Modification of the Microstructure and Transport Properties of La2CuO4−δ Electrodes via Halogenation Routes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Materials
2.2. Structural, Morphological and Electrochemical Characterization
3. Results
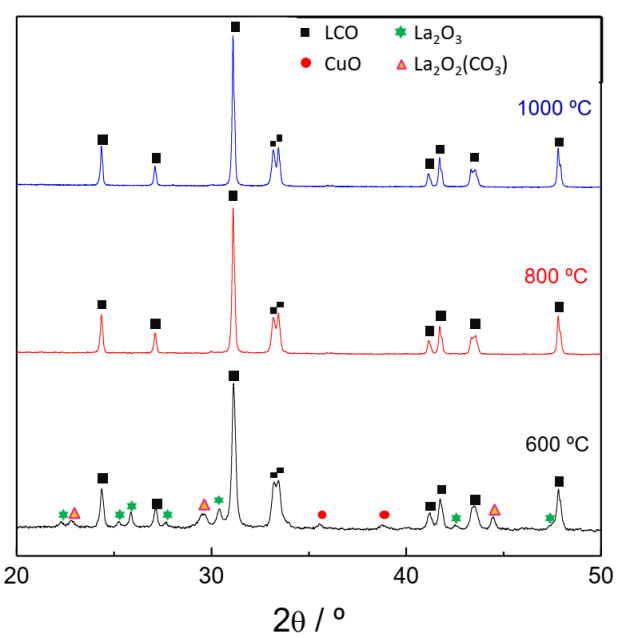
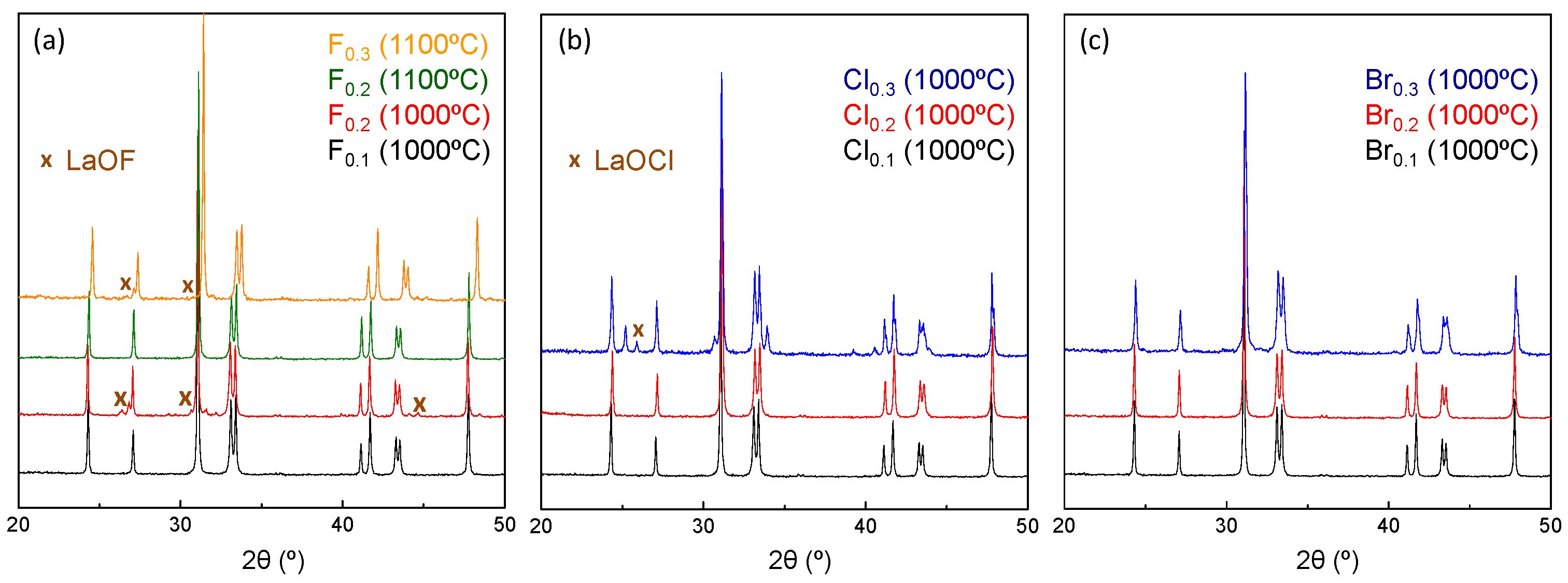
3.1. Structural Properties of La2CuO4−0.5xAx (A = F, Cl, Br; x = 0–0.3) Series
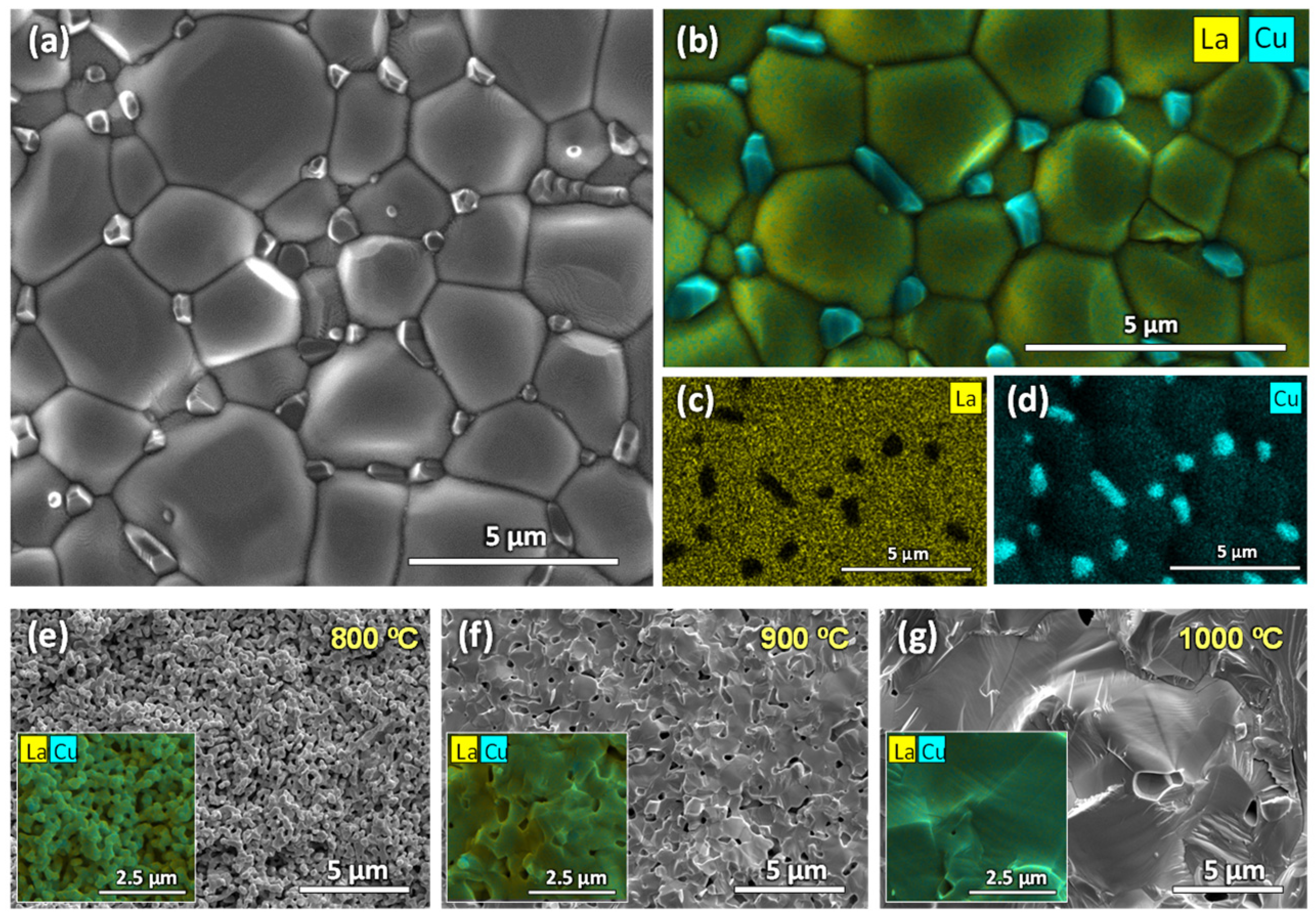
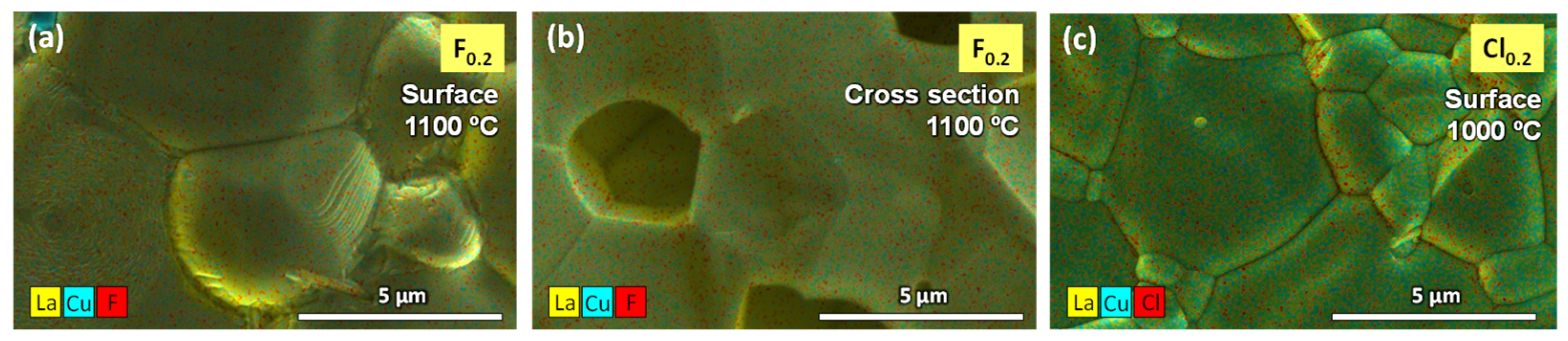
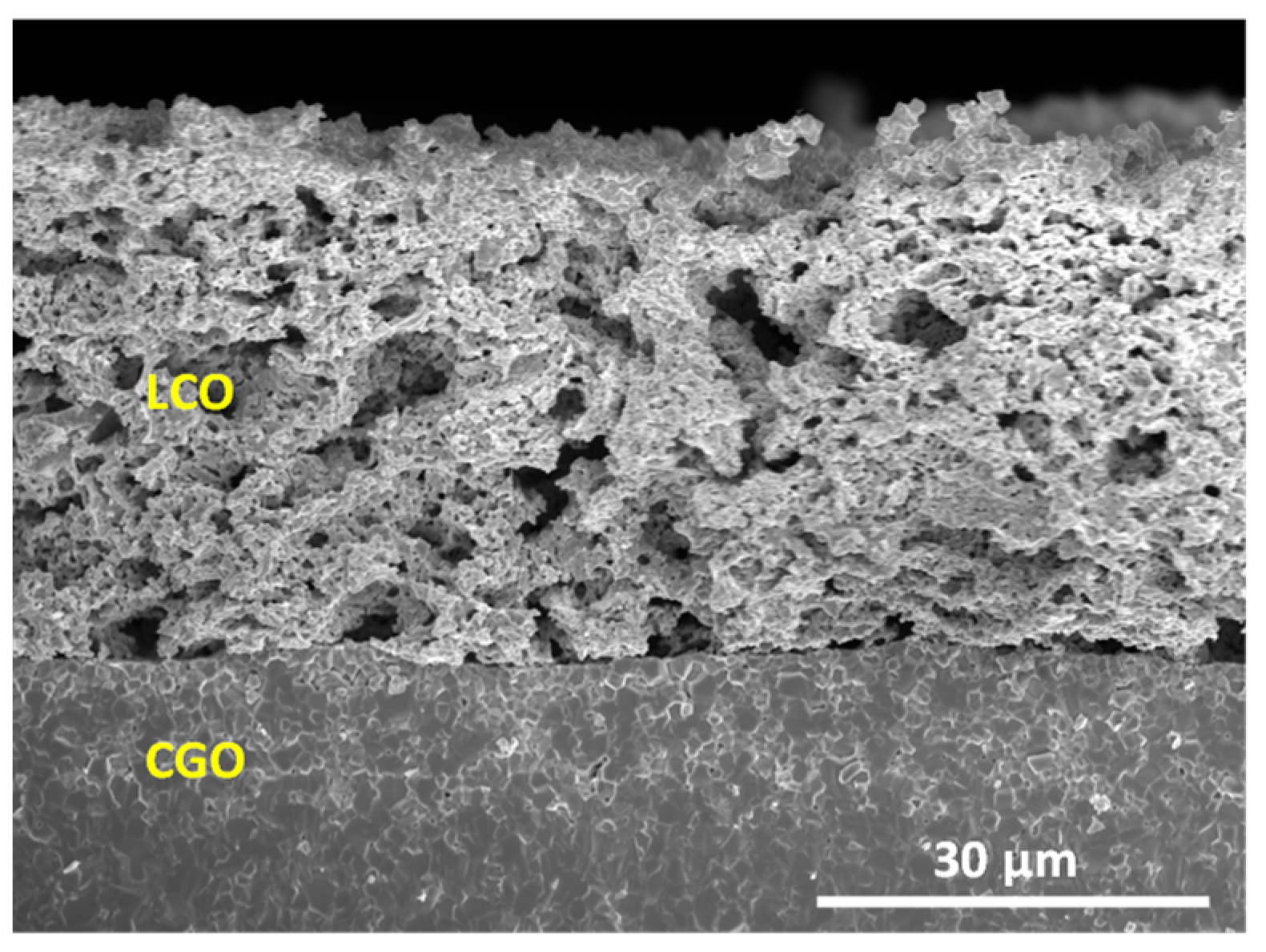
3.2. Microstructural Analysis
3.3. Electrochemical Properties
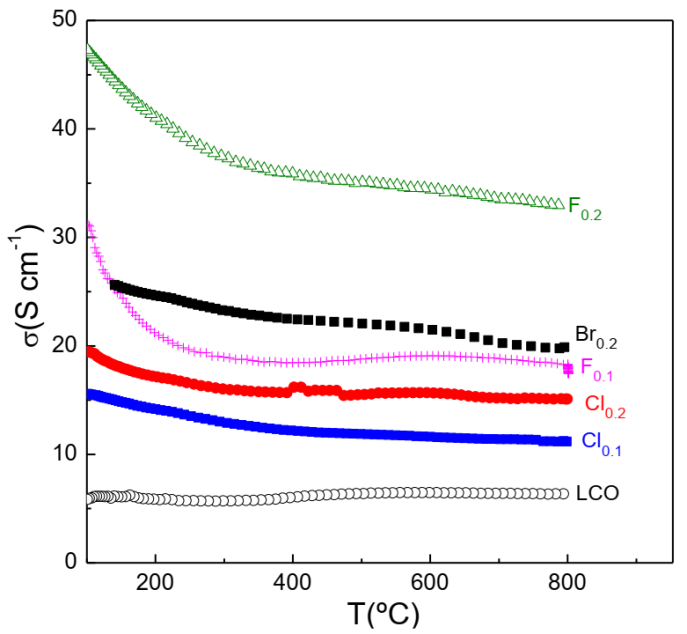
3.3.1. Conductivity
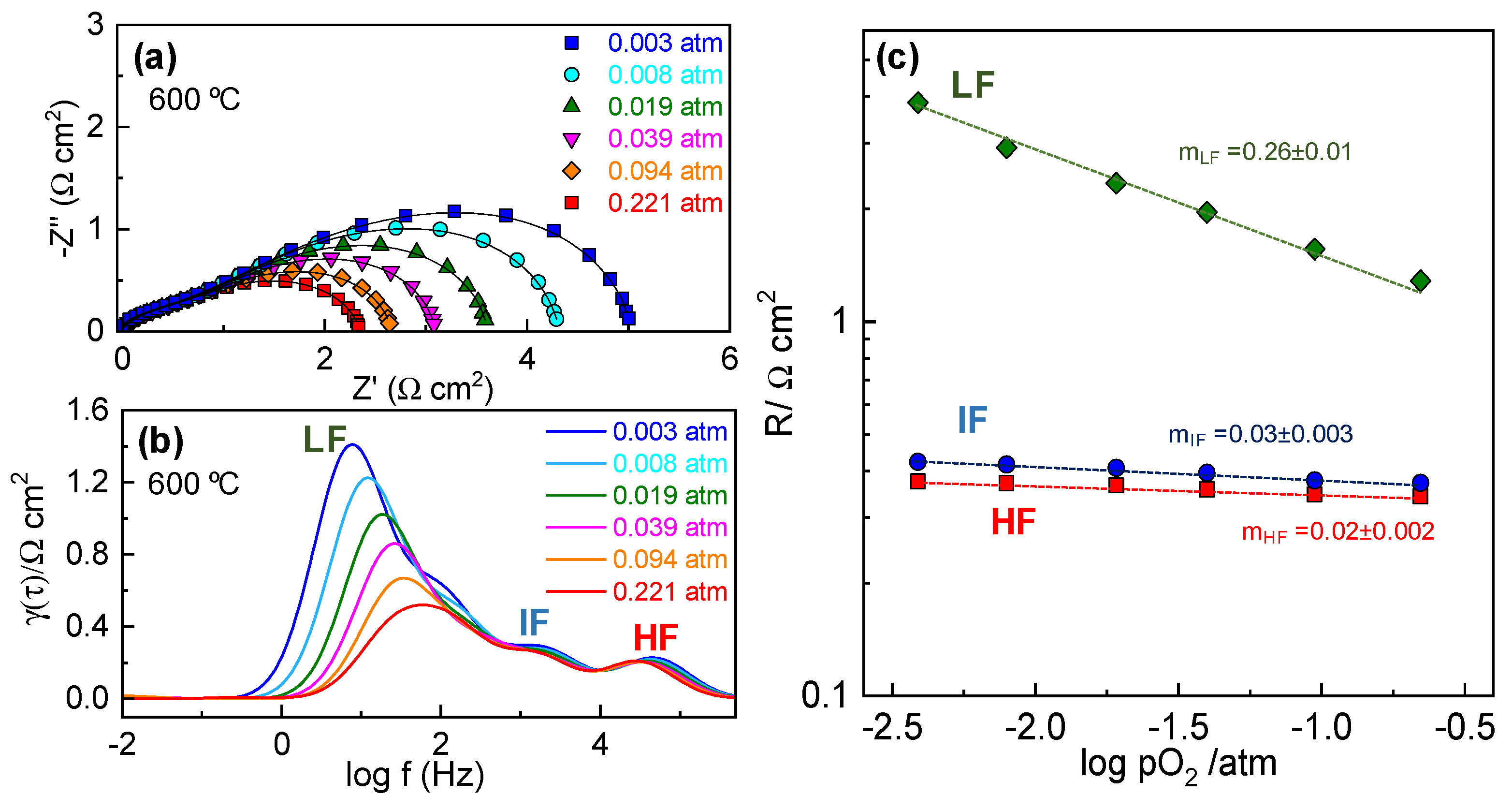
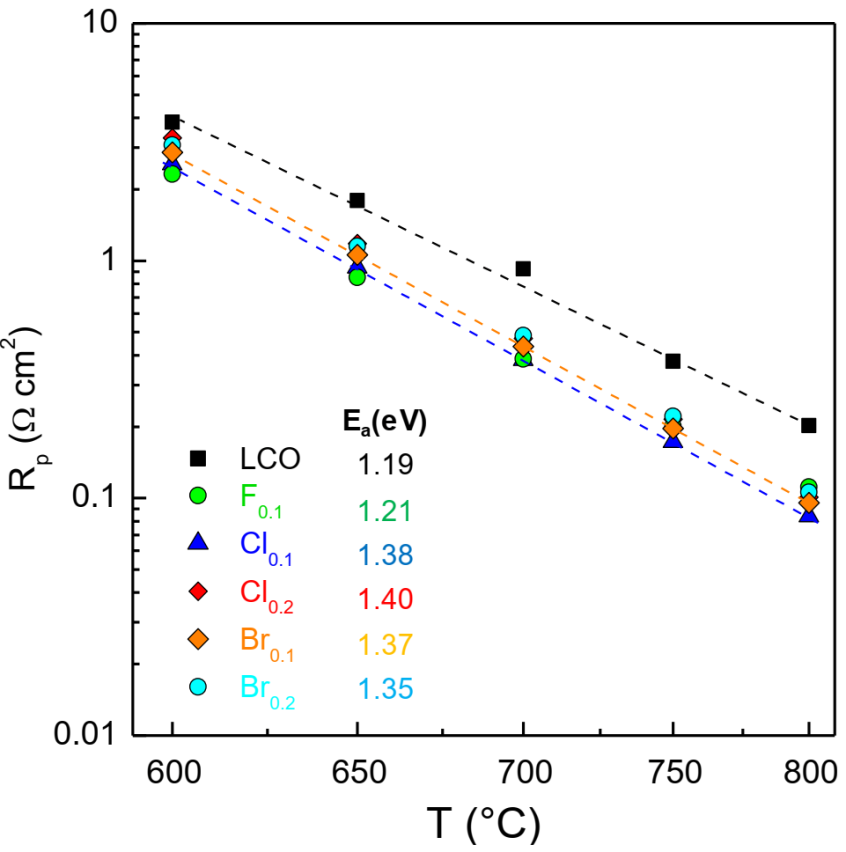
3.3.2. Electrode Polarization Resistance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minh, N.Q. Ceramic Fuel Cells. J. Am. Ceram. Soc. 1993, 76, 563–588. [Google Scholar] [CrossRef]
- Tahir, N.N.M.; Baharuddin, N.A.; Samat, A.A.; Osman, N.; Somalu, M.R. A review on cathode materials for conventional and proton-conducting solid oxide fuel cells. J. Alloys Compd. 2022, 894, 162458. [Google Scholar] [CrossRef]
- Sabri, N.S.M.; Izman, S.; Kurniawan, D. Perovskite materials for intermediate temperature solid oxide fuel cells cathodes: A review. AIP Conf. Proc. 2020, 2262, 030013. [Google Scholar]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.G.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Xu, Q.; Ni, M. Scientometric review of advancements in the development of high-performance cathode for low and intermediate temperature solid oxide fuel cells: Three decades in retrospect. Int. J. Hydrog. Energy 2021, 46, 26518–26536. [Google Scholar] [CrossRef]
