Thermal Stability Determination of Propylene Glycol Sodium Alginate and Ammonium Sulfate with Calorimetry Technology
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Materials
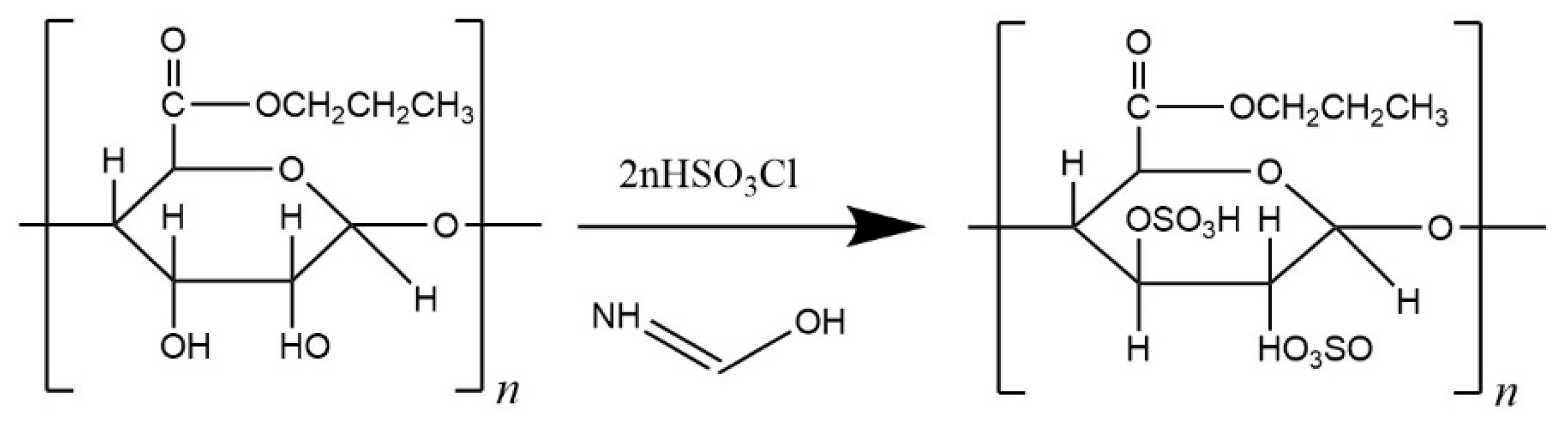
2.2. Sulfonation Synthesis of PSS
2.3. Differential Scanning Calorimetry Experiments
2.4. Thermogravimetric Analysis Experiments
