Codonopsis laceolata Water Extract Ameliorates Asthma Severity by Inducing Th2 Cells’ and Pulmonary Epithelial Cells’ Apoptosis via NF-κB/COX-2 Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Codonopsis laceolata (C. laceolata) Extract Preparation
2.2. Animal Experiment and Ethics Statement
2.3. Bronchioalveolar Fluid (BALF) and Serum Analysis
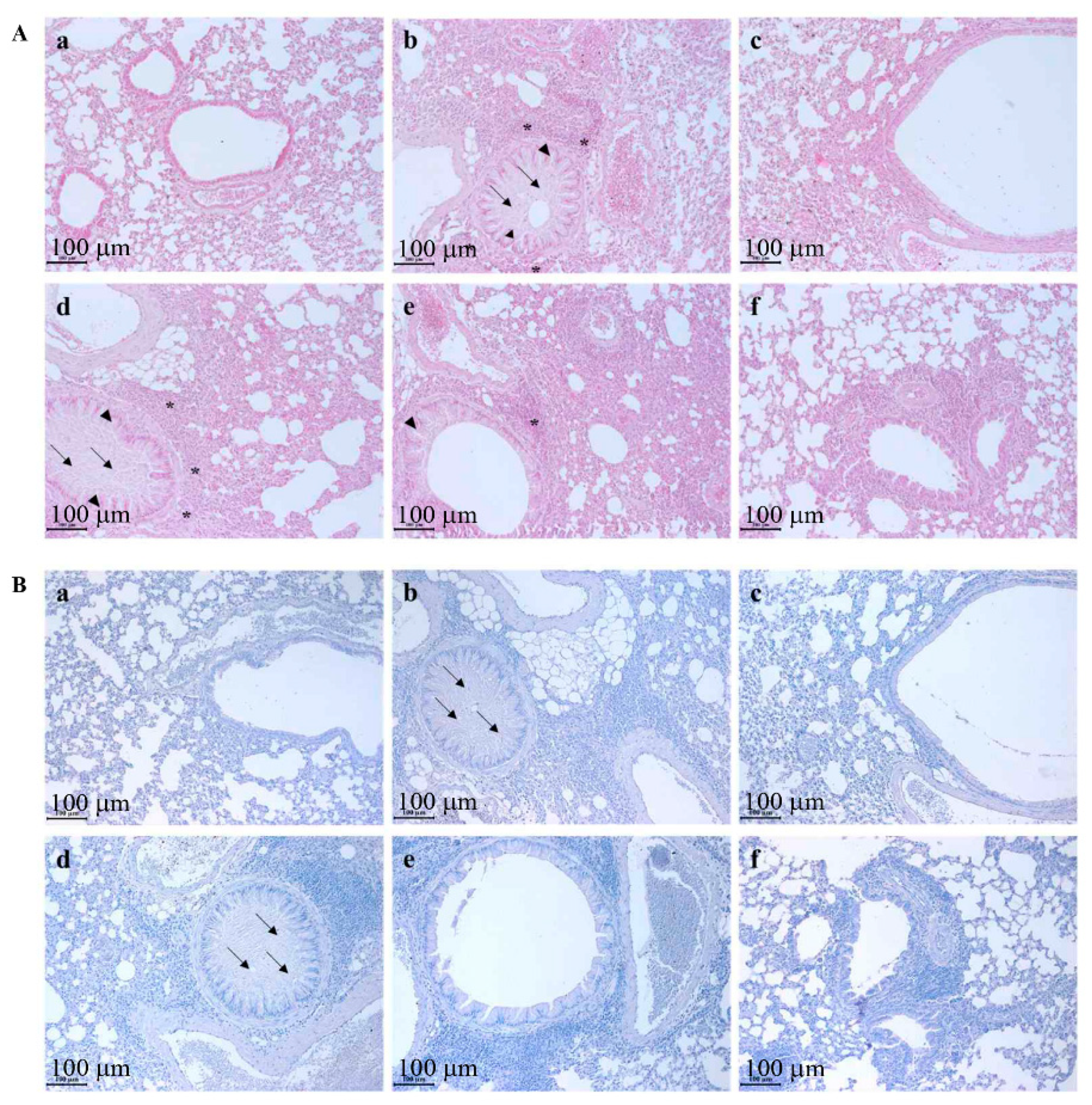
2.4. Histopathological Anlaysis
2.5. Immunofluorescent Analysis
2.6. Real Time-Poly Chain Reaction (RT-PCR) Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Statistical Analysis
3. Results
3.1. Codonopsis laceolata Extract Effectively Suppressed the Population Increments of White Blood Cells (WBC) and Lymphocytes (LY) in Bronchioalveolar Fluid (BALF) and the Upregulated Level of Serum IgE, Which Are Induced by Ovalbumin Treatment
3.2. Codonopsis laceolata Extract Significantly Prevented the Representative Morphological Changes in the Respiratory System, Which Was Induced by Ovalbumin Treatment Such as Airway Remodeling, Mucous Hypersecretion, Pulmonary Epithelial Cell Hyperplasia, and Inflammatory Cell Infiltration
3.3. Codonopsis laceolata Extract Completely Controlled the Levels of cDNA and Protein of IL-4 and IL-13 via the Inactivation of Th2 Cell Transcription Factor, GATA-3
3.4. Codonopsis laceolata Extract Induced Apoptosis of Th2 Cells and Pulmonary Epithelial Cells via the NF-κB/COX-2 Pathway
