 0.9902, Limit of detection = 0.02 gDW L−1, Limit of quantification = 0.07 gDW L−1).
(R2 = 0.9902, Limit of detection = 0.02 gDW L−1, Limit of quantification = 0.07 gDW L−1).
2.1.2. Flocculants
The inorganic flocculants aluminum sulfate, ferric sulfate, and ferric chloride were supplied by VWR International, and solutions of 50, 12, and 20 g L−1, respectively, were prepared in distilled water. Chitosan power with a deacetylation degree ≥75% was obtained from Sigma–Aldrich (St. Louis, MO, US), and a solution of 2 g L−1 was prepared according to the process described by Divakaran and Pillai [30]. A 20 g L−1 solution of Tanfloc SG, obtained from TANAC (Montenegro, Brazil), and a 2 g L−1 Zetag 8185 solution, supplied by BASF (Porto, Portugal), were also prepared by dissolving the required amount in distilled water.
2.2. Methods
2.2.1. Chemical Flocculation Assays
Flocculation assays were conducted in batch mode using Jar test equipment with six stirrers and a fluorescent lamp to observe floc formation. In all assays, 800 mL beakers containing 500 mL of microalgal culture were used. Preliminary assays were performed to determine the minimum dosages of each flocculant that generated primary flocs at pH levels of 5, 6, 7, 8, and 9. Increments of the coagulants were successively added to the 500 mL microalgal culture. After each addition, the solutions were stirred at 150 rpm for 3 min (RM) and at 20 rpm for 15 min (SM). Floc formation was then evaluated by visual observation. The assays were performed in duplicate.
Additional studies were conducted for the flocculants that had a better performance in the preliminary assays—Ferric chloride at pH 9, Zetag 8185 at pH 6, and Tanfloc SG at pH 9. Different flocculant dosages from 10 to 200 mg L−1 were tested. The experiments also included the RM and SM stages, followed by 15 min of sedimentation, and were performed in duplicate. Flocculation efficiency (η) was calculated with Equation (2), knowing the absorbance at 680 nm and measured using a UV-vis spectrophotometer through wavelength scanning. The maximum absorbance was observed at this value. ODi corresponds to the optical density of the microalgal culture prior to any flocculant addition, and ODf is the optical density after the settling period. The samples to determine ODf were taken from the supernatant, approximately 2 cm below the surface. The absorbance was measured in a UV-vis spectrophotometer (UV-6300PC double beam, VWR).
2.2.2. Zetag 8185 Optimization Assays
A 24 full factorial experimental design was performed to determine the effects of the flocculant dosage, settling time, RM time, and SM time on Zetag 8185 harvesting efficiency. Each factor was tested at low (−1) and high (+1) levels in duplicate. Table 1 presents the coded values with the respective real values in parentheses.

Table 1.
Experimental design data for optimizing C. vulgaris harvesting with Zetag 8185 (coded levels and real values in parentheses).
A four-way ANOVA (p < 0.05) was conducted, and an empirical first-order model characterizing flocculation was established. The model was then used to create a contour diagram to analyze the effects of the variables on Zetag flocculation efficiency. The statistical analysis and the model were constructed using Minitab® Statistical Software 21.1.0.0.
3. Results and Discussion
3.1. Minimum Dosage Determination
Preliminary studies aimed to determine the minimum flocculant dosage required to start the formation of primary flocs and the optimal pH for each flocculant. It was also our intent to select the flocculants with the most promising results to study the optimization of other parameters, such as dosage, in more detail. The results are presented in Table 2.

Table 2.
Optimal pH and minimum dosage for flocculation obtained for aluminum sulfate, ferric sulfate, ferric chloride, chitosan, Zetag 8185, and Tanfloc SG.