- Ahmad, M.Z.; Ahmad, H.S.; Chen, R.S.; Ismail, A.F.; Hazan, R.; Baharuddin, N.A. Review on recent advancement in cathode material for lower and intermediate temperature solid oxide fuel cells application. Int. J. Hydrog. Energy 2022, 47, 1103–1120. [Google Scholar] [CrossRef]
- Paydar, S.; Shariat, M.H.; Javadpour, S. Investigation on electrical conductivity of LSM/YSZ8, LSM/Ce0.84Y0.16O0.96 and LSM/Ce0.42Zr0.42Y0.16O0.96 composite cathodes of SOFCs. Int. J. Hydrog. Energy 2016, 41, 23145–23155. [Google Scholar] [CrossRef]
- Chandran, P.R.; Arjunan, T. A review of materials used for solid oxide fuel cell. Int. J. ChemTech Res. 2015, 7, 488–497. [Google Scholar]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A review of the chemical compatibility between oxide electrodes and electrolytes in solid oxide fuel cells. J. Power Sources 2021, 492, 229630. [Google Scholar] [CrossRef]
- Kumar, R.V.; Khandale, A.P. A review on recent progress and selection of cobalt-based cathode materials for low temperature-solid oxide fuel cells. Renew. Sustain. Energy Rev. 2022, 156, 111985. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Zheng, Y.; Chen, J.; Yu, B.; Chen, Y.; Liu, M. Controlling cation segregation in perovskite-based electrodes for high electro-catalytic activity and durability. Chem. Soc. Rev. 2017, 46, 6345–6378. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.S.; de Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrog. Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef] [Green Version]