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Slejko, J.F.; Ghushchyan, V.H.; Sucher, B.; Globe, D.R.; Lin, S.L.; Globe, G.; Sullivan, P.W. Asthma control in the United States, 2008–2010: Indicators of poor asthma control. J. Allergy Clin. Immunol. 2014, 133, 1579–1587. [Google Scholar] [CrossRef]
- Ioachimescu, O.C.; Janocko, N.J.; Ciavatta, M.M.; Howard, M.; Warnock, M.V. Obstructive lung disease and obstructive sleep apnea (OLDOSA) cohort study: 10-year assessment. J. Clin. Sleep Med. 2020, 16, 267–277. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, M.H.; Kim, S.H.; Ahn, T.; Kim, S.W.; Kwak, Y.S.; Cho, I.H.; Nah, S.Y.; Cho, S.S.; Park, K.M.; et al. Korean red ginseng affects ovalbumin-induced asthma by modulating IL-12, IL-4, and IL-6 levels and the NF-κB/COX-2 and PGE2 pathways. J. Ginseng Res. 2021, 45, 482–489. [Google Scholar] [CrossRef]
- Van den Bosch, W.; James, A.L.; Tiddens, H.A.W.M. Structure and function of small airways in asthma patients revisited. Eur. Respir. Rev. 2021, 30, 200186. [Google Scholar] [CrossRef]
- World Health Organization. Asthma. Fact Sheet in 3 May 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/asthma (accessed on 22 March 2022).
- Ferrante, G.; Grutta, S.L. The burden of pediatric asthma. Front. Pediatr. 2018, 6, 186. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals. Available online: https://apps.who.int/iris/handle/10665/342703 (accessed on 22 March 2022).
