Effects of Tetracycline and Copper on Water Spinach Growth and Soil Bacterial Community
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Material
2.2. Experimental Design and Treatment Plan
2.3. Analytic Methods
2.3.1. Assay of Physiological and Biochemical Indexes
2.3.2. Soil Physical and Chemical Indicators
2.4. 16S rRNA Gene Analysis via Illumina High-Throughput Sequencing and Data Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effects of TC and Cu Complex Contamination on Physiological and Biochemical Indicators of Water Spinach
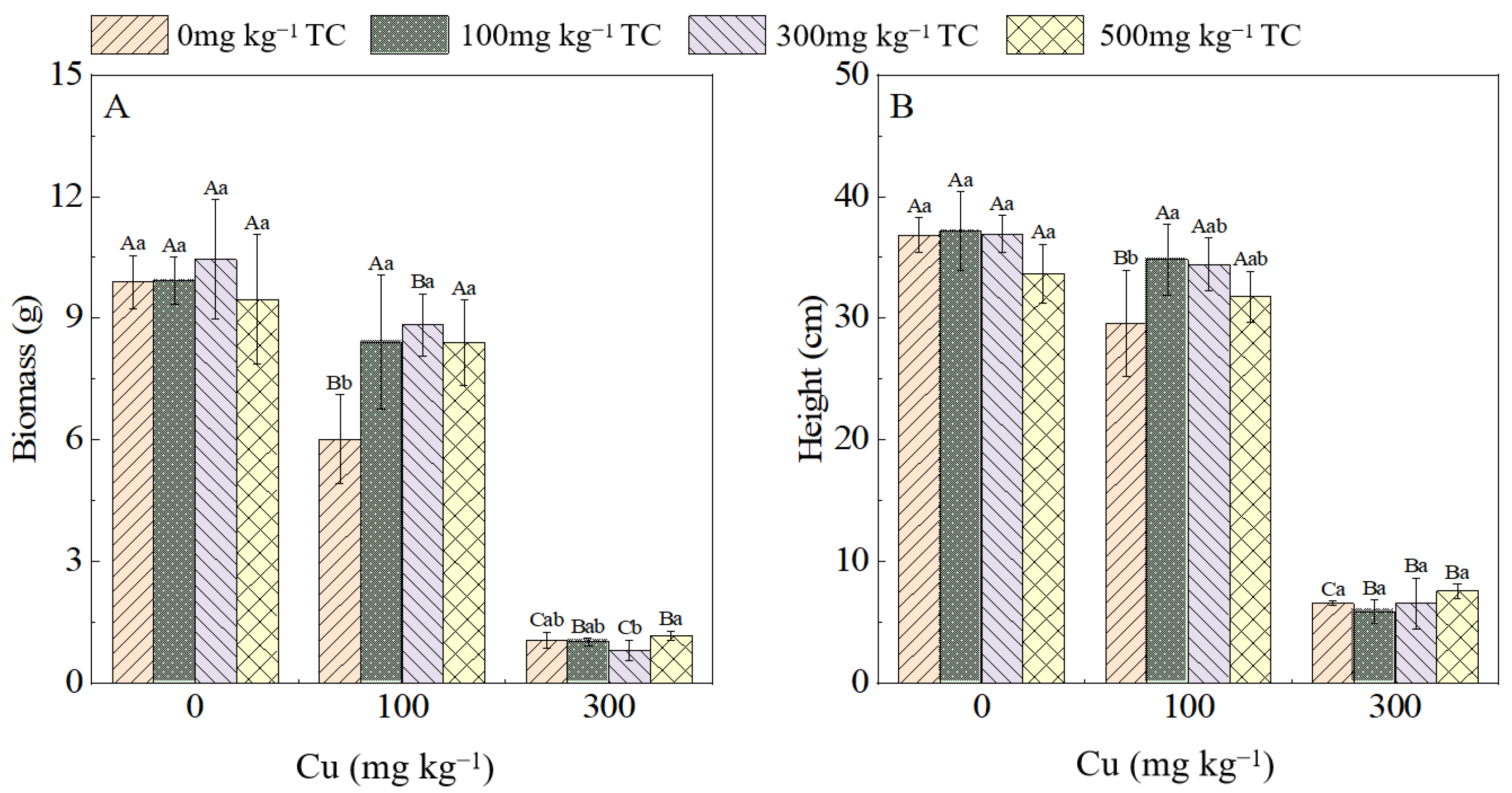
3.1.1. Plant Height and Biomass of Water Spinach
3.1.2. Antioxidant Enzyme Activity and MDA Content in Leaves of the Water Spinach
3.2. Effect of TC and Cu Composed Pollution on Soil Bacterial Community
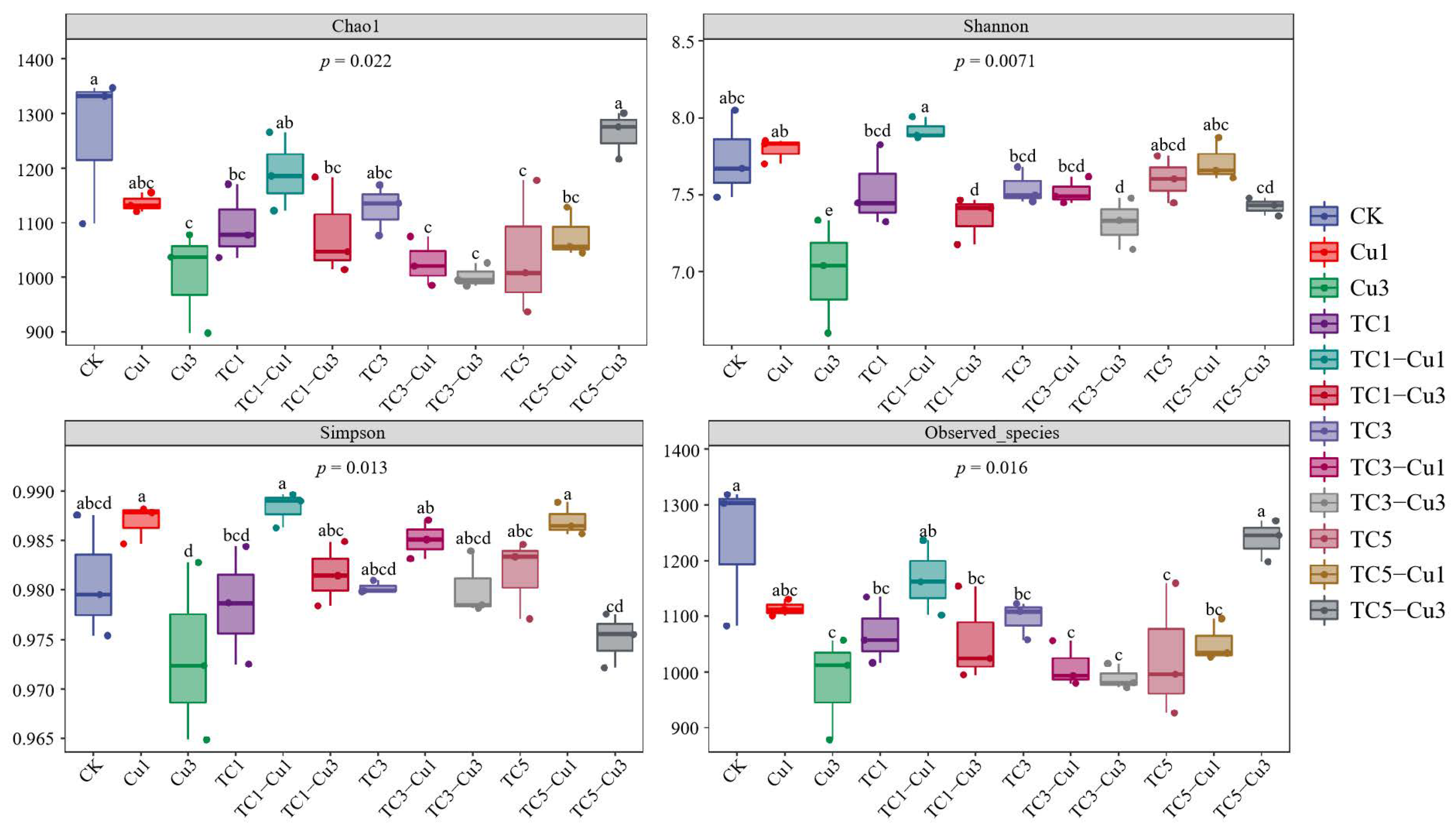
3.2.1. Alpha Diversity of Soil Bacterial Communities
3.2.2. Taxonomic Composition of Soil Bacterial Communities
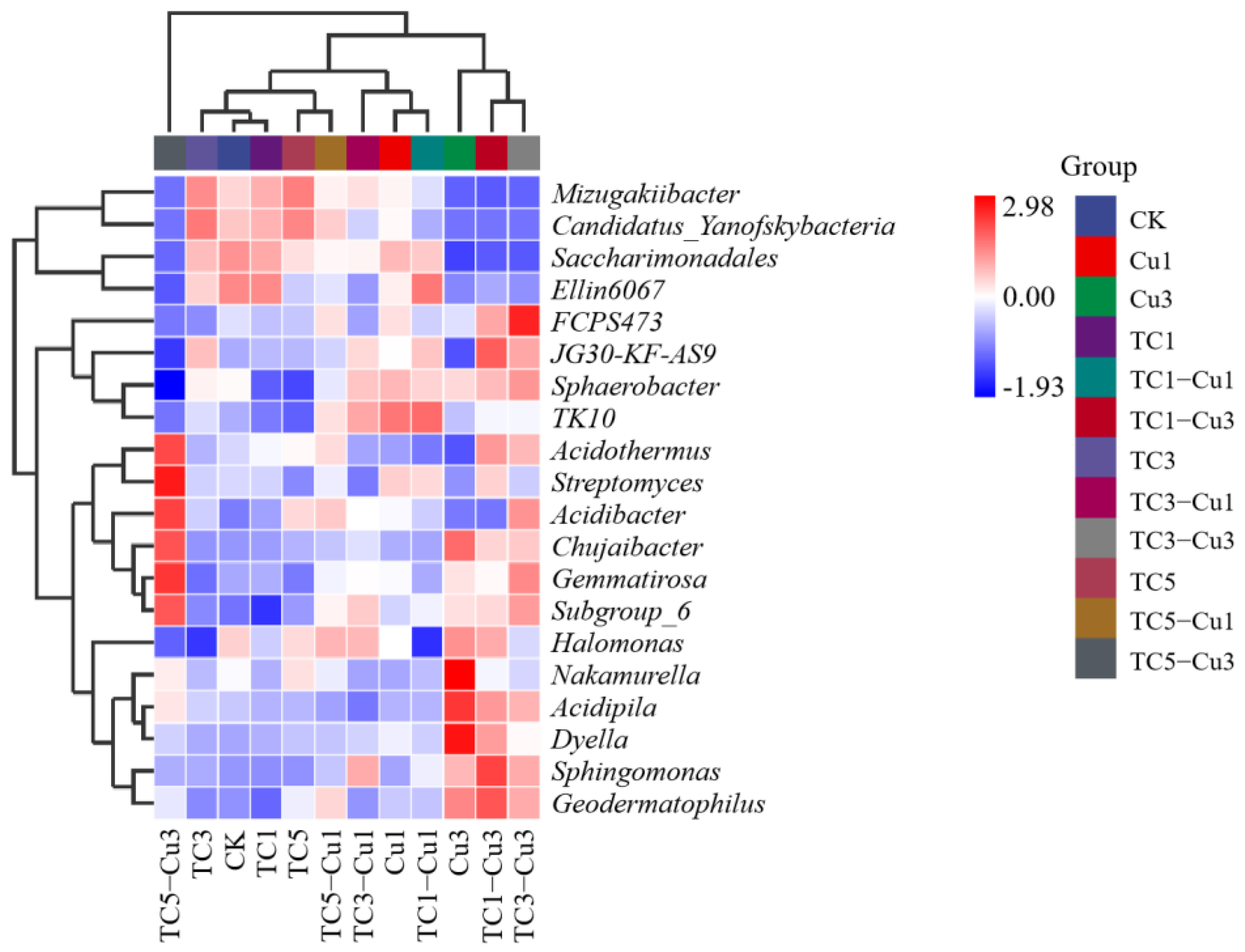
3.2.3. Clustering and Correlation Analysis between Treatment Groups and Soil Microbial Communities at the Genus Level
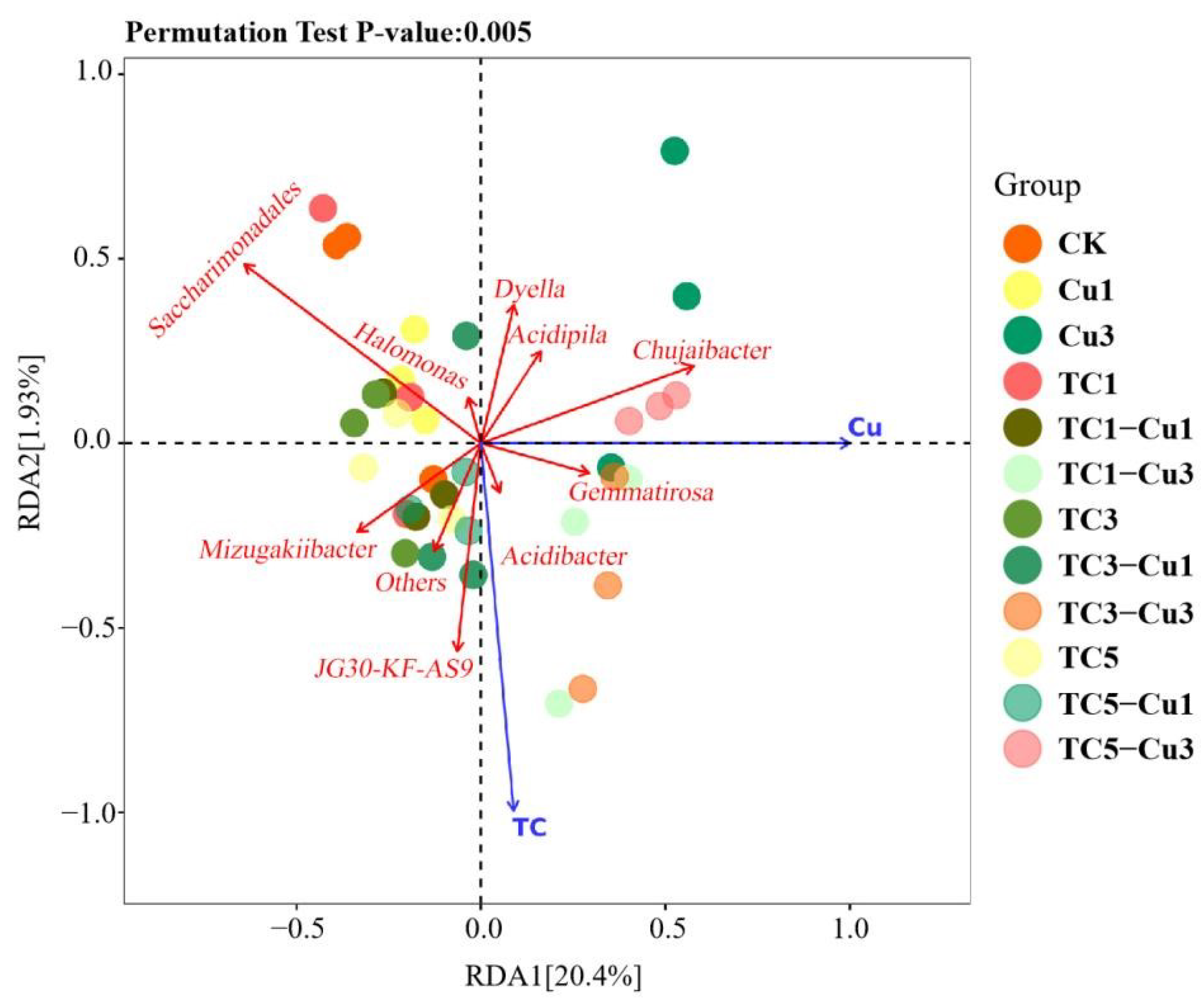
3.2.4. Redundancy Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mog, M.; Ngasotter, S.; Tesia, S.; Waikhom, D.; Panda, S.P.; Sharma, S.; Varshney, S. Problems of antibiotic resistance associated with oxytetracycline use in aquaculture: A review. J. Entomol. Zool. Stud. 2020, 8, 1075–1082. [Google Scholar]