- Majewski, A.J.; Khodimchuk, A.; Zakharov, D.; Porotnikova, N.; Ananyev, M.; Johnson, I.D.; Darr, J.A.; Slater, P.R.; Steinberger-Wilckens, R. Oxygen surface exchange properties and electrochemical activity of lanthanum nickelates. J. Solid State Chem. 2022, 312, 123228. [Google Scholar] [CrossRef]
- Banner, J.; Akter, A.; Wang, R.; Pietras, J.; Sulekar, S.; Marina, O.A.; Gopalan, S. Rare earth Nickelate electrodes containing heavily doped ceria for reversible solid oxide fuel cells. J. Power Sources 2021, 507, 230248. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen diffusion and surface exchange in La2−xSrxNiO4+δ. Solid State Ion. 2000, 135, 709–712. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Muchtar, A.; Somalu, M.R. Short review on cobalt-free cathodes for solid oxide fuel cells. Int. J. Hydrog. Energy 2017, 42, 9149–9155. [Google Scholar] [CrossRef]
- Ding, P.; Li, W.; Zhao, H.; Wu, C.; Zhao, L.; Dong, B.; Wang, S. Review on Ruddlesden–Popper perovskites as cathode for solid oxide fuel cells. J. Phys. Mater. 2021, 4, 022002. [Google Scholar] [CrossRef]
- Benamira, M.; Ringuedé, A.; Cassir, M.; Horwat, D.; Lenormand, P.; Ansart, F.; Bassat, J.M.; Viricelle, J.P. Enhancing oxygen reduction reaction of YSZ/La2NiO4+δ using an ultrathin La2NiO4+δ interfacial layer. J. Alloys Compd. 2018, 746, 413–420. [Google Scholar] [CrossRef] [Green Version]
- Tarutin, A.P.; Lyagaeva, J.G.; Medvedev, D.A.; Bi, L.; Yaremchenko, A.A. Recent advances in layered Ln2NiO4+δ nickelates: Fundamentals and prospects of their applications in protonic ceramic fuel and electrolysis cells. J. Mater. Chem. A 2021, 9, 154–195. [Google Scholar] [CrossRef]