- Baxi, S.N.; Phipatanakul, W. The role of allergen exposure and avoidance in asthma. Adolesc. Med. State Art Rev. 2010, 21, 57-ix. [Google Scholar]
- Kouro, T.; Takatsu, K. IL-5- and eosinophil-mediated inflammation: From discovery to therapy. Int. Immunol. 2009, 21, 1303–1309. [Google Scholar] [CrossRef]
- Monteseirin, J. Neutrophils and asthma. J. Investig. Allergol. Clin. Immunol. 2009, 19, 340–354. [Google Scholar]
- Platts-Mills, T.A.E. The role of immunoglobulin E in allergy and asthma. Am. J. Respir. Crit. Care Med. 2001, 164, S1–S5. [Google Scholar] [CrossRef]
- Zedan, M.M.; El-Chennawi, F.A.; Fouda, A.E. Interleukin-12 and peripheral blood invariant natural killer T cells as an axis in childhood asthma pathogenesis. Iran. J. Allergy Asthma Immunol. 2010, 9, 43–48. [Google Scholar]
- Bok, S.H.; Cho, S.S.; Bae, C.S.; Kang, B.; Son, H.S.; Park, D.H. Socheongryongtang modulates asthma-related changes via modulation of TNF-α and T-bet & IFN-γ in an asthma murine model. Processes 2020, 8, 1167. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Sozener, Z.C.; Akdis, M.; Akdis, C.A. The role of Treg cell subsets in allergic disease. Asian Pac. J. Allergy Immunol. 2020, 38, 139–149. [Google Scholar] [CrossRef]
- Larche, M.; Robinson, D.S.; Kay, A.B. The role of T lymphocytes in the pathogenesis of asthma. J. Allergy Clin. Immunol. 2003, 111, 450–463. [Google Scholar] [CrossRef]
- Romagnani, S. T-cell subsets (Th1 versus Th2). Ann. Allergy Asthma Immunol. 2000, 85, 9–21. [Google Scholar] [CrossRef]
- Lighvani, A.A.; Frucht, D.M.; Jankovic, D.; Yamane, H.; Aliberti, J.; Hissong, B.D.; Nguyen, B.V.; Gadina, M.; Sher, A.; Paul, W.E.; et al. T-bet is rapidly induced by interferon-γ in lymphoid and myeloid cells. Proc. Natl. Acad. Sci. USA 2001, 98, 15137–15142. [Google Scholar] [CrossRef]
- Yagi, R.; Zhu, J.; Paul, W.E. An updated view on transcription factor GATA3-mediated regulation of Th1 and Th2 cell differentiation. Int. Immunol. 2011, 23, 415–420. [Google Scholar] [CrossRef]
- Mitchell, C.; Provost, K.; Niu, N.; Homer, R.; Cohn, L. Interferon-gamma acts on the airway epithelium to inhibit local and systemic pathology in allergic airway disease. J. Immunol. 2011, 187, 3815–3820. [Google Scholar] [CrossRef]
- Chung, F. Anti-inflammatory cytokines in asthma and allergy: Interleukin-10, interleukin-12, interferon-γ. Mediat. Inflamm. 2001, 10, 51–59. [Google Scholar] [CrossRef]
- Gubernatorova, E.O.; Namakanova, O.A.; Gorshkova, E.A.; Medvedovskaya, A.D.; Nedospasov, S.A.; Drutskaya, M.S. Novel anti-cytokine strategies for prevention and treatment of respiratory allergic diseases. Front. Immunol. 2021, 12, 601842. [Google Scholar] [CrossRef]
- Walter, M.J.; Morton, J.D.; Kajiwara, N.; Agapov, E.; Holtzman, M.J. Viral induction of a chronic asthma phenotype and genetic segregation from the acute response. J. Clin. Investig. 2002, 110, 165–175. [Google Scholar] [CrossRef]