Ferric sulfate and ferric chloride showed better performance at a basic pH, with the optimum being pH 9. Despite the minimum dosages being very similar, ferric chloride allowed for the formation of larger flocs. Sanyano et al. [18] also obtained higher harvesting efficiency with ferric chloride at a basic pH (>8) for the harvest of Chlorella sp. The optimal pH for aluminum sulfate was 9 as well. Gani et al. [31] investigated the effect of pH and alum dosage in Botrycoccus sp. Harvesting, and the optimal pH was measured to be 9.2. Table 2 shows that aluminum sulfate needed a much higher dosage than the other inorganic flocculants to form flocs. Ferric salts were more effective than aluminum sulfate since these flocculants are converted to ferric hydroxide at pH 9, causing microalgae to establish bridges together, forming flocs more easily [32]. In the study performed by Sanyano et al. [18], ferric chloride was also more efficient than aluminum sulfate, requiring an inferior dosage to generate denser flocs. Except for chitosan, organic flocculants required lower dosages than did inorganic flocculants to form flocs. Chitosan and Zetag allowed for the formation of larger flocs at an acidic pH, while Tanfloc worked best at a basic pH. In a study by Kirnev et al. [28] on the harvest of N. oleoabundans, the flocculation efficiency increased for Zetag 8185 and chitosan when the pH was below 6, while the maximum efficiency for Tanfloc was achieved at pH 10. Chitosan also showed better performance at pH < 7 on the harvest of Chlorella sorokiniana [33]. Chitosan, Zetag 8185, and Tanfloc SG are cationic polymers, so they are likely to bind to and between the negatively charged C. vulgaris cells. However, chitosan needed a higher dosage in order to neutralize cells. The polymer size and charge density may have influenced flocculation, since chitosan is less charged than the other organic flocculants studied [28]. On the other hand, being a flocculant that requires a lower dosage to initiate floc formation, Zetag 8185 has a high charge density.
3.2. Dosage Optimization
The flocculants with the most promising preliminary tests were selected for the dosage optimization tests. The organic flocculants Zetag 8185 and Tanfloc SG were selected, as they present with the lowest minimum dosages for the formation of flocs. Of the inorganic flocculants, ferric chloride was chosen. Despite the minimum dosage being very similar to that of ferric sulfate, ferric chloride allowed for the formation of larger flocs, which could enable a faster sedimentation process. The assays were performed at the optimal pH previously determined for each flocculant (Table 2), and the results related to the harvesting efficiency are shown in Table 3.

Table 3.
Harvesting efficiency obtained in flocculation assays using concentrations of 10–200 mg L−1 of Zetag 8485 at pH 6 and Tanfloc SG and ferric chloride at pH 9.
Table 3 shows that the dosage significantly affects the efficiency of the flocculation harvesting process. Once again, Zetag 8185 achieved the best results, reaching 98.8% and 97.9% efficiencies at 50 and 100 mg L−1, respectively. The appearance of the culture after flocculation can be observed in Figure 1, which clearly shows the flocculated biomass and the transparency of the medium achieved with 50 and 100 mg Zetag L−1. From 100 mg L−1, there is a slight decrease in the efficiency, which indicates that the optimal dosage for this flocculant, under the conditions studied, is between 10 and 100 mg L−1.
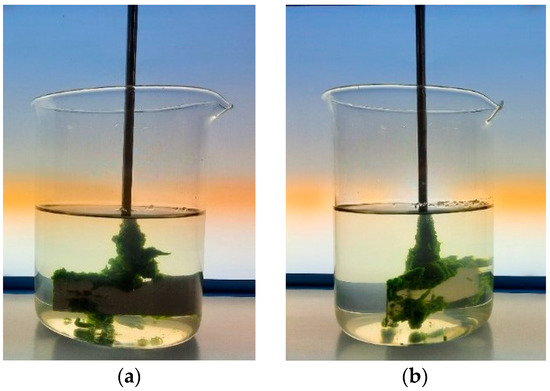
Figure 1.
Flocculated C. vulgaris biomass after harvesting at: (a) 50 mg L−1 Zetag 8185; (b) 100 mg L−1 Zetag 8185.
The harvesting efficiency achieved with the Tanfloc SG enhanced with the increasing dosage was 24.4% with a dosage of 200 mg L−1. However, this value is still very low, especially when compared to the results obtained with Zetag. Even though some neutralization occurred, it was not enough to cause significant aggregation. Kirnev et al. [28] applied different organic flocculants to harvest N. oleoabundans and reported Zetag 8185 as the best flocculant at pH 6 and a concentration of 16 mg L−1, reaching efficiencies above 95%. In the same study, Tanfloc SG only achieved a maximum efficiency of 13% at pH 6 and 14 mg L−1. Tanfloc has a lower charge density compared to Zetag, requiring higher dosages to achieve cell neutralization [28]. In a study performed by Niemi and Gentili [34] to harvest C. vulgaris, grown in municipal wastewater for 2–6 days, a tannin-based flocculant achieved efficiencies between 26% and 71% at 100 mg L−1. Increasing the flocculant dosage to 300 mg L−1 allowed for reaching harvesting efficiencies from 81% to 99%. Therefore, a dosage superior to 200 mg L−1 Tanfloc is expected to achieve better efficiencies.
Ferric chloride was the flocculant with the worst performance, only reaching a maximum efficiency of 9.4% with a concentration of 100 mg L−1. For dosages above 100 mg L−1, there was also a decrease in the efficiency. Due to the excessive positive charge around the negative charged cells, the higher flocculant concentration may have slowed down cell aggregation, destabilizing the microalgal cells because of electrostatic repulsion [31].