- Zinkevich, M.; Aldinger, F. Thermodynamic analysis of the ternary La–Ni–O system. J. Alloys Compd. 2004, 375, 147–161. [Google Scholar] [CrossRef]
- Singh, K.; Ganguly, P.; Rao, C. Structural transitions in (La, Ln)2CuO4 and La2(Cu,Ni)O4 systems. Mater. Res. Bull. 1982, 17, 493–500. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Huo, L.; Sun, L.; Cheng, X.; Grenier, J.-C. Electrode properties of Sr doped La2CuO4 as new cathode material for intermediate-temperature SOFCs. Electrochem. Commun. 2007, 9, 1508–1512. [Google Scholar] [CrossRef]
- Shijie, Z.; Na, L.; Liping, S.; Qiang, L.; Lihua, H.; Hui, Z. A novel high-entropy cathode with the A2BO4-type structure for solid oxide fuel cells. J. Alloys Compd. 2022, 895, 162548. [Google Scholar] [CrossRef]
- Bo, L.; Na, L.; Liping, S.; Qiang, L.; Lihua, H.; Hui, Z. Rare-earth elements doped Nd2CuO4 as Cu-based cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2021, 870, 159397. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, Z.; Niemczyk, A.; Li, K.; Zhao, H.; Świerczek, K. A-site nonstoichiometry and B-site doping with selected M3+ cations in La2−xCu1−y−zNiyMzO4−δ layered oxides. Solid State Ion. 2018, 317, 26–31. [Google Scholar] [CrossRef]
- Goodenough, J.; Manthiram, A. Crystal chemistry and superconductivity in the copper oxides. In Chemistry of High Temperature Superconductors; World Scientific: Singapore, 1991; pp. 1–56. [Google Scholar]
- Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Hurtado, J.; Losilla, E.R.; Marrero-López, D. Stability and electrochemical performance of nanostructured La2CuO4+δ cathodes. J. Alloys Compd. 2019, 788, 565–572. [Google Scholar] [CrossRef]
- Huang, X.; Shin, T.H.; Zhou, J.; Irvine, J.T.S. Hierarchically nanoporous La1.7Ca0.3CuO4−δ and La1.7Ca0.3NixCu1−xO4−δ (0.25 ≤ x ≤ 0.75) as potential cathode materials for IT-SOFCs. J. Mater. Chem. A 2015, 3, 13468–13475. [Google Scholar] [CrossRef] [Green Version]
- Kanai, H.; Mizusaki, J.; Tagawa, H.; Hoshiyama, S.; Hirano, K.; Fujita, K.; Tezuka, M.; Hashimoto, T. Defect chemistry of La2− xSrxCuO4−δ: Oxygen nonstoichiometry and thermodynamic stability. J. Solid State Chem. 1997, 131, 150–159. [Google Scholar] [CrossRef]
- Mazo, G.; Savvin, S. The molecular dynamics study of oxygen mobility in La2−xSrxCuO4−δ. Solid State Ion. 2004, 175, 371–374. [Google Scholar] [CrossRef]
- Zhang, L.; Sun, W.; Xu, C.; Ren, R.; Yang, X.; Qiao, J.; Wang, Z.; Sun, K. Attenuating a metal–oxygen bond of a double perovskite oxide via anion doping to enhance its catalytic activity for the oxygen reduction reaction. J. Mater. Chem. A 2020, 8, 14091–14098. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Zhong, Y.; Zhou, W.; Shao, Z. Anion Doping: A New Strategy for Developing High-Performance Perovskite-Type Cathode Materials of Solid Oxide Fuel Cells. Adv. Energy Mater. 2017, 7, 1700242. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Z.; Gu, Y.; Chen, H.; Zheng, Y.; Ge, L. Effect of Cl doping on the electrochemical performance of Sr2Fe1.5Mo0.5O6−δ cathode material for solid oxide fuel cells. Ceram. Int. 2020, 46, 22787–22796. [Google Scholar] [CrossRef]