- Johnson, P.R.A.; Roth, M.; Tamm, M.; Hughes, M.; Ge, Q.; King, G.; Burgess, J.; Black, J.L. Airway smooth muscle cell proliferation is increased in asthma. Am. J. Respir. Crit. Care Med. 2001, 164, 474–477. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.P.; Tyner, J.W.; Holtzman, M.J. Apoptosis in the airways. Another balancing act in the epithelial program. Am. J. Respir. Cell Mol. Biol. 2003, 29, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Moitra, S.; Faruk, S.M.O.; Das, P.K.; Mondal, S.; Hazra, I.; Basu, A.K.; Tripathi, S.K.; Chaudhuri, S. Unravelling the apoptotic mechanisms in T-lymphocytes in an animal model for pollen induced airway allergy and studying the impact of specific immunotherapy. Immunobiology 2019, 224, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, X.; Zhu, J. Beauveria attenuates asthma by inhibiting inflammatory response and inducing lymphocytic cell apoptosis. Oncotarget 2016, 7, 74557–74568. [Google Scholar] [CrossRef][Green Version]
- Nagai, H. Recent research and developmental strategy of anti-asthma drugs. Pharmacol. Ther. 2012, 133, 70–78. [Google Scholar] [CrossRef]
- Fahy, J.V. Type 2 inflammation in asthma—Present in most, absent in many. Nat. Rev. Immunol. 2015, 15, 57–65. [Google Scholar] [CrossRef]
- Crompton, G. A brief history of inhaled asthma therapy over the last fifty years. Prim. Care Respir. J. 2006, 15, 326–331. [Google Scholar] [CrossRef]
- Blake, K.; Lima, J. Pharmacogenomics of long-acting β2-agonsits. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1733–1751. [Google Scholar] [CrossRef]
- Ciriaco, M.; Ventrice, P.; Russo, G.; Scicchitano, M.; Mazzitello, G.; Scicchitano, F.; Russo, E. Corticosteroid-related central nervous system side effects. J. Pharmacol. Pharmacother. 2013, 4 (Suppl. 1), S94–S98. [Google Scholar] [CrossRef]
- Wang, L.; Xu, M.L.; Hu, J.H.; Rasmussen, S.K.; Wang, M.H. Codonopsis lanceolate extract induces G0/G1 arrest and apoptosis in human colon tumor HT-29 cells—Involvement of ROS generation and polyamine depletion. Food Chem. Toxicol. 2011, 49, 149–154. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, K.J.; Kim, Y.H.; Kim, D.B.; Shin, G.H.; Cho, J.H.; Kim, B.K.; Lee, B.-Y.; Lee, O.-H. Codonopsis lanceolate extract prevents diet-induced obesity in C57BL/6 mice. Nutrients 2014, 6, 4663–4677. [Google Scholar] [CrossRef]
- Cha, A.; Choi, Y.; Jin, Y.; Sung, M.K.; Koo, Y.C.; Lee, K.W.; Park, T. Antilipogenic and anti-inflammatory activities of Codonopsis lanceolate in mice hepatic tissues after chronic ethanol feeding. J. Biomed. Biotechnol. 2012, 2012, 141395. [Google Scholar] [CrossRef]
- Lee, Y.G.; Kim, J.Y.; Lee, J.Y.; Byeon, S.E.; Hong, E.K.; Lee, J.; Rhee, M.H.; Park, H.J.; Cho, J.Y. Regulatory effects of Codonopsis lanceolate on macrophage-mediated immune responses. J. Ethnopharmacol. 2007, 112, 180–188. [Google Scholar] [CrossRef]
- Song, S.Y.; Bok, S.H.; Lee, S.H.; Kim, M.H.; Boo, H.O.; Kim, H.H.; Park, D.-H.; Cho, S.-S. Standardization of diploid Codonopsis laceolata root extract as an anti-hyperuricemic source. Processes 2021, 9, 2065. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cho, S.S.; Li, Y.; Bae, C.S.; Park, K.M.; Park, D.H. Anti-inflammatory effect of Curcuma longa and Allium hookeri co-treatment via NF-κB and COX-2 pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Barnes, P.J. Targeting cytokines to treat asthma and chronic obstructive pulmonary disease. Nat. Rev. Immunol. 2018, 18, 454–466. [Google Scholar] [CrossRef]