2.5. Thermokinetic Analysis
3. Results and discussion
3.1. Process Safety of Sulfonation Synthesis of PSS
3.2. Thermal Analysis Technology
- (1)
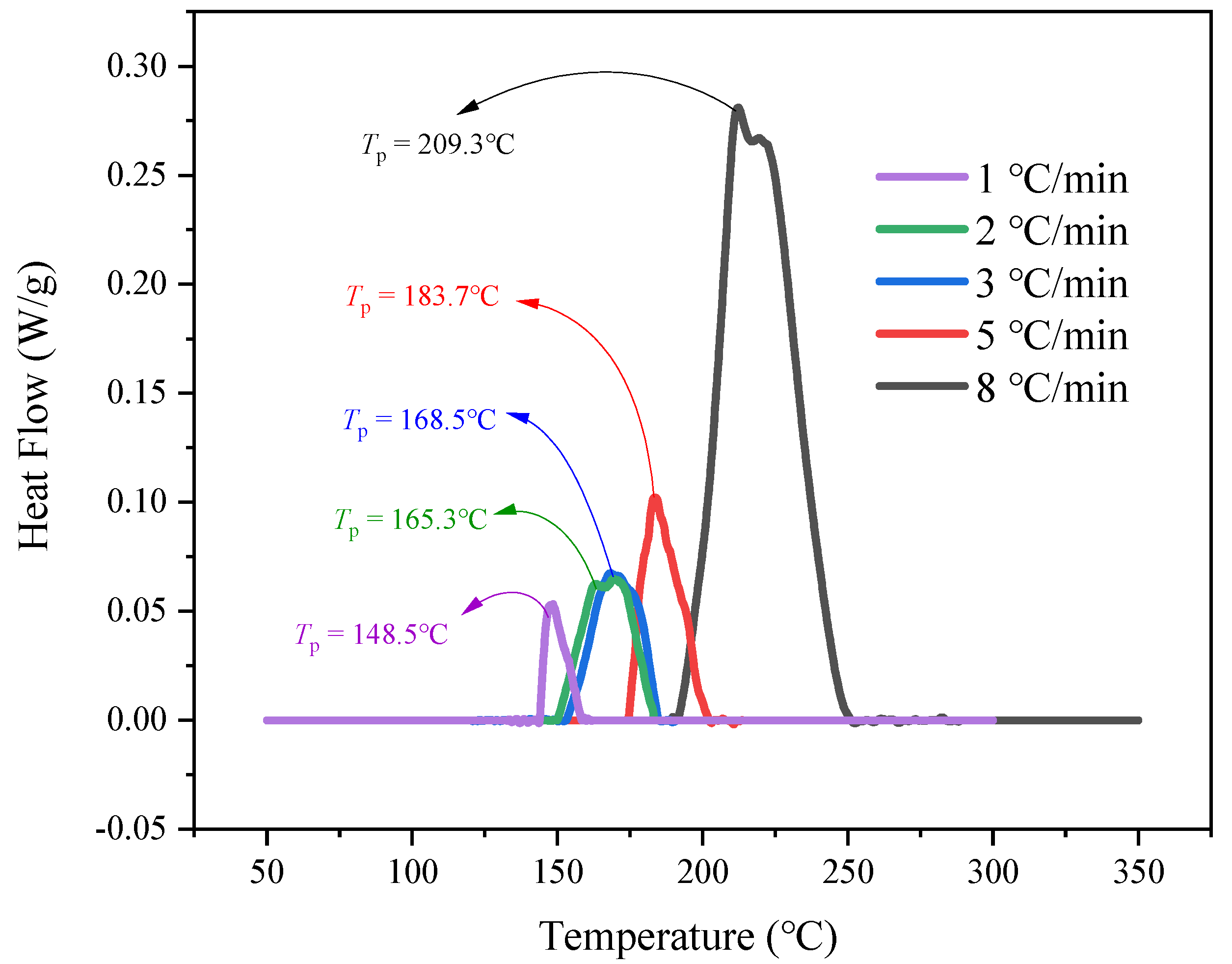
- Thermal decomposition analysis by DSC
- (2)
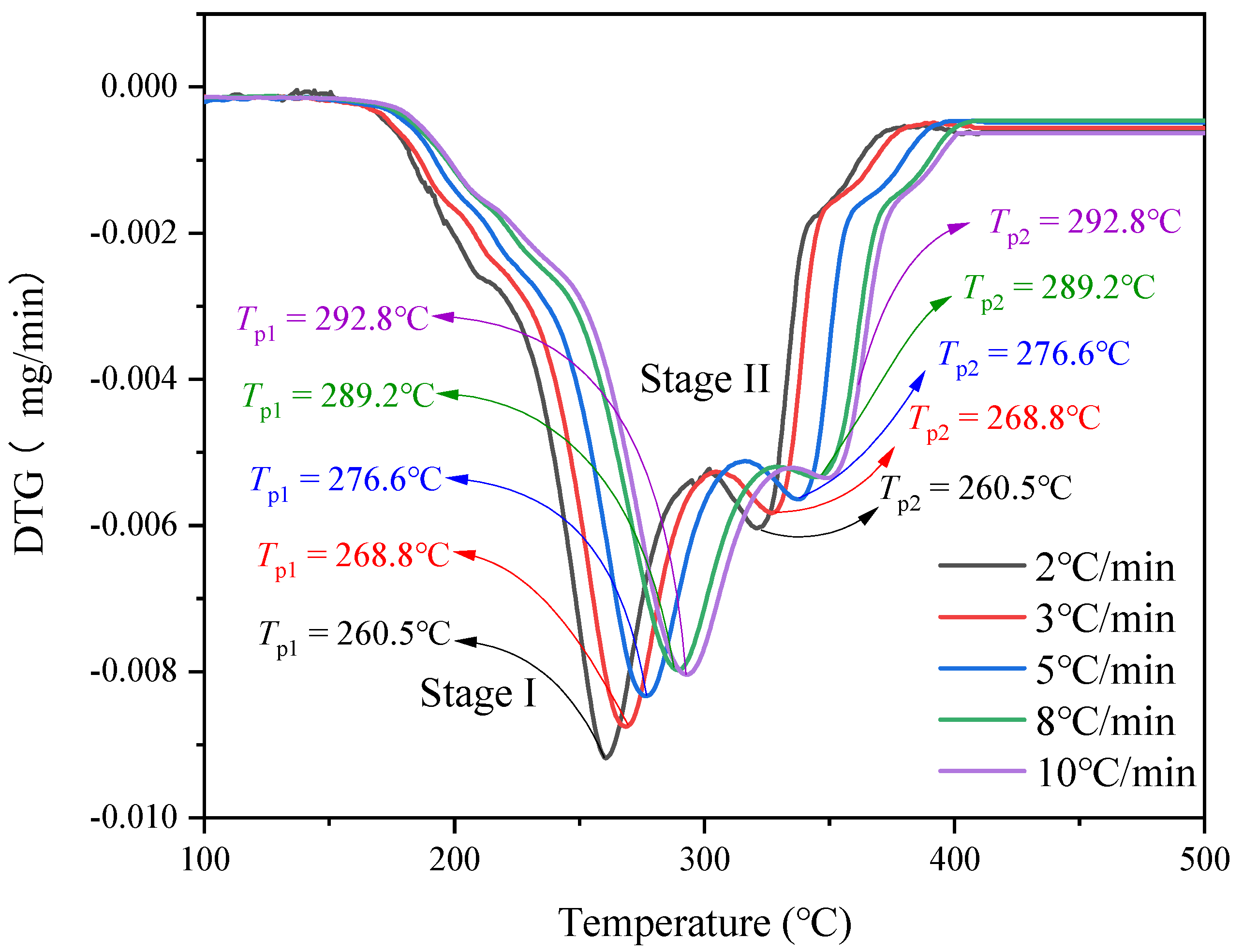
- Thermal decomposition analysis by TG.