- Hou, N.; Gan, J.; Yan, Q.; Zhao, Y.; Li, Y. Improved electrochemical oxidation kinetics of La0.5Ba0.5FeO3−δ anode for solid oxide fuel cells with fluorine doping. J. Power Sources 2022, 521, 230932. [Google Scholar]
- Guan, R.; Wang, Z.; Xu, H.; Hao, X.; Yang, L.; Liu, J.; Yu, S.; He, T. Manipulating the Activity and Thermal Compatibility of NdBaCoFeO5+δ Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells via Fluorine Doping. ACS Appl. Energy Mater. 2022, 5, 481–491. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Zhang, D.; Zeng, Q.; Jiang, Y.; Lin, B. Highly promoted performance of triple-conducting cathode for YSZ-based SOFC via fluorine anion doping. Ceram. Int. 2020, 46, 23964–23971. [Google Scholar] [CrossRef]
- Li, G.; Gou, Y.; Ren, R.; Xu, C.; Qiao, J.; Sun, W.; Wang, Z.; Sun, K. Fluorinated Pr2NiO4+δ as high-performance air electrode for tubular reversible protonic ceramic cells. J. Power Sources 2021, 508, 230343. [Google Scholar] [CrossRef]
- Adachi, S.; Wu, X.J.; Tamura, T.; Tatsuki, T.; Tokiwa-Yamamoto, A.; Tanabe, K. Synthesis and superconducting properties of fluorinated La2CuO4 using NH4F. Phys. C Supercond. 1997, 291, 59–66. [Google Scholar] [CrossRef]
- Tressaud, A.; Robin, C.; Chevalier, B.; Lozano, L.; Etourneau, J. Superconductivity in chlorine-treated La2CuO4. Phys. C Supercond. 1991, 177, 330–336. [Google Scholar] [CrossRef]
- Mi, S.; Liu, Z.; Luo, C.; Cai, L.; Zhang, Z.; Li, L. A review on preparing new energy ultrafine powder materials by freeze-drying. Dry. Technol. 2020, 38, 1544–1564. [Google Scholar] [CrossRef]
