1. Introduction
In line with the recent trend toward personalized or precision medicine, the concept of radiotheranostics has become popular in the field of nuclear medicine. In this concept, a diagnosis is made using nuclides suitable for PET/SPECT, and the subsequent targeted radioisotope/radionuclide therapy (TRT) or the targeted alpha therapy (TAT) is performed after replacement with therapeutic nuclides [
1]. In 2013, the world’s first alpha-particle-emitting radionuclide, the
223Ra pharmaceutical Xofigo™, was approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for introduction to the market [
2]. The approval of Xofigo™ substantially increased the number of diseases that could be treated (from rare diseases to common diseases such as prostate cancer), so the potential for TRT and TAT greatly increased. Since the
Journal of Nuclear Medicine (JNM) reported two surprising cases of complete remission (CR) in the treatment of metastatic prostate cancer in 2016 [
3], the potentials for
225Ac-labeled pharmaceuticals and other alpha-particle-emitting radionuclide therapies have been receiving a great deal of attention [
1]. However, for the effective application of these alpha-emitting radiopharmaceuticals, it is important to not only develop the drugs but also to develop radionuclide production methods. Securing sufficient supplies of target raw materials for each manufacturing method is an important issue. The supply of target raw materials, such as
233U,
229Th, and other nuclear-fuel-derived nuclides, as well as other thorium isotopes (
232Th,
230Th,
226Th, etc.), has attracted attention. Furthermore,
226Ra, which has previously been treated as medical waste in various countries around the world, has been found to have potential as a useful target material, and therefore, international competition has developed in securing a reliable supply.
Japan has a large-scale economy in terms of gross domestic product and is generally regarded as a rich country. On the other hand, it is a “resource-poor country” that depends on foreign countries for about 90% of its energy resources. Food self-sufficiency is also regarded as a security risk for Japan because it has been at around only 40% for many years [
4]. Overseas dependence is conspicuous in the pharmaceutical field, with 50% of the raw materials necessary for general generic drug production dependent on imports. It was estimated that the fraction of generic drugs that could be covered by domestically produced raw materials was only about 30%, so self-sufficiency is limited [
5]. This trend is even more pronounced in the supply of radionuclides and radio-labeled pharmaceuticals. Since the supply of
99mTc, which is a diagnostic radionuclide, is 100% dependent on foreign imports, the suspension of clinical procedures with
99mTc-labeled pharmaceuticals has occurred several times in the past due to unplanned shutdowns of overseas nuclear reactors and eruptions of volcanos in Iceland [
6]. After each incident, the Cabinet Office of Japan issued an action plan promoting the idea of domestic self-sufficiency [
7]. However, there is no sign of improvement to date.
In recent years, the use of the diagnostic nuclide
68Ga and the therapeutic nuclide
177Lu for radiopharmaceuticals has been expanding worldwide.
68Ga is used not only in Europe and North America, but also in 10 Asian, 1 African, and 3 South American countries [
8]. Multiple
68Ga- and
177Lu-labelled pharmaceuticals have already been approved and are becoming widely used as insurance-supported medical treatments [
9]. In contrast, Japan is far behind in terms of insurance support and clinical use. Lutathera ™ was finally approved in 2021, and a few doctor-initiated clinical trials using
68Ga-labelled pharmaceuticals also began in 2021 [
10,
11]. Of the 14 radiopharmaceuticals (including those for diagnosis and treatment) approved in Japan since 2000, there have been no products developed by domestic pharmaceutical companies [
12]. In addition, all five of the radiopharmaceuticals approved as TRT agents by the regulatory authority (the Pharmaceuticals and Medical Devices Agency, PMDA) depend on overseas imports. Moreover, their labeling nuclides (
131I,
90Y,
223Ra,
177Lu) must also be imported, so there is no prospect of domestically researched and developed pharmaceuticals. Despite the recent advent of a super-aging society unlike any other in the world, it can be said that Japan continues to be reliant on foreign resources for the pharmaceutical field and, in particular, for radionuclides. From the perspectives of the current COVID-19 pandemic, the decline in international transportation capacity due to Russia’s invasion of Ukraine, and other security risks, the Cabinet Office of Japan and other domestic authorities are beginning to move toward building a self-sufficiency system for medical resources. For the field of nuclear medicine, this means developing domestic sources for the diagnostic nuclide
99mTc and the therapeutic alpha-emitter
225Ac [
12].
In this paper, we, the National Institutes for Quantum Science and Technology (QST), would like to present the status of research and development in the TRT and TAT fields in Japan, a country lacking natural resources for medical applications. In particular, we wish to discuss the efforts of the QST towards the domestic production and development of the new therapeutic alpha nuclide 225Ac.
2. Towards Development of Alpha-Emitting Radiopharmaceuticals at QST
At the National Institute of Radiological Sciences (NIRS), the predecessor to QST, research and development for the domestic production of alpha-emitters started in 2011. This research was inspired by the results announced for Alpharadin ™ (
223radium chloride; the name used for Xofigo ™ at that time), for which clinical trials began around 2008–2010 [
13]. In 2012, public research funds were obtained so that full-scale research and development of alpha-emitter production, as the “Radiation Therapy Strategic Infrastructure Project for Internal Cancer,” could begin for the first time in Japan. At that time, a therapeutic study was planned with
211At as the first candidate for alpha-emitter because the target material,
209Bi, was easily accessible, even in Japan. We devised a QST-original vertical target system for producing
211At and proceeded to lay the foundation for domestic alpha-emitter research [
14].