- (3)
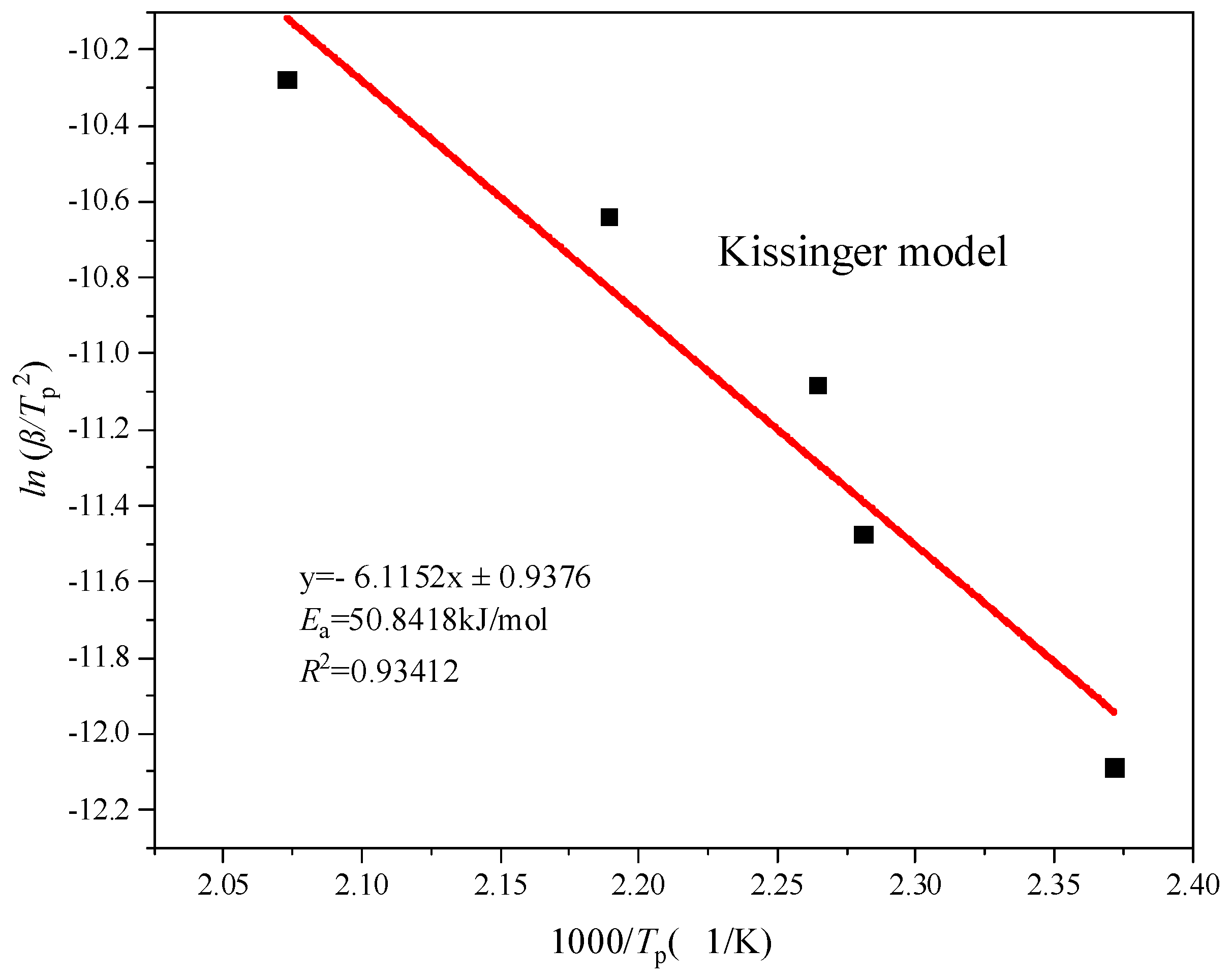
- Analysis of thermokinetic results
3.3. Thermokinetic Parameters Determined by Autocatalytic Model
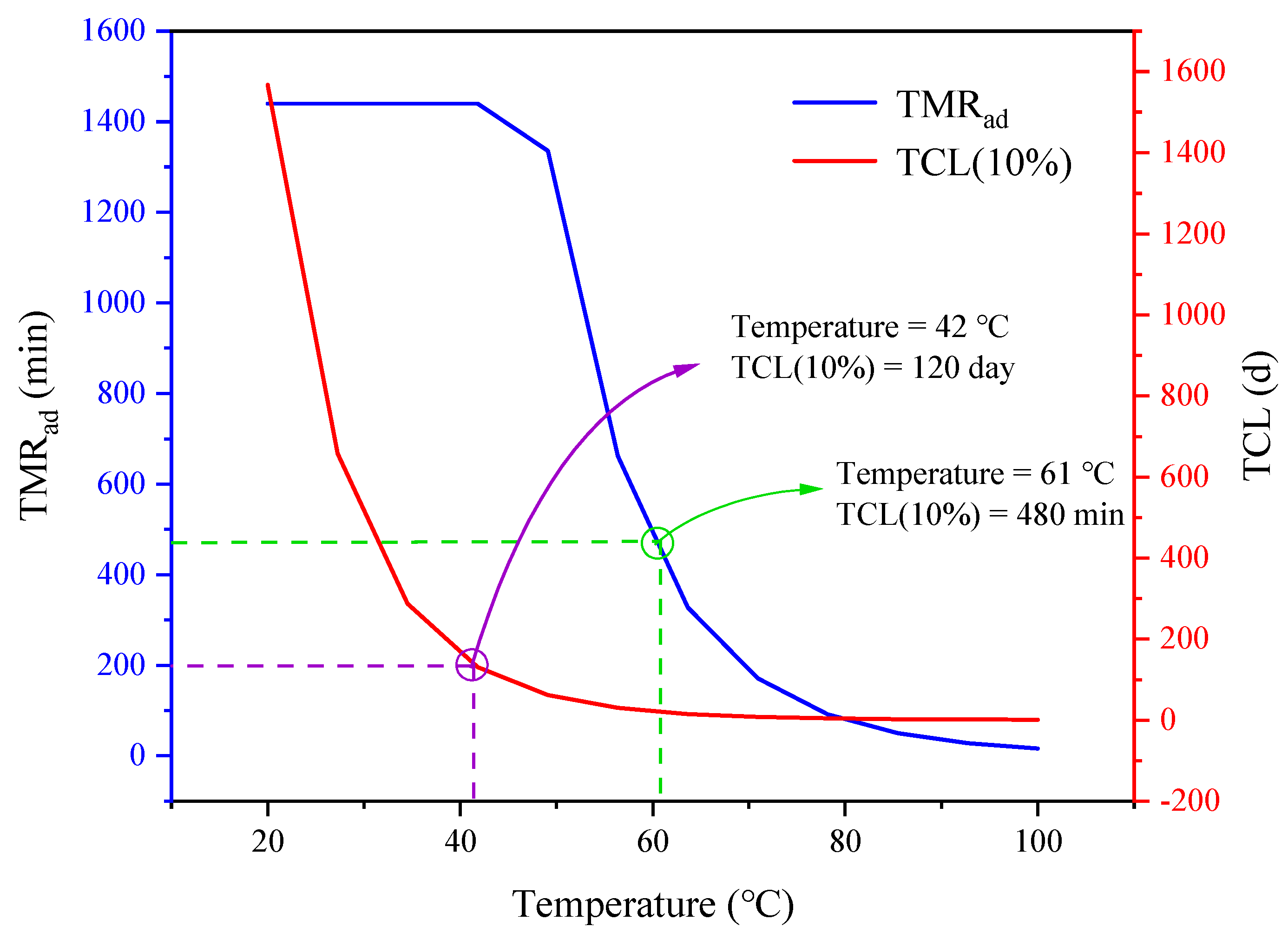
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xue, Y.-T.; Ren, L.; Li, S.; Wang, L.-L.; He, X.-X.; Zhao, X.; Yu, G.-L.; Guan, H.-S.; Li, C.-X. Study on quality control of sulfated polysaccharide drug, propylene glycol alginate sodium sulfate (PSS). Carbohydr. Polym. 2016, 144, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Ren, L.; Sun, Y.; Li, H.-H.; Guan, H.-S.; He, X.-X.; Li, C.-X. Anticoagulant and antithrombotic activities of low-molecular-weight propylene glycol alginate sodium sulfate (PSS). Eur. J. Med. Chem. 2016, 114, 33–40. [Google Scholar] [CrossRef]
- Xue, Y.-T.; Li, S.; Liu, W.-J.; Xin, M.; Li, H.-H.; Yu, G.-L.; Guan, H.-S.; He, X.-X.; Li, C.-X. The mechanisms of sulfated polysaccharide drug of propylene glycol alginate sodium sulfate (PSS) on bleeding side effect. Carbohydr. Polym. 2018, 194, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Uma, K.; Lalithamba, H.S.; Revanasiddappa, B.C. Synthesis and characterization of biologically active N-protected formamides employing Nano TiO2 and their utilization for the synthesis of thioformamides. Chem. Data Collect. 2020, 30, 100591. [Google Scholar] [CrossRef]
- Norval, G.W. Analysis of a blast due to inadvertant mixing of ammonium sulfate and sodium hyplochlorite. Process Saf. Prog. 2016, 35, 92–95. [Google Scholar] [CrossRef]
- Sun, Q.; Jiang, L.; Li, M.; Sun, J. Assessment on thermal hazards of reactive chemicals in industry: State of the Art and perspectives. Prog. Energy Combust. Sci. 2020, 78, 100832. [Google Scholar] [CrossRef]
- Guan, R.; Zou, H.; Lu, D.; Gong, C.; Liu, Y. Polyethersulfone sulfonated by chlorosulfonic acid and its membrane characteristics. Eur. Polym. J. 2005, 41, 1554–1560. [Google Scholar] [CrossRef]
- Huang, A.-C.; Li, Z.-P.; Liu, Y.-C.; Tang, Y.; Huang, C.-F.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C. Essential hazard and process safety assessment of para-toluene sulfonic acid through calorimetry and advanced thermokinetics. J. Loss Prev. Process Ind. 2021, 72, 104558. [Google Scholar] [CrossRef]