- Zakaria, Z.; Awang Mat, Z.; Hassan, S.H.A.; Kar, Y.B. A review of solid oxide fuel cell component fabrication methods toward lowering temperature. Int. J. Energy Res. 2020, 44, 594–611. [Google Scholar] [CrossRef]
- Zamudio-García, J.; Porras-Vázquez, J.M.; dos Santos-Gómez, L.; Losilla, E.R.; Marrero-López, D. Effect of Zn addition on the structure and electrochemical properties of co-doped BaCe0.6Zr0.2Ln0.2O3−δ (Ln = Y, Gd, Yb) proton conductors. Ceram. Int. 2018, 44, 14113–14121. [Google Scholar] [CrossRef]
- Amelo, B. Pert HighScore Plus, Version 3.0 e.; Malvern Panalytical: Almelo, The Netherlands, 2012. [Google Scholar]
- Larson, A.; Von Dreele, R. Los Alamos National Laboratory Report No. LA-UR-86-748. 1994. Available online: https://11bm.xray.aps.anl.gov/documents/GSASManual.pdf (accessed on 12 May 2022).
- Zhang, Y.; Shen, L.; Wang, Y.; Du, Z.; Zhang, B.; Ciucci, F.; Zhao, H. Enhanced oxygen reduction kinetics of IT-SOFC cathode with PrBaCo2O5+δ/Gd0.1Ce1.9O2−δ coherent interface. J. Mater. Chem. A 2022, 10, 3495–3505. [Google Scholar] [CrossRef]
- Dos Santos-Gómez, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Improving the efficiency of layered perovskite cathodes by microstructural optimization. J. Mater. Chem. A 2017, 5, 7896–7904. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the Discretization Methods on the Distribution of Relaxation Times Deconvolution: Implementing Radial Basis Functions with DRTtools. Electrochim. Acta 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Inorganic Crystal Structure Database (ICSD), v2018-01. General Structure Analysis System; FIZ Karlsruhe GmbH: Karlsruhe, Germany, 2018.