- Van, T.T.H.; Yidana, Z.; Smooker, P.M.; Coloe, P.J. Antibiotic use in food animals worldwide, with a focus on Africa: Pluses and minuses. J. Global Antimicrob. Resist. 2020, 20, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Santás-Miguel, V.; Arias-Estévez, M.; Díaz-Raviña, M.; Fernández-Sanjurjo, M.J.; Álvarez-Rodríguez, E.; Núñez-Delgado, A.; Fernández-Calviño, D. Interactions between soil properties and tetracycline toxicity affecting to bacterial community growth in agricultural soil. Appl. Soil Ecol. 2020, 147, 103437. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef]
- Jayalakshmi, K.; Veeraselvam, M.; Ramkumar, P.K.; Venkatesan, M.; Yogeshpriya, S.; Premalatha, N. A review on antimicrobial resistance, diagnosis and an alternative approach. J. Entomol. Zool. Stud. 2021, 9, 1058–1071. [Google Scholar]
- Ronquillo, M.G.; Hernandez, J.C.A. Antibiotic and synthetic growth promoters in animal diets: Review of impact and analytical methods. Food control 2017, 72, 255–267. [Google Scholar] [CrossRef]
- Scaria, J.; Anupama, K.V.; Nidheesh, P.V. Tetracyclines in the environment: An overview on the occurrence, fate, toxicity, detection, removal methods, and sludge management. Sci. Total Environ. 2021, 771, 145291. [Google Scholar] [CrossRef]
- Albero, B.; Tadeo, J.L.; Escario, M.; Miguel, E.; Pérez, R.A. Persistence and availability of veterinary antibiotics in soil and soil-manure systems. Sci. Total Environ. 2018, 643, 1562–1570. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.; Lu, C.; Liao, Q.; Gudda, F.O.; Ling, W. Antibiotics in animal manure and manure-based fertilizers: Occurrence and ecological risk assessment. Chemosphere 2020, 255, 127006. [Google Scholar] [CrossRef]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Tuyiringire, N.; Tusubira, D.; Munyampundu, J.P.; Tolo, C.U.; Muvuny, C.M.; Ogwang, P.E. Application of metabolomics to drug discovery and understanding the mechanisms of action of medicinal plants with anti-tuberculosis activity. Clin. Transl. Med. 2018, 7, 29. [Google Scholar] [CrossRef]