- Lin, C.-P.; Tseng, J.-M.; Chang, Y.-M.; Cheng, Y.-C.; Lin, H.-Y.; Chien, C.-Y. Green thermal analysis for predicting thermal hazard of storage and transportation safety for tert-butyl peroxybenzoate. J. Loss Prev. Process Ind. 2012, 25, 1–7. [Google Scholar] [CrossRef]
- Castleman, B.A.; van der Merwe, E.M.; Doucet, F.J. Thermochemical purification of talc with ammonium sulphate as chemical additive. Miner. Eng. 2021, 164, 106815. [Google Scholar] [CrossRef]
- Darwish, E.; Mansouri, M.; Yilmaz, D.; Leion, H. Effect of Mn and Cu Substitution on the SrFeO3 Perovskite for Potential Thermochemical Energy Storage Applications. Processes 2021, 9, 1817. [Google Scholar] [CrossRef]
- Tafu, N.N.; Jideani, V.A. Characterization of Novel Solid Dispersions of Moringa oleifera Leaf Powder Using Thermo-Analytical Techniques. Processes 2021, 9, 2230. [Google Scholar] [CrossRef]
- Mohamed, A.; Hussain, S.; Alamri, M.S.; Ibraheem, M.A.; Qasem, A.A.A.; Ababtain, I.A. Physicochemical Properties of Starch Binary Mixtures with Cordia and Ziziphus Gums. Processes 2022, 10, 180. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Burnham, A.K.; Favergeon, L.; Koga, N.; Moukhina, E.; Pérez-Maqueda, L.A.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochim. Acta 2020, 689, 178597. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Huang, A.-C.; Tang, Y.; Ma, X.-M.; Yang, Y.-P.; Wu, Z.-H.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C. Thermokinetic model establishment and numerical simulation of 2,4,6-trinitrophenol based on eco-friendly synthesis method. J. Energ. Mater. 2021, 1–20. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Huang, A.-C.; Tang, Y.; Huang, C.-F.; Shen, Q.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C. Thermokinetic analysis of the stability of acetic anhydride hydrolysis in isothermal calorimetry techniques. J. Therm. Anal. Calorim. 2021, 1–9. [Google Scholar] [CrossRef]
- Lv, G.; Li, K.; Shi, Y.; Zhang, R.; Tang, H.; Tang, C. Effect of Aminosilane Coupling Agent-Modified Nano-SiO2 Particles on Thermodynamic Properties of Epoxy Resin Composites. Processes 2021, 9, 771. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, L.; Zhu, S.; Zheng, H.; Ma, Y.; Liu, Y.; Jia, C.; Zhou, C.; Bie, L.; Zhang, G. Modes of Occurrence of Chromium and Their Thermal Stability in Low-Rank Coal Pyrolysis. Processes 2022, 10, 15. [Google Scholar] [CrossRef]