3.3. Zetag 8185 Optimization
Since Zetag 8185 was the flocculant that achieved better harvesting efficiencies, the impact of the other parameters was also studied. In addition to pH and dosage, the settling time and RM and SM time can also influence the harvesting efficiency. The results obtained regarding the efficiency of microalgae harvesting (response variable), which were obtained for the 24 full factorial experimental design, are in Table 4. Runs 17–32 are duplicates of runs 1–16 to perform statistical analysis.

Table 4.
The harvesting efficiency obtained in the 24 full factorial design experiment.
The results were analyzed by ANOVA (p < 0.05) to assess the goodness of fit, and the main effects and interaction analysis between the factors are presented in Table 5. The effects indicate the significance of each variable. The signs + and − indicate that the variable directly or indirectly influences the harvesting efficiency, respectively. For instance, increasing the settling time from 5 to 15 negatively affects efficiency. According to the p-values obtained, the Zetag dosage and the RM and SM times are the most significant variables affecting the flocculation process.

Table 5.
Effects and factor interaction analysis for C. vulgaris flocculation with Zetag 8185.
An empirical model (Equation (3)) describing the harvesting efficiency was obtained by eliminating the terms that are statistically insignificant (p > 0.05) that correspond to the terms that include the settling time. The R2 coefficient obtained was 0.9987, indicating that the model appropriately fitted the data. Based on the experimental results, the harvesting efficiency of C. vulgaris was found to be optimum at a Zetag 8185 dosage of 100 mg L−1, an RM time of 3 min, and without a SM phase. The settling time was not significantly relevant.
η (%) = 5.374 C + 1.350 D + 0.2784 AC + 0.05252 AD − 0.5823 CD − 0.01649 ACD
The contour diagrams with the effects of the interactions between the statistically significant factors (Dosage vs. RM time; Dosage vs. SM time; and RM time vs. SM time) on the flocculation efficiency are in Figure 2. It is possible to observe that the diagrams Dosage vs. RM time and Dosage vs. SM time are very similar. In fact, from the diagram for RM time vs. SM time, it is clear that there is no need to include two mixing phases when Zetag 8185 is used as a flocculant, a simplification which would reduce time and energy costs at an industrial level, further facilitating the process of harvesting microalgae by flocculation.
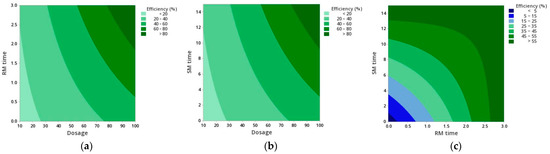
Figure 2.
Contour diagrams of C. vulgaris harvesting efficiency as a function of the statistically significant factors: (a) Dosage vs. RM time; (b) Dosage vs. SM time; (c) RM time vs. SM time.
4. Conclusions
Different organic and inorganic flocculants were studied for the purpose of harvesting C. vulgaris in batch mode. In general, organic flocculants needed small dosages to initiate floc formation. Zetag 8185 showed the best performance, only requiring a dosage of 11 mg gDW−1 (4 mg L−1). Ferric chloride was unable to harvest the strain in question, reaching only an efficiency of 9% for 100 mg L−1. On the other hand, Zetag reached efficiencies close to 100% for 50 and 100 mg L−1 dosages. Based on the empirical model obtained, the harvesting efficiency of C. vulgaris was optimal at a dosage of 100 mg L−1 of Zetag 8185, 3 min of rapid mixing, and without a slow mixing step. The settling time was considered to be statistically insignificant. There is no universal harvesting process, hence, factors such as the microalgal species, cultivation system used, and the purpose of the biomass or product produced by the microalgae must be considered when choosing, for instance, a flocculant.