- Scott, A.; Vadalasetty, K.P.; Chwalibog, A.; Sawosz, E. Copper nanoparticles as an alternative feed additive in poultry diet: A review. Nanotechnol. Rev. 2018, 7, 69–93. [Google Scholar] [CrossRef]
- Keshavarzi, A.; Kumar, V.; Ertunç, G.; Brevik, E.C. Ecological risk assessment and source apportionment of heavy metals contamination: An appraisal based on the Tellus soil survey. Environ. Geochem. Health 2021, 43, 2121–2142. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Gong, Y.; Liu, Q.; Yang, S.; Ma, J.; Zhao, L.; Hou, H. Status of copper accumulation in agricultural soils across China (1985–2016). Chemosphere 2020, 244, 125516. [Google Scholar] [CrossRef] [PubMed]
- Jensen, J.; Larsen, M.M.; Bak, J. National monitoring study in Denmark finds increased and critical levels of copper and zinc in arable soils fertilized with pig slurry. Environ. Pollut. 2016, 214, 334–340. [Google Scholar] [CrossRef]
- Kumar, V.; Pandita, S.; Sidhu, G.P.S.; Sharma, A.; Khanna, K.; Kaur, P.; Bali, A.S.; Setia, R. Copper bioavailability, uptake, toxicity and tolerance in plants: A comprehensive review. Chemosphere 2021, 262, 127810. [Google Scholar] [CrossRef]
- Ghori, N.-H.; Ghori, T.; Hayat, M.Q.; Imadi, S.R.; Gul, A.; Altay, V.; Ozturk, M. Heavy metal stress and responses in plants. Int. J. Environ. Sci. Technol. 2019, 16, 1807–1828. [Google Scholar] [CrossRef]
- Dixit, R.; Wasiullah; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef] [Green Version]