- Jia, G. Combustion Characteristics and Kinetic Analysis of Biomass Pellet Fuel Using Thermogravimetric Analysis. Processes 2021, 9, 868. [Google Scholar] [CrossRef]
- Khelkhal, M.A.; Lapuk, S.E.; Buzyurov, A.V.; Krapivnitskaya, T.O.; Peskov, N.Y.; Denisenko, A.N.; Vakhin, A.V. Thermogravimetric Study on Peat Catalytic Pyrolysis for Potential Hydrocarbon Generation. Processes 2022, 10, 974. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, T.; Qiao, X.; Yang, L.; Zhang, J.; Cui, Y.; Zhang, J. Crystal Structure and Thermal Decomposition of 5-Aminotetrazole Trinitrophloroglucinolate. Acta Phys.-Chim. Sin. 2008, 24, 576–580. [Google Scholar] [CrossRef]
- Huang, A.-C.; Huang, C.-F.; Tang, Y.; Xing, Z.-X.; Jiang, J.-C. Evaluation of multiple reactions in dilute benzoyl peroxide concentrations with additives using calorimetric technology. J. Loss Prev. Process Ind. 2021, 69, 104373. [Google Scholar] [CrossRef]
- Huang, A.-C.; Huang, C.-F.; Xing, Z.-X.; Jiang, J.-C.; Shu, C.-M. Thermal hazard assessment of the thermal stability of acne cosmeceutical therapy using advanced calorimetry technology. Process Saf. Environ. Prot. 2019, 131, 197–204. [Google Scholar] [CrossRef]
- JankoviĆ, B. Isothermal thermo-analytical study and decomposition kinetics of non-activated and mechanically activated indium tin oxide (ITO) scrap powders treated by alkaline solution. Trans. Nonferr. Met. Soc. China 2015, 25, 1657–1676. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Yang, Y.-P.; Zhou, H.-L.; Zhai, J.; Xing, Z.-X.; Huang, C.-F.; Shu, C.-M. Hazard assessment of the thermal stability of nitrification by-products by using an advanced kinetic model. Process Saf. Environ. Prot. 2022, 160, 91–101. [Google Scholar] [CrossRef]
- Sestak, J. (Ed.) 14—Explanation of crystallisation kinetics both during glass heating and melt cooling, while the incorrectness of the Kissinger method is indicated. In Thermal Analysis and Thermodynamic Properties of Solids, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 365–383. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Liu, Z.; Liang, F.; Shen, B. Thermal kinetics analysis of polymerization reaction of styrene-ethylbenzene system. J. Loss Prev. Process Ind. 2021, 73, 104611. [Google Scholar] [CrossRef]