- Singh, R.P.; Arora, P.; Nellaiappan, S.; Shivakumara, C.; Irusta, S.; Paliwal, M.; Sharma, S. Electrochemical insights into layered La2CuO4 perovskite: Active ionic copper for selective CO2 electroreduction at low overpotential. Electrochim. Acta 2019, 326, 134952. [Google Scholar] [CrossRef]
- Zheng, K.; Gorzkowska-Sobas, A.; Swierczek, K. Evaluation of Ln2CuO4 (Ln: La, Pr, Nd) oxides as cathode materials for IT-SOFCs. Mater. Res. Bull. 2012, 47, 4089–4095. [Google Scholar] [CrossRef]
- Chevalier, B.; Tressaud, A.; Brisson, C.; Dance, J.M.; Etourneau, J.; Tuilier, M.H.; Soubeyroux, J.L.; Cassart, M.; Issi, J.P. Anionic Intercalation in La2CuO4 Oxide by Fluorine or Chlorine Treatment. Mol. Cryst. Liq. Cryst. Sci. Technol. Sect. A Mol. Cryst. Liq. Cryst. 1994, 244, 135–142. [Google Scholar] [CrossRef]
- Osinkin, D.A. An approach to the analysis of the impedance spectra of solid oxide fuel cell using the DRT technique. Electrochim. Acta. 2021, 372, 137858. [Google Scholar] [CrossRef]
- Xia, J.; Wang, C.; Wang, X.; Bi, L.; Zhang, Y. A perspective on DRT applications for the analysis of solid oxide cell electrodes. Electrochim. Acta 2020, 349, 136328. [Google Scholar]
- Osinkin, D.A. Detailed analysis of electrochemical behavior of high–performance solid oxide fuel cell using DRT technique. J. Power Sources 2022, 527, 231120. [Google Scholar] [CrossRef]
- Lee, J.G.; Park, M.G.; Yoon, H.H.; Shul, Y.G. Application of GDC-YDB bilayer and LSM-YDB cathode for intermediate temperature solid oxide fuel cells. J. Electroceramics 2013, 31, 231–237. [Google Scholar] [CrossRef]
- Takeda, Y.; Kanno, R.; Noda, M.; Tomida, Y.; Yamamoto, O. Cathodic polarization phenomena of perovskite oxide electrodes with stabilized zirconia. J. Electrochem. Soc. 1987, 134, 2656–2661. [Google Scholar] [CrossRef]
- Jiang, Z.; Lei, Z.; Ding, B.; Xia, C.; Zhao, F.; Chen, F. Electrochemical characteristics of solid oxide fuel cell cathodes prepared by infiltrating (La,Sr)MnO3 nanoparticles into yttria-stabilized bismuth oxide backbones. Int. J. Hydrog. Energy 2010, 35, 8322–8330. [Google Scholar] [CrossRef]
- Almar, L.; Szász, J.; Weber, A.; Ivers-Tiffée, E. Oxygen Transport Kinetics of Mixed Ionic-Electronic Conductors by Coupling Focused Ion Beam Tomography and Electrochemical Impedance Spectroscopy. J. Electrochem. Soc. 2017, 164, F289–F297. [Google Scholar]
- Marrero-López, D.; Romero, R.; Martín, F.; Ramos-Barrado, J.R. Effect of the deposition temperature on the electrochemical properties of La0.6Sr0.4Co0.8Fe0.2O3−δ cathode prepared by conventional spray-pyrolysis. J. Power Sources 2014, 255, 308–317. [Google Scholar] [CrossRef]


| a (Å) | b (Å) | c (Å) | V (Å3) | RWP (%) | RF (%) | |
|---|---|---|---|---|---|---|
| LCO (1000 °C) | 5.3563 (1) | 5.4026 (1) | 13.1543 (3) | 380.64 (2) | 7.78 | 1.74 |
| F0.1 (1000 °C) | 5.3562 (1) | 5.4038 (1) | 13.1517 (3) | 380.68 (2) | 7.32 | 1.42 |
| F0.2 (1100 °C) | 5.3571 (1) | 5.4049 (1) | 13.1515 (3) | 380.76 (2) | 8.14 | 2.36 |
| Cl0.1 (1000 °C) | 5.3567 (1) | 5.4038 (1) | 13.1523 (3) | 380.72 (2) | 7.45 | 1.32 |
| Cl0.2 (1000 °C) | 5.3605 (2) | 5.4078 (2) | 13.1589 (4) | 381.46 (3) | 7.45 | 2.38 |
| Cl0.3 (1000 °C) | 5.3538 (4) | 5.3997 (4) | 13.144 (1) | 380.00 (7) | 12.65 | 3.62 |
| Br0.1 (1000 °C) | 5.3567 (1) | 5.4044 (1) | 13.1510 (3) | 380.72 (2) | 7.91 | 1.65 |
| Br0.2 (1000 °C) | 5.3569 (1) | 5.4050 (1) | 13.1511 (3) | 380.76 (2) | 7.51 | 1.84 |
| Br0.3 (1000 °C) | 5.3572 (2) | 5.4052 (2) | 13.1514 (7) | 380.82 (5) | 7.86 | 1.84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; R. Losilla, E.; Marrero-López, D. Modification of the Microstructure and Transport Properties of La2CuO4−δ Electrodes via Halogenation Routes. Processes 2022, 10, 1206. https://doi.org/10.3390/pr10061206
dos Santos-Gómez L, Zamudio-García J, Porras-Vázquez JM, R. Losilla E, Marrero-López D. Modification of the Microstructure and Transport Properties of La2CuO4−δ Electrodes via Halogenation Routes. Processes. 2022; 10(6):1206. https://doi.org/10.3390/pr10061206
Chicago/Turabian Styledos Santos-Gómez, Lucía, Javier Zamudio-García, José M. Porras-Vázquez, Enrique R. Losilla, and David Marrero-López. 2022. "Modification of the Microstructure and Transport Properties of La2CuO4−δ Electrodes via Halogenation Routes" Processes 10, no. 6: 1206. https://doi.org/10.3390/pr10061206