- Girotto, F.; Cossu, R. Animal waste: Opportunities and challenges. Sustain. Agri. Rev. 2017, 22, 1–13. [Google Scholar]
- Quaik, S.; Embrandiri, A.; Ravindran, B.; Hossain, K.; Ismail, N.; Al-Dhabi, N.A.; Arasu, M.V.; Ignacimuthu, S. Veterinary antibiotics in animal manure and manure laden soil: Scenario and challenges in Asian countries. J. King. Saud. Univ. Sci. 2020, 32, 1300–1305. [Google Scholar] [CrossRef]
- Yaashikaa, P.R.; Kumar, P.S.; Jeevanantham, S.; Saravanan, R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ. Pollut. 2022, 301, 119035. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Berendonk, T.U. Heavy metal driven co-selection of antibiotic resistance in soil and water bodies impacted by agriculture and aquaculture. Front. Microbiol. 2012, 3, 399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Zou, M.; Zhang, B.; Lai, W.; Zeng, X.; Chen, S.; Wang, M.; Yi, X.; Tao, X.; Lu, G. Remediation of Cd-, Pb-, Cu-, and Zn-contaminated soil using cow bone meal and oyster shell meal. Ecotox. Environ. Safe. 2022, 229, 113073. [Google Scholar] [CrossRef]
- Gullberg, E.; Albrecht, L.M.; Karlsson, C.; Sandegren, L.; Andersson, D.I. Selection of a multidrug resistance plasmid by sublethal levels of antibiotics and heavy metals. MBio 2014, 5, e01918-14. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. At the nexus of antibiotics and metals: The impact of Cu and Zn on antibiotic activity and resistance. Trends Microbiol. 2017, 25, 820–832. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Zhu, L.; Wang, J.; Zhu, G. Toxicity of enrofloxacin, copper and their interactions on soil microbial populations and ammonia-oxidizing archaea and bacteria. Sci. Rep. 2018, 8, 5828. [Google Scholar] [CrossRef]