211At is produced by irradiating bismuth with helium ions using an accelerator. Astatine, which is located under iodine in the periodic table, was in 1940 the first element to be artificially synthesized in research to expand the periodic table using an accelerator [
15]. Now, around 80 years later, it is very interesting to see that such an artificial element may be useful for the health and welfare of humankind.
In 2016, the NIRS, the Quantum Beam Science Research Directorate, and the Fusion Energy Directorate of the Japan Atomic Energy Agency were reorganized and integrated as the National Institutes for Quantum Science and Technology. In the 7-year medium-to-long-term research plan that was drafted at that time, a national TRT and TAT research project called “Next Generation Cancer Treatment Research” started as one of the three pillars of the national “Research and development for innovative medical use of radiation” strategy. With the expertise of the Quantum Beam Science Research Directorate, which excels in labeling technology for halogen group elements,
211At-labeled
meta-astato-benzylguanidine (MABG), which is a nuclide conversion agent of
131I-labeled
meta-iodo-benzylguanidine (MIBG) for the treatment of malignant pheochromocytoma, was developed as part of continued research from the NIRS era [
16]. The results of alpha-emitter research have been steadily accumulating over the past 6 years, and a doctor-initiated clinical trial, which is the first human study of MABG, is about to start at Fukushima Medical University in 2022 [
17,
18]. Another doctor-initiated clinical trial of [
211At]NaAt, which is a first-in-human study for the treatment of intractable thyroid cancer, was started in 2021 at Osaka University [
19]. The research and development of alpha-emitting radiopharmaceuticals focusing on
211At is gradually progressing at multiple facilities across Japan. In general, the physical half-life of
211At is relatively short at 7.2 h, which is inconvenient for commercial supply and logistics, so it is not currently regarded as the highest priority for the future development of TRT and TAT worldwide, and only a few countries, such as, the United States, Sweden, Denmark, and Canada, are under development [
1]. However, as a country lacking natural resources for medical applications, Japan is one of the few countries promoting the clinical research and development of
211At-labeled therapeutic agents.
3. R and D for the Production of 225Ac at QST
As noted above, led by Osaka University, the research and development of alpha-emitting radiopharmaceuticals in Japan is focused on mainly
211At. On the other hand, the QST has set itself apart from other domestic research institutes by conducting full-scale research on the production of
225Ac, as well as
211At. In 2016, the only method to produce
225Ac relied on extraction from
229Th derived from
233U, but this
229Th generator method is not feasible in Japan because we do not have access to fissiles of
233U. However, as one of the leading accelerator and medical research facilities in Japan, the QST has focused on a
225Ac production method via proton irradiation first reported by Apostolidis et al. in 2005 [
20]. This advanced technique irradiates an α-emitter,
226Ra, with protons to obtain another α-emitter. Due to difficulties in handling
222Rn, which is a gas that emanates as a daughter of
226Ra, this reaction channel has received little attention. At the QST, we started research and development of
225Ac production with charged particles using
226Ra that had once been used as a sealed source for brachytherapy and was now being kept as radioactive waste. Thanks to the national waste management program, a certain amount of
226Ra was available as a target for proton irradiation, even in our resource-poor country.
In 1901, just three years after the discovery and isolation of
226Ra by Pierre and Marie Curie in 1898 [
21],
226Ra was utilized for medical purposes.
226Ra decays to
222Rn and has been applied to radiotherapy using the beta rays and gamma rays emitted by the daughter nuclides.
226Ra in the form of a brachytherapy needle was inserted into an affected area to suppress tumor growth. However, from the middle of the 20th century, brachytherapy has focused on the use of other, more manageable nuclides, so the existing
226Ra brachytherapy needles were collected and stored as medical waste. In Japan, several tens of grams of sealed
226Ra sources that were no longer used as radium needles or tubes were recovered and were in storage by the 2010s. Since
226Ra has a long half-life of 1600 years, emits high energy gamma rays, and emanates
222Rn, it is difficult to obtain the approval of the local governments for landfill disposal. The handling and storage of
226Ra is a troublesome issue in Japan and many other countries. Nobody in Japan seemed to recognize the potential usefulness of
226Ra at that time.
However, the situation in the United States was quite different. A method that used
226Ra as a target to produce
227Ac via neutron irradiation (i.e.,
226Ra(n,γ)
227Ra) was already known. Beta decay of
227Ra with a half-life of 43 min produced the desired
227Ac [
22]. The DOE was producing
227Ac in this way. Since
227Ac can be used as the parent nuclide of a
223Ra generator in Xofigo
TM production, the DOE had been working with Bayer between 2007 and 2017 to engage in the production of
227Ac, and they made a big profit. Later, in 2018, the DOE and Bayer renewed their cooperation for the provision of nuclides [
23]. We pay homage to the DOE because they were the first to see the potential of collecting medical waste from around the world for the production of
223Ra and other nuclides. We cannot help but be discouraged by Japan’s lack of foresight in not seeing that it might be possible to make effective use of the limited amount of
226Ra that it had as a resource for medical applications.
In 2015, most of the 226Ra stored in Japan was transferred to the US Department of Energy (DOE) for recycling, while a small number of 226Ra needles were provided to us for research purposes. However, besides radioactivity, there was no other information (e.g., manufacturer, chemical form, or presence of additives (carriers)) available for the 226Ra needles. It was a challenging task to develop a suitable refining technology (including de-capping, the identification of the chemical formula, the presence of additives, separation, and isolation of 226Ra from the needle). Our TRT and TAT research project had a big handicap from the beginning.
