The Effect of Cu Additions on the Antibacterial Properties of Metallic Glassy Ni50TM50 (TM; Ti, Zr) Binary Systems
Abstract
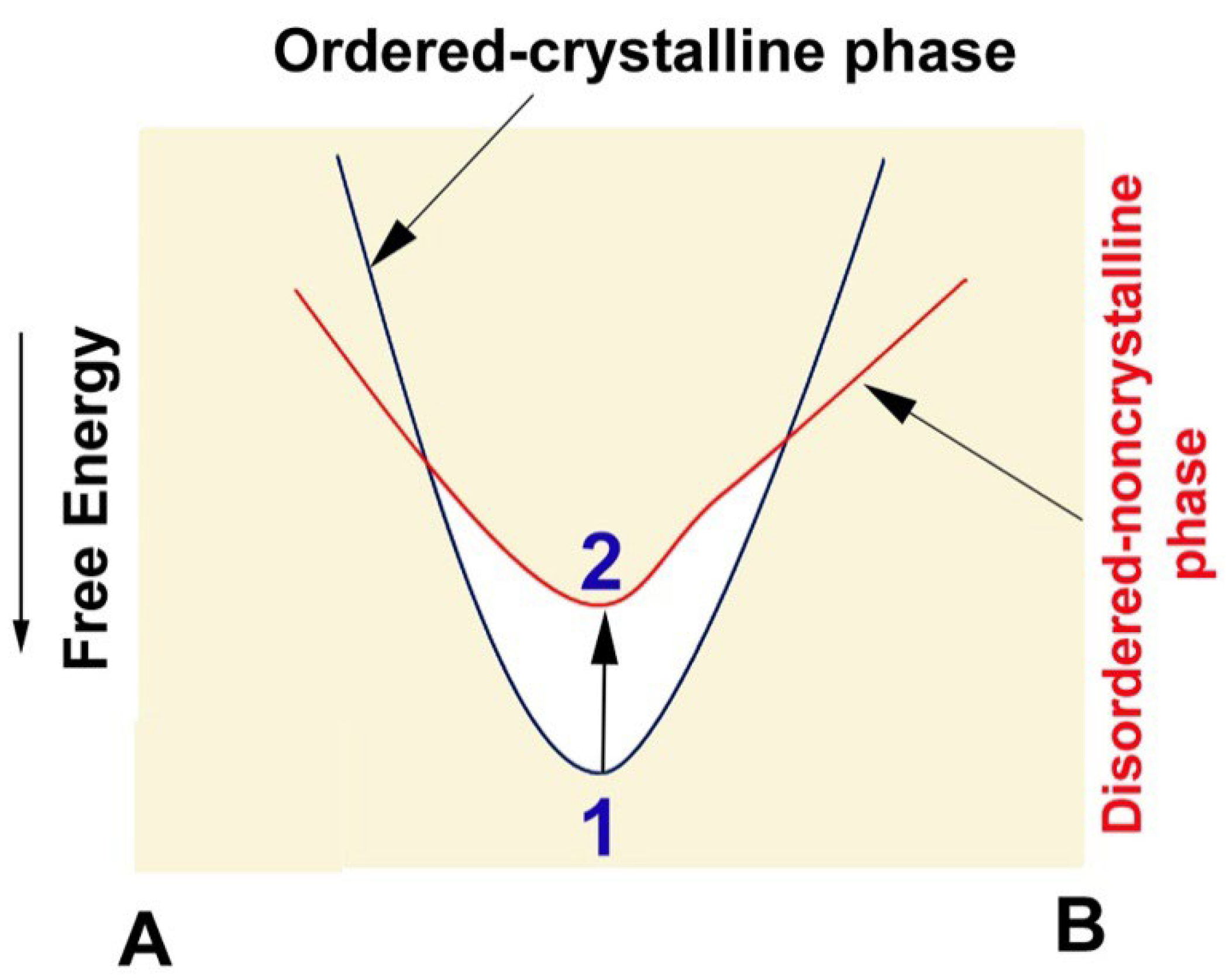