- Xu, Y.; Yu, W.; Ma, Q.; Wang, J.; Zhou, H.; Jiang, C. The combined effect of sulfadiazine and copper on soil microbial activity and community structure. Ecotox. Environ. Safe. 2016, 134, 43–52. [Google Scholar] [CrossRef]
- Lu, X.; Gao, Y.; Luo, J.; Yan, S.; Rengel, Z.; Zhang, Z. Interaction of veterinary antibiotic tetracyclines and copper on their fates in water and water hyacinth (Eichhornia crassipes). J. Hazard. Mater. 2014, 280, 389–398. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, C.; Song, Z.; He, C.; He, J.; Huang, W.; Dang, Z. Influences of tetracycline and cadmium on rice roots: Growth and root exudates. Acta Sci. Circumst. 2019, 41, 1518–1528. [Google Scholar]
- Maleki, M.; Ghorbanpour, M.; Kariman, K. Physiological and antioxidative responses of medicinal plants exposed to heavy metals stress. Plant Gene. 2017, 11, 247–254. [Google Scholar] [CrossRef]
- Aderemi, A.O.; Novais, S.C.; Lemos, M.F.L.; Alves, L.M.; Hunter, C.; Pahl, O. Oxidative stress responses and cellular energy allocation changes in microalgae following exposure to widely used human antibiotics. Aquat. Toxicol. 2018, 203, 130–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, R. Analytical Methods of Soil Agrochemistry; China Agricultural Science and Technology Publishing House: Beijing, China, 1999; pp. 18–99. [Google Scholar]
- Łukaszewicz, P.; Białk-Bielińska, A.; Dołżonek, J.; Caban, M.; Stepnowski, P. A new approach for the extraction of tetracyclines from soil matrices: Application of the microwave-extraction technique. Anal. Bioanal. Chem. 2018, 410, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Gao, C. Evaluation of Combined Toxicity of Heavy Metals and Antibiotics to Microcystis Aeruginosa; North West Agriculture and Forestry University: Xi’an, China, 2020. [Google Scholar]