Research and development into
225Ac production started at the QST via the
226Ra(p,2n)
225Ac reaction, and we eventually succeeded in the trial production of
225Ac. In 2018, the QST, as a contracted research institute, participated in the “Cyclic Innovation for Clinical Empowerment (CiCLE)” project of the Japanese Agency for Medical Research and Development (AMED). The project was led by Nihon Medi-Physics (NMP) [
24]. As a result, a large amount of AMED research funding was invested in the QST so that a full-scale
225Ac production research and development project could begin.
4. Joint IAEA-JRC Workshop 2018—“Supply of Actinium-225”
After the 2016 report on prostate cancer treatment by Kratochwil et al., global competition had begun towards the aim of establishing a stable mass production method for both
225Ac by itself and
225Ac-labeled compounds that were sufficient for clinical use. In 2018, the International Atomic Energy Agency (IAEA) and the Joint Research Center (JRC) of the European Commission jointly held an international workshop [
25]. As far as we know, this was the world’s first international conference dedicated to
225Ac. Meeting attendees included representatives from the IAEA, JRC, and the DOE. Chemistry, pharmacy, engineering, and medical professionals from 15 countries, (i.e., Argentina, Belgium, Canada, Cuba, Czech Republic, Finland, France, Germany, Japan, Republic of Korea, Poland, Russia, South Africa, Switzerland, and the United States) attended. From Japan, Professor Jun Hatazawa (then at Osaka University) and two of the authors (Higashi and Nagatsu, QST) participated. Technical issues and opinions related to
225Ac production were widely discussed, and information on the current state of research and regulatory issues for clinical use in each country was exchanged. It was a valuable meeting. A report by the IAEA summarizing the workshop was published on the web for those readers who would like to know more details [
25].
It should be noted that the following sentences that we include here are from the oral presentations that we personally witnessed as participants at the workshop and may differ from the details that are presently officially approved by each facility or institution.
(Comment: As of 2022, four years after the workshop, it is interesting to look back at these presentations because, while there are some projects that have made steady progress, there are also some development plans that have not been heard of since then. We think there may be a number of cases where the results have not been announced due to reasons such as economic strategy. Please refer to the latest data for the latest development status of each country.)
4.1. Outline of Workshop Discussion
At that time, the main production method for 225Ac was the conventional generator extraction method from 229Th (Th-GEx method). Other known methods that used an accelerator included: ① spallation via the 232Th(p,x)225Ac reaction, ② transmutation by protons targeted at 226Ra, and ③ photonuclear reaction induced by high-energy electrons bombarding 226Ra. One production method that relied on the reaction of 226Ra(3n,2β)229U→229Pa→229Th→225Ra→225Ac induced by high neutron flux using a nuclear reactor (abbreviated as the reactor method) was also known.
It was noted that the Th-GEx method was performed at a large scale at the JRC and Oak Ridge National Laboratory (ORNL) in the United States. Method ① was conducted on an experimental scale at Los Alamos National Laboratory (LANL) and Brookhaven National Laboratory (BNL) in the United States; the Conseil Européen pour la Recherche Nucléaire (CERN), TRIUMF, in Canada; and a few institutes in Russia. Method ② was originally developed by the JRC in Europe, but many other facilities around the world, including the QST in Japan, were investigating the method at a small scale. The JRC and Munich University were preparing to work at a medium scale. Method ③ was conducted at an experimental scale in the United States and Australia, and the reactor method was being investigated at a similar scale at ORNL in the United States.
(
Note:
Table 1 lists the alternative
225Ac production methods that used an accelerator. It was created by adding the latest data [
26,
27,
28] to the 2018 document [
25].
In 2018, the
225Ac yield via the Th-GEx method was 33 GBq (0.9Ci) per year from ORNL in the US [
29], 11 GBq (0.3Ci) per year from the JRC in Europe, and 22 GBq (0.6Ci) per year from the Institute of Physics and Power Engineering (IPPE) in Obrinsk, Russia. The overall global annual production capacity was, thus, 66 GBq (1.8Ci) per year.
(Note: The supply from Russia, which corresponded to about one-third of the world’s production capacity in 2018, was suspended due to the invasion of Ukraine in 2022, and the impact on future global TRT and TAT development is not negligible.)
Considering future global clinical demand, it is necessary to increase the production capacity of
225Ac. It was estimated that 1850 GBq (50Ci) would be required annually per pharmaceutical, which is roughly about sub-tens of TBq (hundreds of Ci) in total [
30]. It was, therefore, predicted that the Th-GEx method is not able to meet all of the future
225Ac demand. As a consequence, it is a matter of urgency to establish new production methods (as well as quality evaluation, dose evaluation, cost benefit, logistics, etc.) for
225Ac.
It was noted that there were 3 tons of
233U in the United States, of which 1 ton was refined and the remaining 2 tons were contained in spent reactor fuel. In other words, from the perspective of a secure supply of raw materials, a strategy to expand commercial supply via the Th-GEx method in the US is highly promising [
31].
There was also a strong opinion that it was meaningless to increase production without considering quality, cost, purity, and current good manufacturing practice (cGMP). Considering future regulatory approval, the concentration of impurities, acceptable thresholds for possible contaminants, and (radio)toxicity are key factors for the addition of potential alternative production methods to the FDA Drug Master File.
(
Note: In 2021, the Th-GEx method was listed in the FDA Drug Master File [
32].)
In particular, the handling of 227Ac (T1/2 = 21.8 years), which is considered to be radiotoxic, was an issue. Thus, the separation and purification of 227Ac were also important. Since the waste exemption level of 227Ac is extremely low, radioactive waste and sewerage management should be also considered.
There was also a strong opinion that Method ① had a potential issue with 227Ac contamination. As the FDA is quite strict in regards to purity, it was thought to be unlikely that this method would be approved.