Author Contributions
Conceptualization, C.A.M., A.F.E. and J.C.M.P.; methodology, C.A.M., A.F.E. and J.C.M.P.; investigation, C.A.M.; resources, J.C.M.P.; writing-original draft preparation, C.A.M.; writing-review and editing, C.A.M., A.F.E. and J.C.M.P.; supervision, A.F.E. and J.C.M.P.; project administration, J.C.M.P.; funding acquisition, J.C.M.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by: (i) LA/P/0045/2020 (ALiCE) and UIDB/00511/2020-UIDP/00511/2020 (LEPABE), funded by national funds through FCT/MCTES (PIDDAC); (ii) Project PIV4Algae (Ref. PTDC/BTA-BTA/31736/2017; POCI-01-0145-FEDER-031736), funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES; and (iii) Project PhotoBioValue (ref. PTDC/BTA-BTA/2902/2021), funded by FEDER funds through COMPETE2020—Programa Operacional Competitividade e Internacionalização (POCI) and by national funds (PIDDAC) through FCT/MCTES. A.F. Esteves thanks FCT for financially supporting her work through the FCT PhD Research Scholarships 2020.05477.BD.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Barros, A.I.; Gonçalves, A.L.; Simões, M.; Pires, J.C. Harvesting techniques applied to microalgae: A review. Renew. Sustain. Energy Rev. 2015, 41, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.I.; Esteves, A.F.; Pires, J.C.; Gonçalves, A.L. Microalgal Biorefineries: Key Processes and Main Challenges. In Microalgal Biotechnology: Recent Advances, Market Potencial, and Sustainability; Shekh, A., Schenk, P., Sarada, R., Eds.; Royal Society of Chemistry: London, UK, 2021; pp. 36–76. [Google Scholar]
- Ru, I.T.K.; Sung, Y.Y.; Jusoh, M.; Wahid, M.E.A.; Nagappan, T. Chlorella vulgaris: A perspective on its potential for combining high biomass with high value bioproducts. Appl. Phycol. 2020, 1, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Yun, Y.S.; Park, J.M. Kinetic modeling of the light-dependent photosynthetic activity of the green microalga Chlorella vulgaris. Biotechnol. Bioeng. 2003, 83, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.M.; Ji, X.W.; He, Y.J.; Li, Z.F.; Wang, M.Z.; Chen, B.L.; Huang, J. Simultaneous fixation of carbon dioxide and purification of undiluted swine slurry by culturing Chlorella vulgaris MBFJNU-1. Algal Res. 2020, 47. [Google Scholar] [CrossRef]
- Khan, M.I.; Shin, J.H.; Kim, J.D. The promising future of microalgae: Current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Factories 2018, 17, 36. [Google Scholar] [CrossRef]
- Roselet, F.; Vandamme, D.; Muylaert, K.; Abreu, P.C. Harvesting of microalgae for biomass production. In Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment; Alam, M., Wang, Z., Eds.; Springer: Singapore, 2019; pp. 211–243. [Google Scholar]
- Branyikova, I.; Prochazkova, G.; Potocar, T.; Jezkova, Z.; Branyik, T. Harvesting of microalgae by flocculation. Fermentation 2018, 4, 93. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, D.; Ferreira, A.; Quelhas, P.; Schulze, P.S.; Gouveia, L. Nannochloropsis oceanica harvested using electrocoagulation with alternative electrodes—An innovative approach on potential biomass applications. Bioresour. Technol. 2022, 344, 126222. [Google Scholar] [CrossRef]
- Gutierrez, R.; Ferrer, I.; Uggetti, E.; Arnabat, C.; Salvado, H.; Garcia, J. Settling velocity distribution of microalgal biomass from urban wastewater treatment high rate algal ponds. Algal Res. 2016, 16, 409–417. [Google Scholar] [CrossRef] [Green Version]
- Gough, R.; Holliman, P.J.; Cooke, G.M.; Freeman, C. Characterisation of algogenic organic matter during an algal bloom and its implications for trihalomethane formation. Sustain. Water Qual. Ecol. 2015, 6, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Hua, L.-C.; Lai, C.-H.; Wang, G.-S.; Lin, T.-F.; Huang, C. Algogenic organic matter derived DBPs: Precursor characterization, formation, and future perspectives—A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1803–1834. [Google Scholar] [CrossRef]
- Gonzalez-Camejo, J.; Pachés, M.; Marín, A.; Jiménez-Benítez, A.; Seco, A.; Barat, R. Production of microalgal external organic matter in a Chlorella-dominated culture: Influence of temperature and stress factors. Environ. Sci. Water Res. Technol. 2020, 6, 1828–1841. [Google Scholar] [CrossRef]