- Fatmi, M.; Ghebouli, B.; Ghebouli, M.A.; Chihi, T.; Abdul Hafiz, M. The kinetics of precipitation in Al-2.4wt% Cu alloy by Kissinger, Ozawa, Bosswel and Matusita methods. Phys. B Condens. Matter 2011, 406, 2277–2280. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, C.; Dong, Z. Comparison of the Ozawa and modified Avrami models of polymer crystallization under nonisothermal conditions using a computer simulation method. Thermochim. Acta 2007, 466, 22–28. [Google Scholar] [CrossRef]
- Lu, Z.; Yang, L.; Guo, Y. Thermal behavior and decomposition kinetics of six electrolyte salts by thermal analysis. J. Power Sources 2006, 156, 555–559. [Google Scholar] [CrossRef]
- Stephy, A.; Antony, A.M.; Francis, T. Thermal decomposition kinetics of melt-mixed ethylene-co-vinyl acetate—Based bio-composites. Mater. Today Chem. 2021, 21, 100544. [Google Scholar] [CrossRef]
- Costamagna, P.; Giordano, A.; Lazzarini, Y.; Delucchi, M.; Busca, G. Process of ammonia removal from anaerobic digestion and associated ammonium sulphate production: Pilot plant demonstration. J. Environ. Manag. 2020, 259, 109841. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Pan, Y.; Ran, Z.; Li, Y.; Jiang, J.; Wang, Q.; Jiang, J. Thermal hazard and decomposition kinetics of 1-butyl-2,3-dimethylimidazolium nitrate via TGA/DSC and FTIR. J. Loss Prev. Process Ind. 2021, 72, 104562. [Google Scholar] [CrossRef]
- Ben Talouba, I.; Balland, L.; Mouhab, N.; Abdelghani-Idrissi, M.A. Kinetic parameter estimation for decomposition of organic peroxides by means of DSC measurements. J. Loss Prev. Process Ind. 2011, 24, 391–396. [Google Scholar] [CrossRef]
- Yao, B.; Zhao, D.; Zhang, Z.; Huang, C. Safety Study on Wax Deposition in Crude Oil Pipeline. Processes 2021, 9, 1572. [Google Scholar] [CrossRef]
- Ryou, M.-H.; Lee, J.-N.; Lee, D.J.; Kim, W.-K.; Jeong, Y.K.; Choi, J.W.; Park, J.-K.; Lee, Y.M. Effects of lithium salts on thermal stabilities of lithium alkyl carbonates in SEI layer. Electrochim. Acta 2012, 83, 259–263. [Google Scholar] [CrossRef]
- Zhou, H.-L.; Jiang, J.-C.; Huang, A.-C.; Tang, Y.; Zhang, Y.; Huang, C.-F.; Liu, S.-H.; Shu, C.-M. Calorimetric evaluation of thermal stability and runaway hazard based on thermokinetic parameters of O,O–dimethyl phosphoramidothioate. J. Loss Prev. Process Ind. 2022, 75, 104697. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Chen, X.; Song, X.; Zhang, Y.; Tang, K. Thermal decomposition mechanism of 65% lysine sulfate powder and its thermal stability based on thermal analysis. J. Loss Prev. Process Ind. 2020, 64, 104089. [Google Scholar] [CrossRef]