(
Note: The issue of
227Ac contamination was resolved, and in 2020, Method ① was added to the FDA Drug Master File [
33]. Since the Th-GEx method and Method ① are now listed in the FDA Drug Master File, these two production paths will be mainly used in the manufacture of
225Ac-labelled radiopharmaceutical investigational drugs developed by the “mega pharmas”. It is expected that these two
225Ac production paths will be the main paths for commercial
225Ac-labelled therapeutic agents in the global pharmaceutical market. It is necessary in the future to register the original
225Ac production protocol developed in Japan with the FDA or a similar official authority.)
4.2. European Situation
The JRC found it difficult to completely eliminate 225Ra from the 225Ac that it supplied six times a year using the Th-GEx method. However, using various resins and columns (anion exchange resin, UTEVA column, TEHDGA column, etc.), a radionuclidic purity of >99.98% was achieved for 225Ac. In addition, the JRC also demonstrated a prototype 225Ac/213Bi generator (3.7 GBq (100 mCi) of 225Ac). The JRC’s strategies were clear, and it had already established itself as an international research center for drug discovery using 225Ac.
(
Note: In 2020, Endocyte (owned by Novartis Pharmaceuticals) started to use JRC-supplied
225Ac, and a phase I clinical trial of TRT and TAT for advanced prostate cancer with the
225Ac-labeled PSMA-617 radiopharmaceutical (initially developed and characterized at JRC Karlsruhe in 2013) started in South Africa and Australia. It is our impression that the trial is proceeding smoothly now [
34].)
It was noted that SCK-CEN (Belgian Nuclear Research Center) was able to produce high-quality, GMP-grade 225Ac and continuously supplied it with a backup system. SCK-CEN was also affiliated with Global Morpho Pharma (France) and IRE Elit in the production of GMP-grade 225Ac. SCK-CEN, having a BR2 reactor and an accelerator-driven subcritical reactor called MYRRHA (Multi-purpose hYbrid Research Reactor for High-tech Application), already had several hundred grams of 226Ra, and irradiation would start from 2019. Furthermore, SCK-CEN would be able to produce GMP-grade 225Ac at 37 GBq (1000 mCi)/week using an IBA (Ion Beam Applications S.A., EURONEXT) Rhodotron by irradiating with 40 MeV electrons at 125 kW. In addition, another cyclotron belonging to IBA, KIUBE, was able to supply GMP-grade 177Lu.
(
Note: In 2021, SCK-CEN and IBA signed an R and D partnership agreement for the joint production of
225Ac, and future trends should be watched [
35].)
4.3. The Situation in the United States
Since the 1990s, ORNL has produced 50 GBq (1.4 Ci) of 225Ac using the Th-GEx method. To separate and purify 225Ac, various resins and columns (anion exchange, resin, MP1 column, AG50W microcolumn, etc.) were used. It was claimed that 740 MBq (20 mCi) of 225Ac could be prepared every 2–3 months and a maximum of 5.2 GBq (140 mCi) annually. In addition, ORNL also performed the reactor method in a high-flux reactor, and a maximum of 5.2 GBq (140 mCi) of 225Ac could be produced annually with that method. However, the production efficiency was poor, and 228Th and 227Ac were often mixed.
The United States has been working on development under the framework of the Tri-Lab project led by the DOE since 2009. Production via Method ① was also investigated in cooperation with the NIDC (National Isotope Development Center), ORNL, BNL, and LANL. At that time, ORNL had already produced a total of 67 GBq (1800 mCi) of
225Ac and exported a total of 8.7 GBq (235 mCi) to Europe for research purposes. Their production procedure had already been submitted for consideration to the FDA Drug Master File. They said that
227Ac (T
1/2 = 21.8 years) contamination could be an issue, but related studies showed that
227Ac in the
225Ac had little or no impact on dosimetry and toxicity [
36], and therefore, it was expected that GMP production and certification would be possible.
(
Note: Method ① was listed in the FDA Drug Master File in 2020 [
33].)
TERRA POWER is a company that was established in the United States by Bill Gates in 2007. It is developing next-generation nuclear reactors, such as a traveling wave reactor or a molten salt reactor, to reduce CO2 emission from electric power plants. They aimed to expand 225Ac production using the Th-GEx method, and they already had 45 g, or 370 GBq (10 Ci), of 229Th, which was derived from stored 233U. The parent 233U for 229Th contains 220 ppm of 232U as an impurity that breeds 208Tl (T1/2 = 3 min)-emitting 2.6 MeV gammas, so it was troublesome to handle. The process to isolate 229Th from 233U and extract 225Ac from 229Th was still in the development phase. They expected that they would be able to ship 225Ac by 2020 or later.
(
Note: The Th-GEx method was listed in the FDA Drug Master File in 2021, and as of 2022, TerraPower is aiming to build a supply network in partnership with the US DOE [
37].)
NIOWAVE promoted Method ③. An electron accelerator had already been licensed in accordance with local and national laws (Radioactive Material Act, Financial Act, National Environmental Policy Act (NEPA), and the US Department of Transportation (DoT) regulations). The company expected to receive future approval for cGMP registration from the FDA. Their 225Ac-labeled compounds were expected to complete phase I by April 2019, phase II by September 2020, and then proceed to phase III and commercialization. NIOWAVE aimed to produce 370 GBq (10 Ci) of 225Ac annually. They stated that they had already secured sub-ten GBq (several hundred mCi) of 226Ra devolved from sources obtained at nearby hospitals. Two huge chambers that were developed as an accumulation system were installed at the top of the hot lab stores for 222Rn (T1/2 = 3.8 d) management. The chambers effectively reduced the 222Rn burden to the environment by allowing physical decay in the chambers.