- Cao, Y.; Ma, C.; Chen, H.; Chen, G.; White, J.C.; Xing, B. Copper stress in flooded soil: Impact on enzyme activities, microbial community composition and diversity in the rhizosphere of Salix integra. Sci. Total Environ. 2020, 704, 135350. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, J.; Gao, L.; Kong, F.; Shen, G.; Wang, R.; Gao, J.; Zhang, J. The effects of tetracycline residues on the microbial community structure of tobacco soil in pot experiment. Sci. Rep. 2020, 10, 8804. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.K.; Singh, N.R.; Kumar, U.; Mishra, S.R.; Perumal, R.C.; Benny, J.; Thatoi, H. Illumina MiSeq based assessment of bacterial community structure and diversity along the heavy metal concentration gradient in Sukinda chromite mine area soils, India. Ecol. Genet. Genomics. 2020, 15, 100054. [Google Scholar] [CrossRef]
- Shaw, J.L.A.; Ernakovich, J.G.; Judy, J.D.; Farrell, M.; Whatmuff, M.; Kirby, J. Long-term effects of copper exposure to agricultural soil function and microbial community structure at a controlled and experimental field site. Environ. Pollut. 2020, 263, 114411. [Google Scholar] [CrossRef]
- Reddy, B.; Dubey, S.K. River Ganges water as reservoir of microbes with antibiotic and metal ion resistance genes: High throughput metagenomic approach. Environ. Pollut. 2019, 246, 443–451. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ahn, J.-H.; Weon, H.-Y.; Hong, S.-B.; Seok, S.-J.; Kim, J.-S.; Kown, S.-W. Chujaibacter soli gen. nov., sp. nov., isolated from soil. J. Microbiol. 2015, 53, 592–597. [Google Scholar] [CrossRef]