- Tankov, I.; Yankova, R.; Mitkova, M.; Stratiev, D. Non-isothermal decomposition kinetics of pyridinium nitrate under nitrogen atmosphere. Thermochim. Acta 2018, 665, 85–91. [Google Scholar] [CrossRef]
- Tiwari, S.; Gehlot, C.L.; Srivastava, K.; Srivastava, D. Simulation of the thermal degradation and curing kinetics of fly ash reinforced diglycidyl ether bisphenol A composite. J. Indian Chem. Soc. 2021, 98, 100077. [Google Scholar] [CrossRef]
- Huang, A.-C.; Liao, F.-C.; Huang, C.-F.; Tang, Y.; Zhang, Y.; Shu, C.-M.; Xing, Z.-X.; Jiang, J.-C.; Hsieh, W.-Y. Calorimetric approach to establishing thermokinetics for cosmeceutical benzoyl peroxides containing metal ions. J. Therm. Anal. Calorim. 2021, 144, 373–382. [Google Scholar] [CrossRef]
- Cao, C.-R.; Liu, S.-H.; Das, M.; Shu, C.-M. Evaluation for the thermokinetics of the autocatalytic reaction of cumene hydroperoxide mixed with phenol through isothermal approaches and simulations. Process Saf. Environ. Prot. 2018, 117, 426–438. [Google Scholar] [CrossRef]
- Wu, S.-H.; Chi, J.-H.; Wu, Y.-T.; Huang, Y.-H.; Chu, F.-J.; Horng, J.-J.; Shu, C.-M.; Charpentier, J.-C. Thermal hazard analysis of triacetone triperoxide (TATP) by DSC and GC/MS. J. Loss Prev. Process Ind. 2012, 25, 1069–1074. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Kossoy, A.A.; Yao, Y.-D.; Chen, L.-P.; Chen, W.-H. Kinetics-based simulation approach to evaluate thermal hazards of benzaldehyde oxime by DSC tests. Thermochim. Acta 2017, 655, 319–325. [Google Scholar] [CrossRef]




| Reagent | CAS | Reagent Purity | Usage | Manufacturers |
|---|---|---|---|---|
| Propylene glycol alginate(PGA) | 9005-37-2 | CP | 10 g | Zhejiang Yinuo Biotechnology Co., Ltd. (Hangzhou, China) |
| Formamide | 75-12-7 | AR | 100 mL | Yatai Chemical Co., Ltd. (Wuxi, China) |
| Chlorosulfonic acid | 7790-94-5 | AR | 30 mL | Qianyan Chemical Technology Co., Ltd. (Wuhan, China) |
| β (°C /min) | T0 (°C) | Tp (°C) | Te (°C) |
|---|---|---|---|
| 1 | 132.7 | 148.5 | 161.7 |
| 2 | 146.9 | 165.3 | 182.9 |
| 3 | 120.5 | 168.5 | 190.7 |
| 5 | 174.4 | 183.7 | 213.7 |
| 8 | 189.5 | 209.3 | 288.7 |
| Stage I | Stage II | |||
|---|---|---|---|---|