(
Note: NIOWAVE’s production capacity is currently scaled up to produce 370 GBq (10 Ci) of
225Ac every week using Method ③. In addition, they also started
225Ac production via Method ① and aim to produce
225Ac at 18,500 GBq (500 Ci) annually [
38]).
4.4. The Situation in Canada
The Canadian Nuclear Lab (CNL) held a maximum of 400 g of high-purity 233U in the form of UO2 (232U <10 ppm; however, 20% was insoluble → regeneration required) formerly used in a thorium fuel cycle. It was said that a 229Th/225Ac generator that combined UTEVA resin and DGA resin was prepared using a thorium solution separated from uranium (AGMP resin). An automated milking system using a commercially available device (OMNI Lab) was under development, and it was said that the Th-GEx method could produce 225Ac at a yield of 0.4–0.8 MBq (10–20 µCi)/day from a load of 37 MBq (1 mCi) of 229Th. Having four of these automated milking devices, they succeeded in labeling a monoclonal antibody targeting epidermal growth factor receptor (EGFR) with 225Ac. However, the specific activity of 229Th was low, which caused a breakthrough of thorium from the generator. As part of a national strategy for 225Ac supply, the CNL was affiliated with TRIUMF, AECL/EACL, and Saskatchewan University.
TRIUMF (Canada) promoted Method ③. They planned to use an eLINAC (superconducting RF accelerator technology, 30 MeV, 10 mA at maximum) at the Advanced Rare Isotope Laboratory (ARIEL, a government-funded facility in collaboration with 21 Canadian universities). As such, it was theoretically possible to produce up to 74 TBq (2000 Ci) of 225Ac every month. Potential issues were high-level radiation exposure during 226Ra handling (8.1 mSv/h at 1 m) and 222Rn management. They had not considered applying for FDA approval at that time. They were also considering Method ①.
TRIUMF (Canada) has the world’s largest cyclotron, which can provide 500 MeV protons at 110 µA at maximum. With the settings they were using (480 MeV proton, 85 µA × 31 h on 10 g Th), 0.44 GBq (12 mCi) of 225Ac was successfully produced in 2017, and plans were in place to increase to 3.7 GBq (100 mCi) in a batch-yield using 500 MeV x 110 µA on a 100 g-Th-232 target. It was said that GMP approval would be obtained, but the quality control of 225Ac, especially the removal of 227Ac, was still an issue.
(
Note: TRIUMF and Osaka University have been strengthening their cooperation in recent years, and an Osaka-University-based venture company, Alpha Fusion Inc., initiated the import of
225Ac for preclinical use from TRIUMF [
39].)
4.5. The Situation in Russia
At IPPE (Obrinsk, Russia), 225Ac was produced by the Th-GEx method at about 15.5 GBq (0.4 Ci) per year, and all of that was shipped overseas at that time. The purity of the parent nuclide, 233U, was 90.55% (9.45% contaminants, including 232U = 26 ppm and Th = 0.24%), and 187 mg of 229Th (approximately 1.55 GBq (40 mCi)) could be extracted from 1 kg of uranium (approximately 370 GBq (10 Ci)). The isotopic purity of 229Th was only 6.81% (232Th = 93.11%, 230Th = 0.08%, and 228Th = trace level), but the quality of the obtained 225Ac was excellent (229Th < 0.007, 228Th < 0.007, 224Ra < 0.02, 225Ra < 0.02, and other nuclides < 0.02, as percentages of 225Ac radioactivity). In addition, a plan for increasing 229Th inventory via the 229Ac → 229Th path combined with the 228Ra(n,γ)229Ra → 229Ac reaction was proposed. The 228Ra target could be obtained from 232Th (T1/2 = 1.4 × 1010 y; it is estimated that 0.4 mg of 228Ra is contained in 1 ton of natural 232Th). They were also considering the production of 229Th by irradiating natural 230Th (20 g of 230Th in 1 ton of 238U) with fast neutrons, and this production method was patented in Russia. There was also a presentation from the Institute of Nuclear Research (INR).
(Note: As mentioned above, the supply from Russia, which was equivalent to about one-third of the world production capacity at that time, was stopped due to the invasion of Ukraine in 2022. The influence of that change to the supply is not be small for future TRT and TAT development around the world.)
4.6. Possibilities of Method ②, Transmutation by Protons Targeted at 226Ra
The JRC has been promoting low- or mid-energy proton irradiation of a 226Ra target for 20 years and already obtained patents in Europe in 1996 and in the United States in 2001. This method has the advantages of high yield and high radionuclidic purity, as validated by the JRC and the Munich group, as well as the fact that the reaction can be performed with protons of energies of 20 MeV or less on a moderate accelerator platform. As a result, this method has already been validated at many facilities around the world. Possible disadvantages are the limited supply and handling of 226Ra, targeting preparation (solid and liquid), optimizing the energy window, and management of 222Rn (T1/2 = 3.8 d). Further technical improvements, cost, waste, cGMP, quality assurance, and so on still needed to be considered.
The mining, purification, and commercial distribution of 226Ra had already stopped by the 1960s, but the amount in storage around the globe was estimated to be several kilograms. Considering the possibility of collecting 226Ra as a co-extracted product in uranium mining, it was thought that 257 mg of 226Ra could be recovered from 1 ton of uranium-containing ore, suggesting that the annual productivity for fresh 226Ra could reach 12.85 kg.