- Roselet, F.; Burkert, J.; Abreu, P.C. Flocculation of Nannochloropsis oculata using a tannin-based polymer: Bench scale optimization and pilot scale reproducibility. Biomass Bioenergy 2016, 87, 55–60. [Google Scholar] [CrossRef]
- Davis, M.L. Coagulation and Flocculation. In Water and Wastewater Engineering: Design Principles and Practice; McGraw-Hill Education: New York, NY, USA, 2010. [Google Scholar]
- Machado, G.; Dos Santos, C.A.; Gomes, J.; Faria, D.; Santos, F.; Lourega, R. Chemical modification of tannins from Acacia mearnsii to produce formaldehyde free flocculant. Sci. Total Environ. 2020, 745, 140875. [Google Scholar] [CrossRef] [PubMed]
- Sanyano, N.; Chetpattananondh, P.; Chongkhong, S. Coagulation–flocculation of marine Chlorella sp. for biodiesel production. Bioresour. Technol. 2013, 147, 471–476. [Google Scholar] [CrossRef]
- Schmitt, F.O.; Rodrigues, R.T.; Oliveira, C. Efficacy of two natural tannins-based polymers in contrast to aluminum sulfate for drinking water production. Clean. Eng. Technol. 2021, 3, 100099. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Z.; Hiltunen, E. Microalgae Chlorella vulgaris biomass harvesting by natural flocculant: Effects on biomass sedimentation, spent medium recycling and lipid extraction. Biotechnol. Biofuels 2018, 11, 183. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-Y.; Lee, K.; Lee, J.; Lee, Y.-H.; Han, J.-I.; Park, J.-Y.; Oh, Y.-K. Acidified-flocculation process for harvesting of microalgae: Coagulant reutilization and metal-free-microalgae recovery. Bioresour. Technol. 2017, 239, 190–196. [Google Scholar] [CrossRef]
- Ang, W.L.; Mohammad, A.W. State of the art and sustainability of natural coagulants in water and wastewater treatment. J. Clean. Prod. 2020, 262, 121267. [Google Scholar] [CrossRef]
- Rossi, S.; Visigalli, S.; Cascino, F.C.; Mantovani, M.; Mezzanotte, V.; Parati, K.; Canziani, R.; Turolla, A.; Ficara, E. Metal-based flocculation to harvest microalgae: A look beyond separation efficiency. Sci. Total Environ. 2021, 799, 149395. [Google Scholar] [CrossRef]
- Subramaniyam, V.; Subashchandrabose, S.R.; Thavamani, P.; Chen, Z.; Krishnamurti, G.; Naidu, R.; Megharaj, M. Toxicity and bioaccumulation of iron in soil microalgae. J. Appl. Phycol. 2016, 28, 2767–2776. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Vallamkondu, J.; Corgiat, E.B.; Gill, K.D. Understanding aspects of Aluminum exposure in a lzheimer’s disease development. Brain Pathol. 2016, 26, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Mold, M.; Linhart, C.; Gómez-Ramírez, J.; Villegas-Lanau, A.; Exley, C. Aluminum and amyloid-β in familial Alzheimer’s disease. J. Alzheimer’s Dis. 2020, 73, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Teh, C.Y.; Budiman, P.M.; Shak, K.P.Y.; Wu, T.Y. Recent advancement of coagulation–flocculation and its application in wastewater treatment. Ind. Eng. Chem. Res. 2016, 55, 4363–4389. [Google Scholar] [CrossRef]
- Kirnev, P.; de Carvalho, J.; Miyaoka, J.; Cartas, L.; Vandenberghe, L.; Soccol, C. Harvesting Neochloris oleoabundans using commercial organic flocculants. J. Appl. Phycol. 2018, 30, 2317–2324. [Google Scholar] [CrossRef]
- Japar, A.S.; Takriff, M.S.; Mohd Yasin, N.H.; Mahmod, S.S. Optimization of Chlorella biomass harvesting by flocculation and its potential for biofuel production. J. Appl. Phycol. 2021, 33, 1621–1629. [Google Scholar] [CrossRef]
- Divakaran, R.; Pillai, V.S. Flocculation of algae using chitosan. J. Appl. Phycol. 2002, 14, 419–422. [Google Scholar] [CrossRef]
- Gani, P.; Mohamed Sunar, N.; Matias-Peralta, H.; Abdul Latiff, A.A. Effect of pH and alum dosage on the efficiency of microalgae harvesting via flocculation technique. Int. J. Green Energy 2017, 14, 395–399. [Google Scholar] [CrossRef]
- Min, K.H.; Kim, D.H.; Ki, M.-R.; Pack, S.P. Recent progress in flocculation, dewatering, and drying technologies for microalgae utilization: Scalable and low-cost harvesting process development. Bioresour. Technol. 2022, 344, 126404. [Google Scholar] [CrossRef]
- Xu, Y.; Purton, S.; Baganz, F. Chitosan flocculation to aid the harvesting of the microalga Chlorella sorokiniana. Bioresour. Technol. 2013, 129, 296–301. [Google Scholar] [CrossRef]
- Niemi, C.; Gentili, F.G. The use of natural organic flocculants for harvesting microalgae grown in municipal wastewater at different culture densities. Physiol. Plant. 2021, 173, 536–542. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).