| β (°C/min) | T1 (°C) | DTG (mg/s) | T2 (°C) | DTG (mg/s) |
| 2 | 260.5 | −0.5511 | 323.9 | −0.3587 |
| 3 | 268.8 | −0.5252 | 326.9 | −0.3499 |
| 5 | 276.6 | −0.4999 | 337.4 | −0.3385 |
| 8 | 289.2 | −0.4787 | 345.2 | −0.3203 |
| 10 | 292.8 | −0.4825 | 349.3 | −0.4821 |
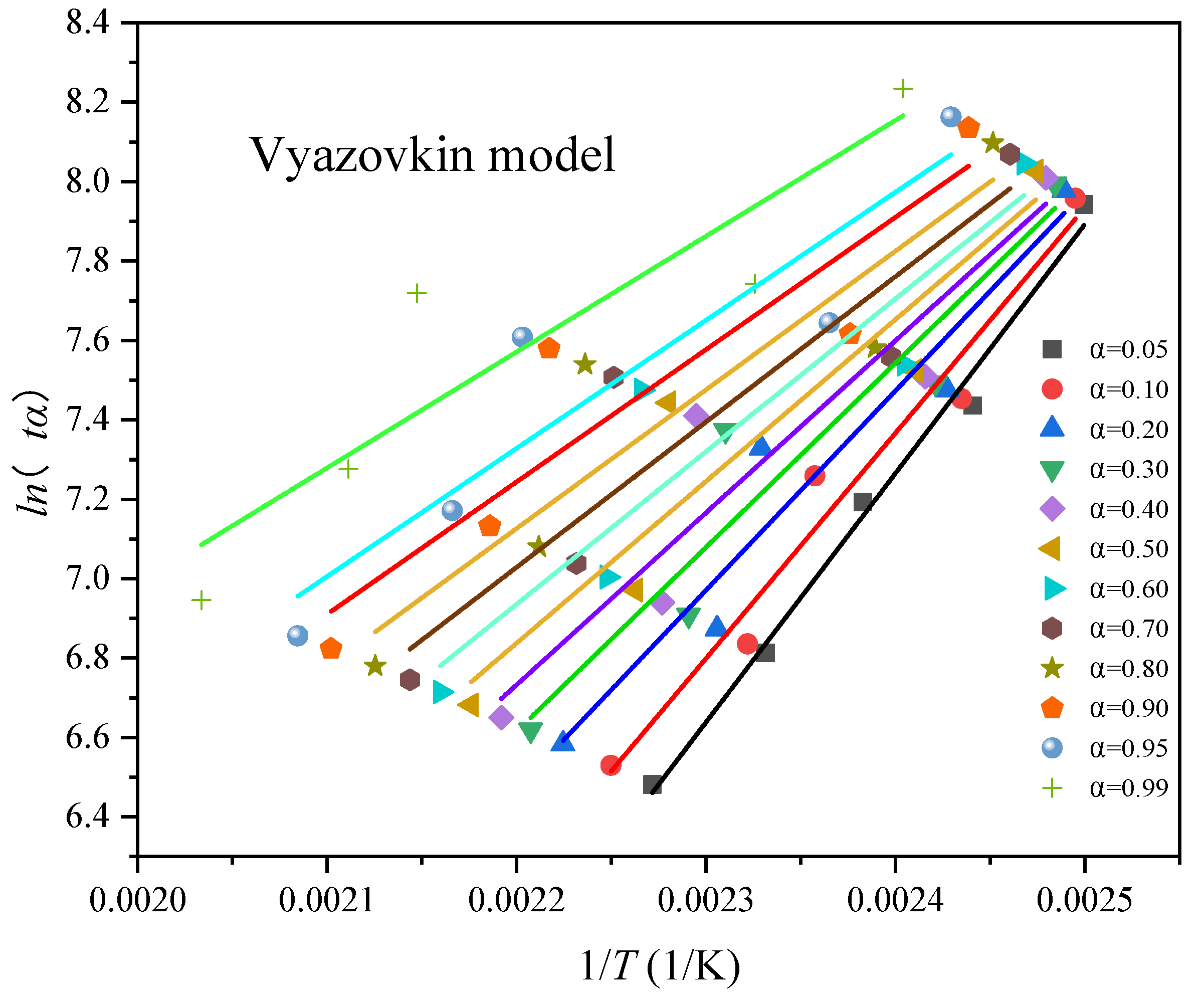
| α | Ea (kJ/mol) | R2 |
|---|---|---|
| 0.05 | 66.2145 | 0.9920 |
| 0.10 | 60.9222 | 0.9789 |
| 0.20 | 55.0361 | 0.9591 |
| 0.30 | 51.5291 | 0.9485 |
| 0.40 | 48.7408 | 0.9407 |
| 0.50 | 46.2739 | 0.9348 |
| 0.60 | 44.0605 | 0.9294 |
| 0.70 | 42.1805 | 0.9247 |
| 0.80 | 40.3987 | 0.9235 |
| 0.90 | 38.5649 | 0.9242 |
| 0.95 | 37.3125 | 0.9261 |
| 0.99 | 33.8099 | 0.9176 |
| Ea (kJ/mol) | R2 | |
|---|---|---|
| Kissinger | 50.8418 | 0.9341 |
| FWO | 51.4959 | 0.9562 |
| Vyazovkin | 46.9087 | 0.9006 |
| KAS | 47.0870 | 0.9417 |
| average value | 49.0834 | 0.9332 |
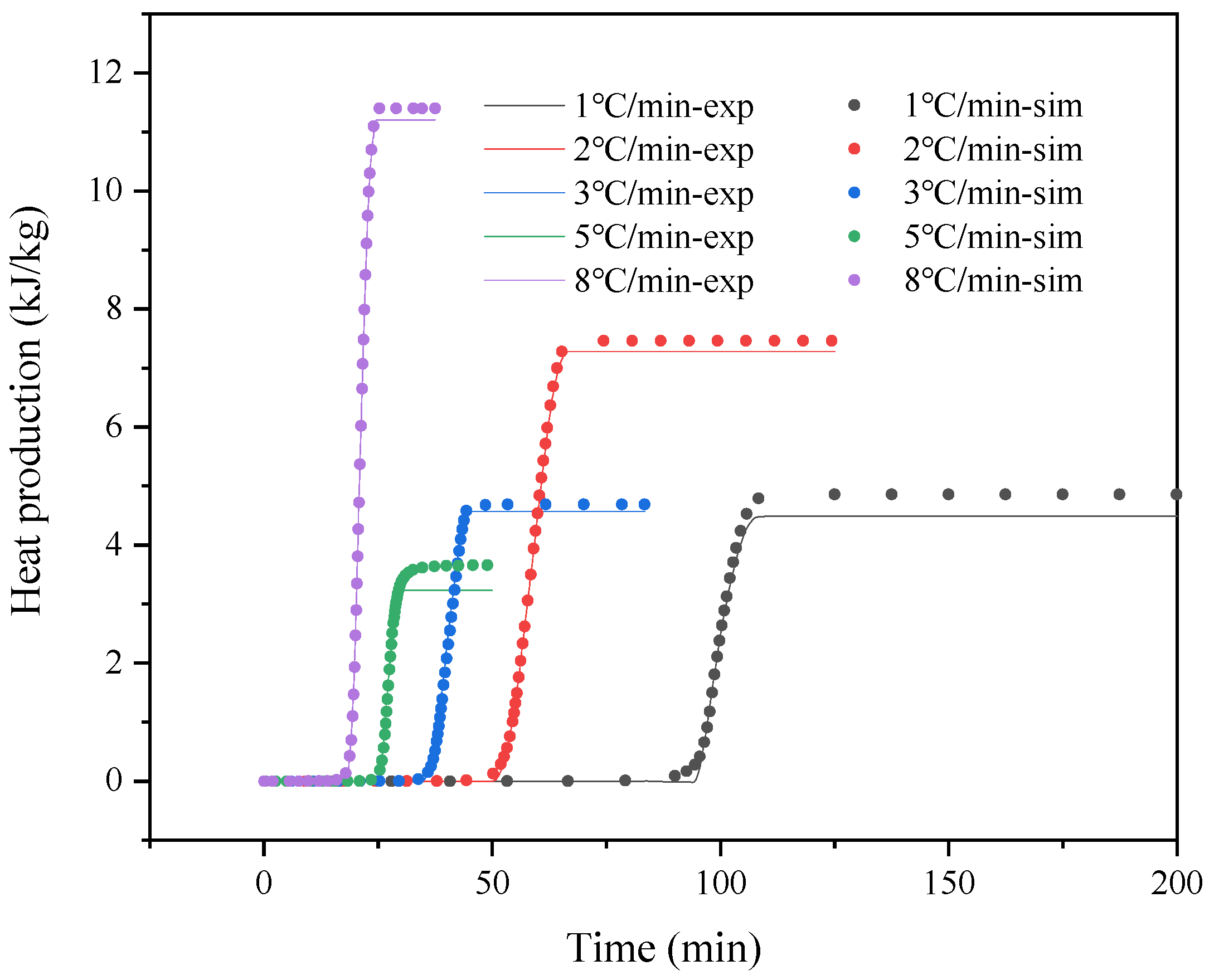
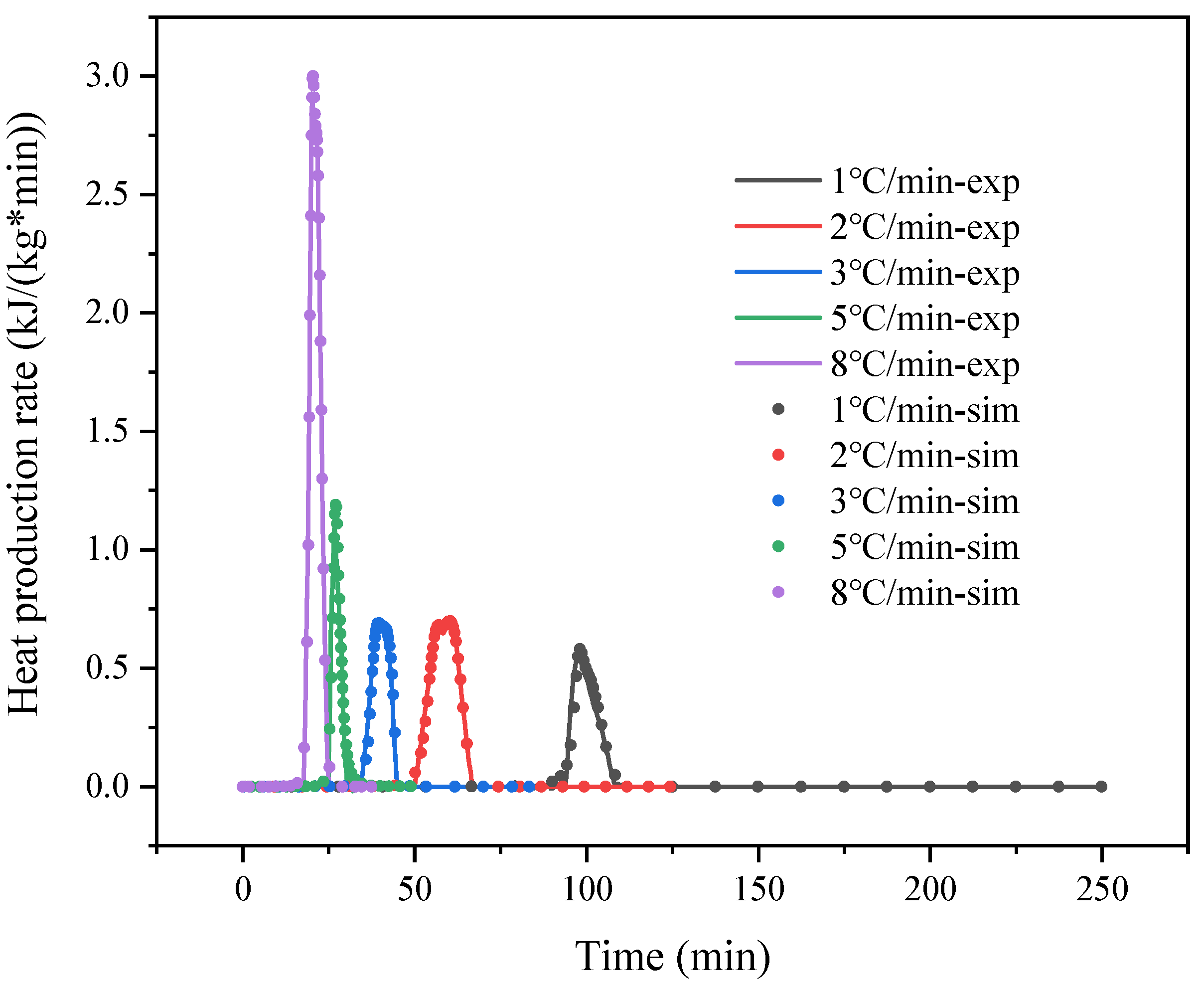
| PSS + (NH4)2SO4 5 °C/min | PSS + (NH4)2SO4 8 °C/min | |||
|---|---|---|---|---|
| Autocatalysis A to B1 | Autocatalysis B1 to B2 | Autocatalysis A to B1 | Autocatalysis B1 to B2 | |
| ln A (ln 1/s) | 15.2094 | 30.9340 | 18.3841 | 20.1172 |
| Ea (kJ/mol) | 70.9749 | 132.3971 | 87.4477 | 100.1652 |
| Reaction order n1 | 1.3955 | 2.4947 | 1.2565 | 1.1530 |
| Reaction order n2 | 0.9987 | 0.5087 | 0.9257 | 0.3684 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, C.; Liu, Y.-C.; Wu, J.; Tang, Y.; Zhai, J.; Shu, C.-M.; Jiang, J.-C.; Xing, Z.-X.; Huang, C.-F.; Huang, A.-C. Thermal Stability Determination of Propylene Glycol Sodium Alginate and Ammonium Sulfate with Calorimetry Technology. Processes 2022, 10, 1177. https://doi.org/10.3390/pr10061177
Yao C, Liu Y-C, Wu J, Tang Y, Zhai J, Shu C-M, Jiang J-C, Xing Z-X, Huang C-F, Huang A-C. Thermal Stability Determination of Propylene Glycol Sodium Alginate and Ammonium Sulfate with Calorimetry Technology. Processes. 2022; 10(6):1177. https://doi.org/10.3390/pr10061177
Chicago/Turabian StyleYao, Chen, Ye-Cheng Liu, Jie Wu, Yan Tang, Juan Zhai, Chi-Min Shu, Jun-Cheng Jiang, Zhi-Xiang Xing, Chung-Fu Huang, and An-Chi Huang. 2022. "Thermal Stability Determination of Propylene Glycol Sodium Alginate and Ammonium Sulfate with Calorimetry Technology" Processes 10, no. 6: 1177. https://doi.org/10.3390/pr10061177