4.7. Our Impression as a Participant from a Country Lacking Natural Resources for Medical Applications
The United States and the European Union are moving to build a supply system for 225Ac as a national policy, and the raw materials (i.e., 229Th and 226Ra) are abundant. Their levels of political strategy, as well as the speed of decision making and the performance capability in R and D, are several orders of magnitude superior to our country.
Japanese nuclear medicine is already behind the world, and the number of clinical procedures with 68Ga- or 177Lu-labelled compounds and therapeutic agents is critically poor. This situation is the same for 225Ac, and urgent action is needed to reinforce domestic production, establish secure import routes, promote drug development, and regulate for clinical application to catch up with other countries. In particular, we have to realize that securing 226Ra as a “medical resource” is an urgent task.
5. Full-Scale Production and Development of 225Ac at QST
Two of the authors, (Higashi and Nagatsu), visited the JRC in Germany prior to participating in the IAEA international workshop held in Vienna. The tour of the JRC facility was conducted under the guidance of Prof. Alfred Morgenstern, who directed the research and wrote the 2005 paper on the Method ②
226Ra(p,2n)
225Ac reaction [
20]. We had several problems that had to be overcome in our own development at that time, but this site visit gave us many valuable hints for solving the problems, which eventually led to the success of our subsequent research and development. We would like to thank Prof. Caciuffo and Prof. Morgenstern for allowing us to visit the JRC.
The “CiCLE” project funded by AMED in 2018 has progressed steadily [
24]. The QST is proceeding with full-scale research and development with a radiopharmaceutical company representing Japan, NMP. The production of
225Ac using the
226Ra(p,2n)
225Ac channel was eventually put into practical use [
40]. In the four years since 2018, the QST has proceeded with many developments (described below) and has applied for international patents for a number of related elemental technologies, (i.e., a target station design that enables high-intensity irradiation, an isolation and recovery method for
226Ra, and a separation and purification method for
225Ac). We believe that the QST and NMP have established a clinically adaptable practical technique for
225Ac production. In addition, NMP established the accelerator facility of “CRADLE”, which is capable of a clinical trial level of production of
225Ac, in September 2019 in Sodegaura City, Chiba, as an achievement of the AMED/CiCLE project. The construction of this facility meant that a domestic clinical-trial-scale supply of
225Ac-labelled radiopharmaceuticals became available in Japan [
41]. With these achievements, NMP was able to establish a commercial platform for the production of alpha-emitters to support TRT- and TAT-related industry, including clinical applications in Japan and overseas. We are proud to say that the Japanese are now ready for a new era of
225Ac-based cancer treatment.
6. Current Status of 225Ac Distribution and Related R and D in Japan
Four years have passed since the above-mentioned international workshop was held. Many
225Ac-related studies have been reported at international conferences, suggesting that
225Ac-labeled radiopharmaceuticals are steadily progressing toward clinical application worldwide.
Table 1 shows the latest information we have about each institute working on the production of
225Ac using an accelerator. Interestingly, two new production paths have been developed since 2018. As mentioned above, Method ① was listed in the FDA Drug Master File in 2020, as was the Th-GEx method in 2021 [
32,
33]. As of 2022, TerraPower is aiming to build a supply network in partnership with the US Department of Energy (DOE) [
37].
Table 2 summarizes the movements in each country in March 2022 regarding clinical trials with α-emitter-labeled radiopharmaceuticals. In 2020, phase I clinical trials for targeting advanced prostate cancer with
225Ac-labeled PSMA-617 were initiated in South Africa and Australia [
34]. These trials seemed to be a stimulant for subsequent clinical trials with
225Ac (e.g.,
225Ac-labeled antibody human kallikrein-2 (hK2) [
42] and an
225Ac-labeled insulin-like growth factor-1 receptor (IGF-1R)-targeting humanized monoclonal antibody radiopharmaceutical called FPI-1175) [
43]. It can be said that the development of
225Ac-labeled radiopharmaceuticals in each country is booming.
It was mentioned earlier that a doctor-initiated, first-in-human, clinical study with
211At-NaAt was started in Japan in 2021 for the treatment of intractable thyroid cancer at Osaka University [
19]. However, what is the status of
225Ac-labeled drug research in Japan?
The QST, which has been a major driving force in domestic
225Ac production research, is also a leading institute for
225Ac-related biological studies. For example, using an anti-podoplanin (PDPN) antibody developed by Prof. Yukinari Kato at Tohoku University, the QST started a TRT and TAT study for treating malignant mesothelioma with
90Y- and
225Ac-PDPN-antibodies aided by
111In for diagnostics [
44]. Malignant mesothelioma, which is frequently found in developed countries, is an intractable malignant tumor that develops mainly in the pleura, and 10,000 new cases are reported annually. The relationship between exposure to asbestos and this disease has been known for many years. In Japan in 2005, there was an incident known as the “Kubota shock”, which revealed the risk of asbestos in the neighborhood surrounding manufacturers of major industrial machinery and construction materials. In response to this, the Japanese government enacted the “Act on Relief of Health Damage Caused by Asbestos” in 2006 and is still taking measures against health damage [
45]. PDPN, a platelet aggregation-inducing protein, is expressed in some normal tissues, but it is also expressed in many cancers, including malignant mesothelioma. Kato’s group succeeded in developing an NZ series of antibodies that selectively recognized PDPN expressed in tumors. Further work eventually obtained NZ-16, which had the highest affinity and stability in vivo. We modified the NZ-16 antibody by labeling it with therapeutic nuclides to validate the efficacy and successfully obtained proof-of-concept (POC) results in a xenograft model [
46]. Based on this achievement, our project was funded by the AMED grants of “Translational Research Program—pre-B” and “Non-clinical study for practical application of innovative cancer therapeutic agents (pharmaceuticals)” [
47]. Since the protocol for these nonclinical trials has been finalized, including consultation on the regulatory science through an interview with PMDA, we are steadily preparing for clinical trials.