- Tarantini, F.; Baiardini, I.; Passalacqua, G.; Braido, F.; Canonica, G.W. Asthma treatment: ‘magic bullets which seek their own targets’. Allergy 2007, 62, 605–610. [Google Scholar] [CrossRef]
- Bang, M.A.; Seo, J.H.; Seo, J.W.; Jo, G.H.; Jung, S.K.; Yu, R.; Park, D.H.; Park, S.J. Bacillus subtilis KCTC 11782BP-produced alginate oligosaccharide effectively suppresses asthma via T-helper cell type 2-related cytokines. PLoS ONE 2015, 10, e0117524. [Google Scholar] [CrossRef]
- Lee, S.Y.; Bae, C.S.; Choi, Y.H.; Seo, N.S.; Na, C.S.; Yoo, J.C.; Cho, S.S.; Park, D.H. Opuntia humifusa modulates morphological changes characteristic of asthma via IL-4 and IL-13 in an asthma murine model. Food Nutr. Res. 2017, 61, 1393307. [Google Scholar] [CrossRef]
- Bok, S.H.; Seo, J.H.; Bae, C.S.; Kang, B.; Cho, S.S.; Park, D.H. Allium hookeri root extract regulates asthmatic changes through immunological modulation of Th1/Th2-related factors in an ovalbumin-induced asthma mouse model. Mol. Med. Rep. 2019, 20, 3215–3223. [Google Scholar] [CrossRef]
- Jang, T.Y.; Jung, A.Y.; Kyung, T.S.; Kim, D.Y.; Hwang, J.H.; Kim, Y.H. Anti-allergic effect of luteolin in mice with allergic asthma and rhinitis. Cent. Eur. J. Immunol. 2017, 42, 24–29. [Google Scholar] [CrossRef]
- Zhu, Z.; Homer, R.J.; Wang, Z.; Chen, Q.; Geba, G.P.; Wang, J.; Zhang, Y.; Elias, J.A. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J. Clin. Investig. 1999, 103, 779–788. [Google Scholar] [CrossRef]
- Chapoval, S.P.; Al-Garawi, A.; Lora, J.M.; Strickland, I.; Ma, B.; Lee, P.J.; Homer, R.J.; Ghosh, S.; Coyle, A.J.; Elias, J.A. Inhibition of NF-κB activation reduces the tissue effects of transgenic IL-13. J. Immunol. 2007, 179, 7030–7041. [Google Scholar] [CrossRef]
- Conde, E.; Bertrand, R.; Balbino, B.; Bonnefoy, J.; Stackowicz, J.; Caillot, N.; Colaone, F.; Hamdi, S.; Houmadi, R.; Loste, A.; et al. Dual vaccination against IL-4 and IL-13 protects against chronic allergic asthma in mice. Nat. Commun. 2021, 12, 2574. [Google Scholar] [CrossRef]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Rockland Immunochemicals, Inc. Apoptosis Pathway Antibodies. Available online: https://www.rockland.com/resources/apoptosis-pathway-antibodies (accessed on 26 April 2022).
- Lim, J.W.; Kim, H.; Kim, K.H. Nuclear factor-κB regulates cyclooxygenase-2 expression and cell proliferation in human gastric cancer cells. Lab. Investig. 2001, 81, 349–360. [Google Scholar] [CrossRef]
- Luo, J.L.; Kamata, H.; Karin, M. IKK/NF-κB signaling: Balancing life and death—A new approach to cancer therapy. J. Clin. Investig. 2005, 115, 2625–2632. [Google Scholar] [CrossRef]
- Vignola, A.M.; Chiappara, G.; Siena, L.; Bruno, A.; Gagliardo, R.; Merendino, A.M.; Polla, B.S.; Arrigo, A.; Bonsignore, G.; Bousquet, J.; et al. Proliferation and activation of bronchial epithelial cells in corticosteroid-dependent asthma. J. Allergy Clin. Immunol. 2001, 108, 738–746. [Google Scholar] [CrossRef]