- Liang, Z.; Zhang, W.; Yang, Y.; Ma, J.; Li, S.; Wen, Z. Soil characteristics and microbial community response in rare earth mining areas in southern Jiangxi Province, China. Environ. Sci. Pollut. Res. 2021, 28, 56418–56431. [Google Scholar] [CrossRef]
- Mason, L.M.; Eagar, A.; Patel, P.; Blackwood, C.B.; DeForest, J.L. Potential microbial bioindicators of phosphorus mining in a temperate deciduous forest. J. Appl. Microbiol. 2021, 130, 109–122. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Z.; Wang, X.; George, T.S.; Feng, G.; Zhang, L. Simulated root exudates stimulate the abundance of Saccharimonadales to improve the alkaline phosphatase activity in maize rhizosphere. Appl. Soil Ecol. 2022, 170, 104274. [Google Scholar] [CrossRef]
- Xie, Z.; Fu, T.; He, T.; Cheng, J.; Fu, D.; Luo, S. Effects of Cd Stress on Bacterial Community Composition and Diversity in Potato Rhizosphere Soil. J. Henan Agric. Sci. 2020, 49, 48–58. [Google Scholar]
- Zhu, F.; Li, J.; Zhang, Y.; Xiao, J.; Liang, Z. Watermelon Rhizosphere Soil Bacterial Diversity Affects the Occurrence of Fusarium Wilt. Chin. Agric. Sci. Bull. 2018, 34, 69–76. [Google Scholar]
- Zhang, M.; Riaz, M.; Xia, H.; Li, Y.; Wang, X.; Jiang, C. Four-year biochar study: Positive response of acidic soil microenvironment and citrus growth to biochar under potassium deficiency conditions. Sci. Total Environ. 2022, 813, 152515. [Google Scholar] [CrossRef] [PubMed]



| Determination Index | Measured Value |
|---|---|
| pH | 5.44 |
| SOM (g kg−1) | 26.18 |
| TC (mg kg−1) | Not Detected |
| Cu (mg kg−1) | 35.00 |
| Total nitrogen (g kg−1) | 0.84 |