Another
225Ac-labeled therapeutic agent,
225Ac-labeled OTSA101, was developed. OTSA101 is a humanized antibody recognizing human FZD10, which is a transmembrane protein belonging to the frizzled family [
48]. It is overexpressed in most synovial sarcomas, indicating a promising therapeutic target for such sarcoma. A phase I trial of beta-emitting
90Y-OTSA101 was conducted in France [
49], and another phase I trial is recruiting in Japan (NCT04176016). Some of the enrolled patients showed a stable-disease response to therapy for recurrent sarcomas. To provide an additional option to such patients, we evaluated the efficacy of
225Ac-OTSA101 in synovial sarcoma mouse models [
50]. The alpha-emitting radiopharmaceutical achieved a complete response in 60% of mice, and no recurrence was observed. Radioimmunotherapy with
225Ac-OTSA101 is a promising therapeutic option for synovial sarcoma.
Based on the above-mentioned AMED/CiCLE research, NMP reported the efficacy of an
225Ac-labeled humanized antibody for pancreatic cancer model mice with a targeting strategy of cancer-cell-related mucin MUC5AC and verified the tumor growth inhibitory effect [
51].
In collaboration with Heidelberg University in Germany, Osaka University developed a fibroblast activation protein (FAP) derivative,
225Ac-labelled FAPI-04, that is attracting worldwide attention. They are proceeding to verify the inhibitory effect in pancreatic cancer model mice [
52].
What is the status of
225Ac production research in Japan? Unfortunately, the biggest concern for Japan is still the prospect of securing raw
226Ra.
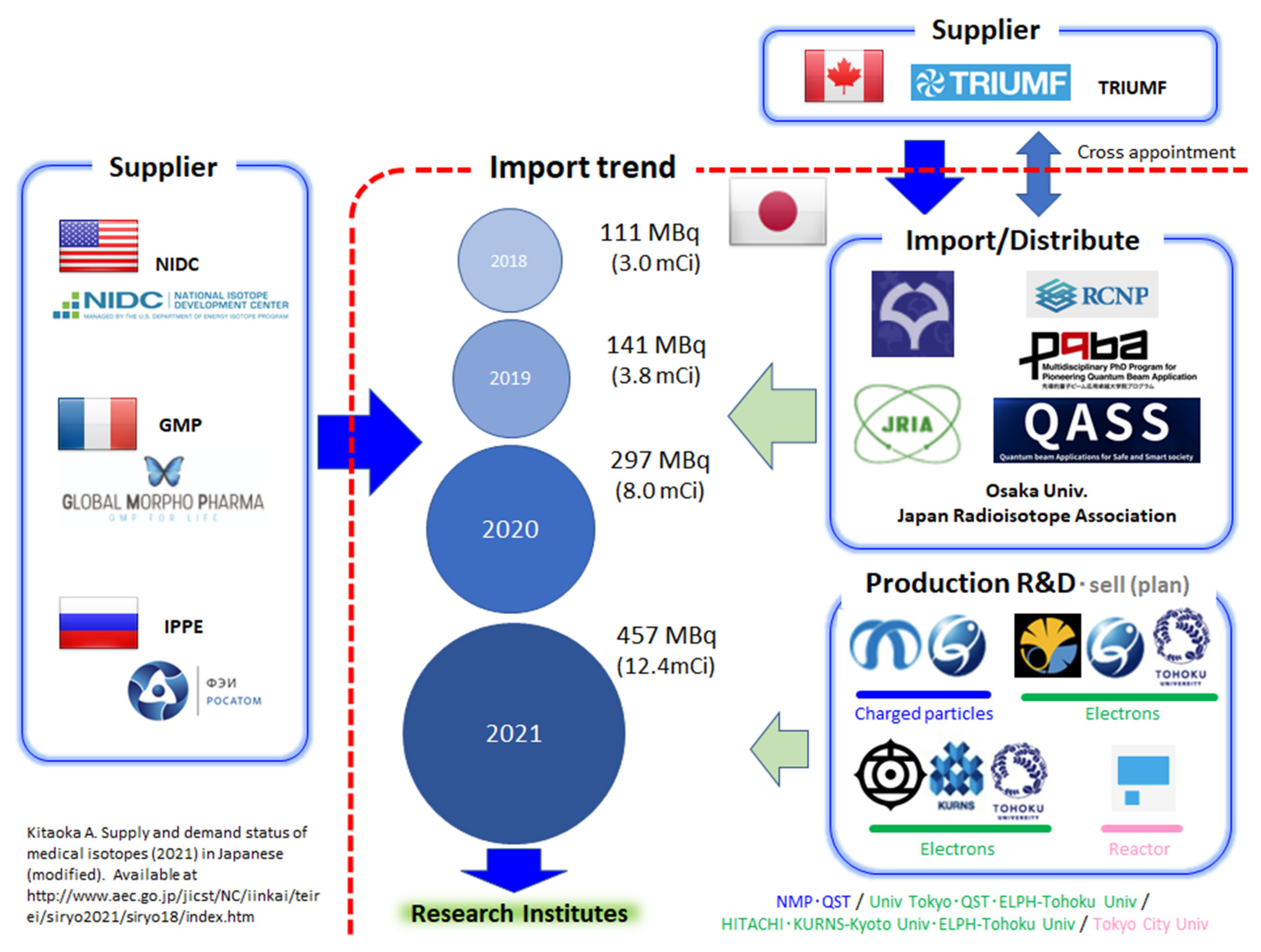
Figure 1 shows Japan’s status with respect to
225Ac supply from 2018 to 2021. At present,
225Ac supply depends on imports from overseas, i.e., the National Isotope Development Center (NIDC) in the United States, Global Morpho Pharma (GMP) in France, and IPPE in Russia. The amount of imported
225Ac has increased year by year, suggesting that basic TAT research is surely progressing in our country. Furthermore, as it has a tight relationship with TRIUMF, Osaka University established a venture company, Alpha Fusion Inc., that launched a research project named Quantum beam Applications for Safe and Smart society (QASS). QASS has a couple of social missions, one of which is to develop novel TAT agents with cyclotron technology. QASS collaborated with the Japan Radioisotope Association (JRIA) to import
225Ac from TRIUMF as one of their initial activities [
39].