- Benayoun, L.; Letuve, S.; Druilhe, A.; Boczkowski, J.; Dombret, M.C.; Mechighel, P.; Megret, J.; Leseche, G.; Aubier, M.; Pretolani, M. Regulation of peroxisome proliferator-activated receptor γ expression in human asthmatic airways: Relationship with proliferation, apoptosis, and airway remodeling. Am. J. Respir. Crit. Care Med. 2001, 164, 1487–1494. [Google Scholar] [CrossRef]
- Hoppenot, D.; Malakauskas, K.; Lavinskiene, S.; Bajoriuniene, I.; Kalinauskaite, V.; Sakalauskas, R. Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina 2015, 51, 10–17. [Google Scholar] [CrossRef]
- Geng, G.; Du, Y.; Dai, J.; Tian, D.; Xia, Y.; Fu, Z. KIF3A knockdown sensitizes bronchial epithelia to apoptosis and aggravates airway inflammation in asthma. Biomed Pharmacother. 2018, 97, 1349–1355. [Google Scholar] [CrossRef]
- Isik, S.; Karaman, M.; Micili, S.C.; Caglayan-Sozmen, S.; Bagriyanik, H.A.; Arikan-Ayyildiz, Z.; Uzuner, N. Beneficial effects of ursodeoxycholic acid via inhibition of airway remodeling, apoptosis of airway epithelia cells, and Th2 immune response in murine model of chronic asthma. Allergol. Immunopathol. 2017, 45, 339–349. [Google Scholar] [CrossRef]



| Group | Mucous Hypersecretion (0~3) | Epithelial Hyperplasia (0~3) | Inflammatory Cell Infiltration (0~3) |
|---|---|---|---|
| CON | 0.1 ± 0.08 | 0.2 ± 0.21 | 0.1 ± 0.13 |
| OVA | 2.6 ± 0.34 ** | 2.5 ± 0.21 ** | 2.6 ± 0.34 ** |
| DEX | 0.2 ± 0.11 $$ | 0.6 ± 0.25 *,$$ | 0.5 ± 0.16 *,$$ |
| 50 mg/kg/day C. laceolata | 2.6 ± 0.38 **,## | 2.3 ± 0.21 **,## | 2.4 ± 0.40 **,## |
| 150 mg/kg/day C. laceolata | 1.4 ± 0.42 **,$,##,@ | 1.3 ± 0.22 **,$$,#,@@ | 1.4 ± 0.38 **,$$,##,@ |
| 300 mg/kg/day C. laceolata | 0.5 ± 0.24 *,$$,#,@@,% | 0.9 ± 0.29 *,$$,@@,% | 0.8 ± 0.31 *,$$,@@,% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bok, S.-H.; Han, K.M.; Boo, H.-O.; Cho, S.-S.; Park, D.-H. Codonopsis laceolata Water Extract Ameliorates Asthma Severity by Inducing Th2 Cells’ and Pulmonary Epithelial Cells’ Apoptosis via NF-κB/COX-2 Pathway. Processes 2022, 10, 1249. https://doi.org/10.3390/pr10071249
Bok S-H, Han KM, Boo H-O, Cho S-S, Park D-H. Codonopsis laceolata Water Extract Ameliorates Asthma Severity by Inducing Th2 Cells’ and Pulmonary Epithelial Cells’ Apoptosis via NF-κB/COX-2 Pathway. Processes. 2022; 10(7):1249. https://doi.org/10.3390/pr10071249
Chicago/Turabian StyleBok, So-Hyeon, Kang Min Han, Hee-Ock Boo, Seung-Sik Cho, and Dae-Hun Park. 2022. "Codonopsis laceolata Water Extract Ameliorates Asthma Severity by Inducing Th2 Cells’ and Pulmonary Epithelial Cells’ Apoptosis via NF-κB/COX-2 Pathway" Processes 10, no. 7: 1249. https://doi.org/10.3390/pr10071249
APA StyleBok, S.-H., Han, K. M., Boo, H.-O., Cho, S.-S., & Park, D.-H. (2022). Codonopsis laceolata Water Extract Ameliorates Asthma Severity by Inducing Th2 Cells’ and Pulmonary Epithelial Cells’ Apoptosis via NF-κB/COX-2 Pathway. Processes, 10(7), 1249. https://doi.org/10.3390/pr10071249