| Available N (A-N, mg kg−1) | 181.81 |
| Available P (A-P, mg kg−1) | 87.16 |
| Available K (A-K, mg kg−1) | 514.27 |
| TC (mg per kg of Soil) | Cu (mg per kg of Soil) | ||
|---|---|---|---|
| 0 | 100 | 300 | |
| 0 | Control | Cu1 | Cu3 |
| 100 | TC1 | TC1-Cu1 | TC1-Cu3 |
| 300 | TC3 | TC3-Cu1 | TC3-Cu3 |
| 500 | TC5 | TC5-Cu1 | TC5-Cu3 |
| Factor | TC | Cu | TC × Cu | |||
|---|---|---|---|---|---|---|
| F | P | F | P | F | P | |
| CAT | 7.988 | 0.001 * | 24.045 | 0.000 * | 11.443 | 0.000 * |
| POD | 24.433 | 0.000 * | 32.009 | 0.000 * | 21.053 | 0.000 * |
| SOD | 1.079 | 0.377 | 18.476 | 0.000 * | 7.370 | 0.000 * |
| MDA | 9.378 | 0.000 * | 56.168 | 0.000 * | 41.781 | 0.000 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, J.; Wang, J.; Zheng, X.; Jia, A.; Zou, M.; Zhang, J.; Tao, X. Effects of Tetracycline and Copper on Water Spinach Growth and Soil Bacterial Community. Processes 2022, 10, 1135. https://doi.org/10.3390/pr10061135
Tao J, Wang J, Zheng X, Jia A, Zou M, Zhang J, Tao X. Effects of Tetracycline and Copper on Water Spinach Growth and Soil Bacterial Community. Processes. 2022; 10(6):1135. https://doi.org/10.3390/pr10061135
Chicago/Turabian StyleTao, Jiadan, Jiayu Wang, Xiongkai Zheng, Aiping Jia, Mengyao Zou, Jinlian Zhang, and Xueqin Tao. 2022. "Effects of Tetracycline and Copper on Water Spinach Growth and Soil Bacterial Community" Processes 10, no. 6: 1135. https://doi.org/10.3390/pr10061135
APA StyleTao, J., Wang, J., Zheng, X., Jia, A., Zou, M., Zhang, J., & Tao, X. (2022). Effects of Tetracycline and Copper on Water Spinach Growth and Soil Bacterial Community. Processes, 10(6), 1135. https://doi.org/10.3390/pr10061135