Regarding domestic research into
225Ac production by the QST and NMP, it can be said that Japan has taken several large steps [
40,
41]. In addition, NMP succeeded in producing the world’s first GBq amount of
225Ac using the transmutation method in April 2022 [
53]. Unfortunately, the QST had a fire accident at its accelerator vault in November 2021. Two cyclotrons were critically damaged. It is expected that recovery will take a considerable time [
54]. Consequently, in-house production of
225Ac at our laboratory has been impossible since then, and related biological studies have also suffered significantly after this accident. The recovery or replacement of our main research equipment is highly desired.
In October 2019, Hitachi Co. Ltd., Tohoku University, and Kyoto University jointly issued a press release about their successful test production of
225Ac with Method ③ [
55]. Another group from the University of Tokyo, the QST, and Tohoku University also employed this reaction channel. Due to the small amount of target material and the low intensity of the electrons, the actual yield of
225Ac from this method is currently insufficient. In summary, the diversification of domestic
225Ac production capacity is good news for Japan, but the fact that all
225Ac production platforms need
226Ra as the target indicates that securing a good supply of
226Ra is a very important issue for Japan.
7. Atomic Energy Commission “Special Subcommittee for Securing Medical Radioisotope Production and Utilization”
In November 2021, the Atomic Energy Commission under the jurisdiction of the Cabinet Office established a special subcommittee, “Medical Radioisotope Production and Utilization,” with the aim of achieving full-scale domestic self-sufficiency and establishing economic security for medically important radioisotopes [
56]. This action was based on the statement that “the government will work towards radioisotope production by means of research reactors or other technology”, which is contained in the Growth Strategy Follow-up (decided by the Cabinet on 18 June 2021) issued by the Prime Minister and the Minister of Education, Culture, Sports, Science, and Technology (MEXT) [
57]. Thus, the subcommittee is deliberating on an action plan for the secure production and utilization of medical radioisotopes, including related policy decisions, with observers from related ministries, such as the Cabinet Office, MEXT, the Ministry of Health, Labor, and Welfare (MHLW), the Ministry of Economy, Trade, and Industry (METI), the Nuclear Regulation Authority (NRA), and the Reconstruction Agency. An action plan is expected to be issued in 2022, and it is hoped that it will have a positive impact on our nuclear medicine and atomic energy society.
As a member of this committee, the QST has pointed out that Japan’s slow progress in the field of nuclear medicine, as evidenced by the inability to use PET nuclide 68Ga and therapeutic nuclide 177Lu, could be caused by the “vertically divided administration” model of the government. We have requested the establishment of a supervisory ministry similar to the US DOE that can provide strategic management for the development and effective utilization of energy and medical resources. It has already been 4 years since we learned about the national policies and strategies of many countries with respect to the R and D for 225Ac and TAT at the international workshop. We have finally established a cross-ministerial subcommittee for the R and D of radioisotopes in Japan. Considering the foresight involved in the US DOE’s swift and appropriate response, as well as similar actions found in other countries, our action seems quite late. However, it is expected that a socially effective action plan will be issued from the subcommittee to enable us to catch up with world progress. The QST made the following two recommendations to the subcommittee as our contribution:
Recommendation 1: Realizing that Japan is a “country lacking natural resources for medical applications”, the government should promote the establishment of an 225Ac supply system as a national policy and also secure important raw materials, such as 226Ra and thorium.
Recommendation 2: Remove the “vertically divided administration” model and build a system centered on the Cabinet Office that can strategically develop and utilize energy resources effectively, including medical resources, and formulate policies related to radioisotopes.
In response to requests from many societies, including cancer patient groups and radiation-related organizations, one of the major goals of the subcommittee is to promote the domestic production of radioisotopes using research reactors, such as the “Joyo” facility in Ibaraki [
58]. If the mass production of radioisotopes in a research test reactor becomes possible in the future, it will be a big step forward for the development of
225Ac in Japan. The core research on reactor-based
225Ac production is advocated by Tokyo City University [
59]. This research has already been adopted in the “National Issue-Based R&D Promotion Project (Nuclear System R&D Project)” in 2020 in collaboration with the Japan Atomic Energy Agency (JAEA), NucMed Inc., Mitsubishi Heavy Industries, Ltd., and Kanazawa University [
60]. The primal step in this R and D project is to restart the currently suspended test reactor “Joyo” and, after that, begin irradiating
226Ra or
230Th to produce
225Ac at test and larger scales [
59]. From the perspective of securing the important raw material of
226Ra, the JAEA may be able to contribute by utilizing the former uranium mine at Ningyo-toge Pass.
The solutions listed above are receiving a lot of attention from cancer patient groups, the Society of Nuclear Medicine, and related political and business circles in Japan. We sincerely hope that these actions will stimulate the development of TRT and TAT so that it will be available to cancer patients.