:1. Introduction
2. Materials and Methods
2.1. Preparations of the Starting Master Alloys
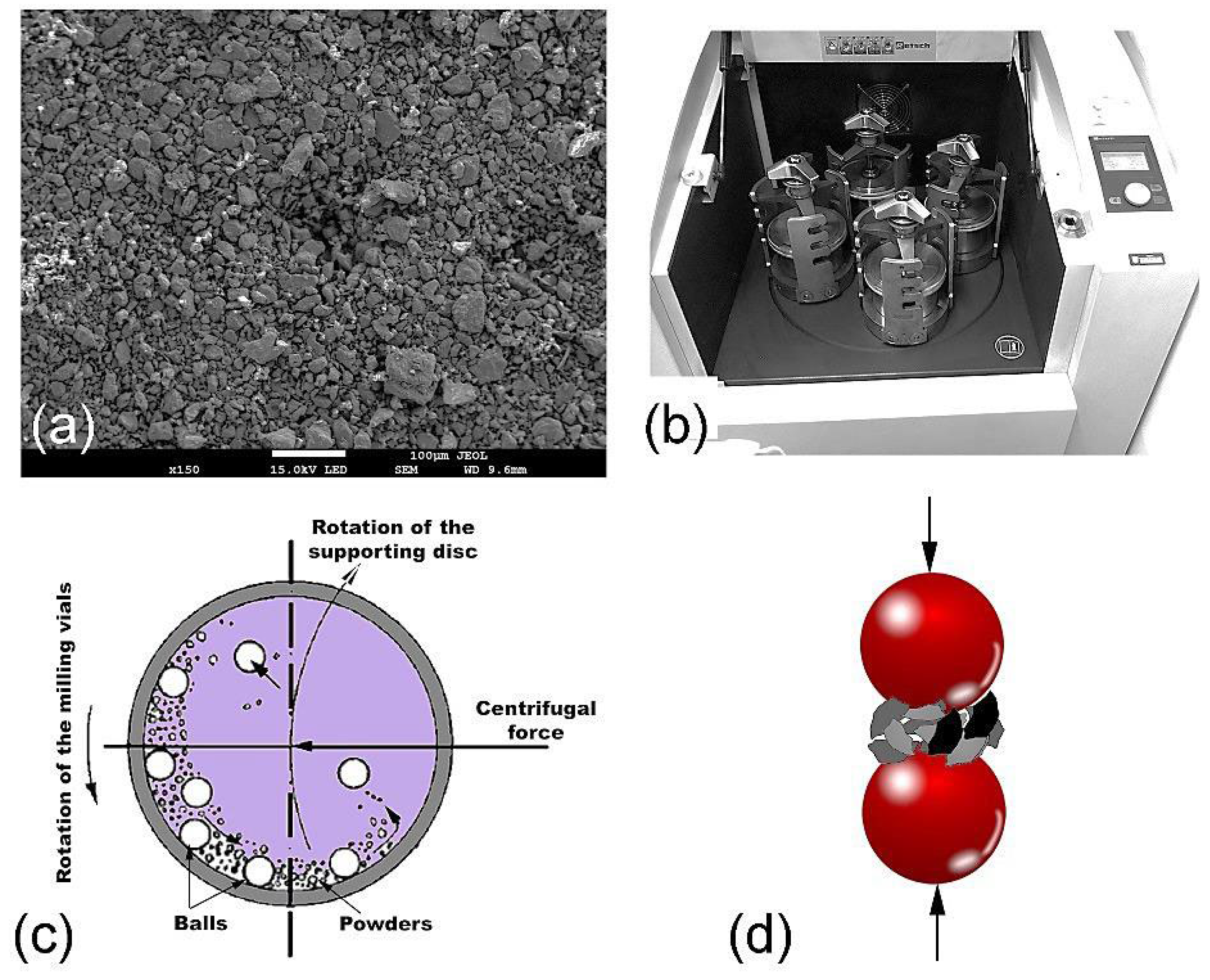
2.2. Preparations of Metallic Glassy Powders
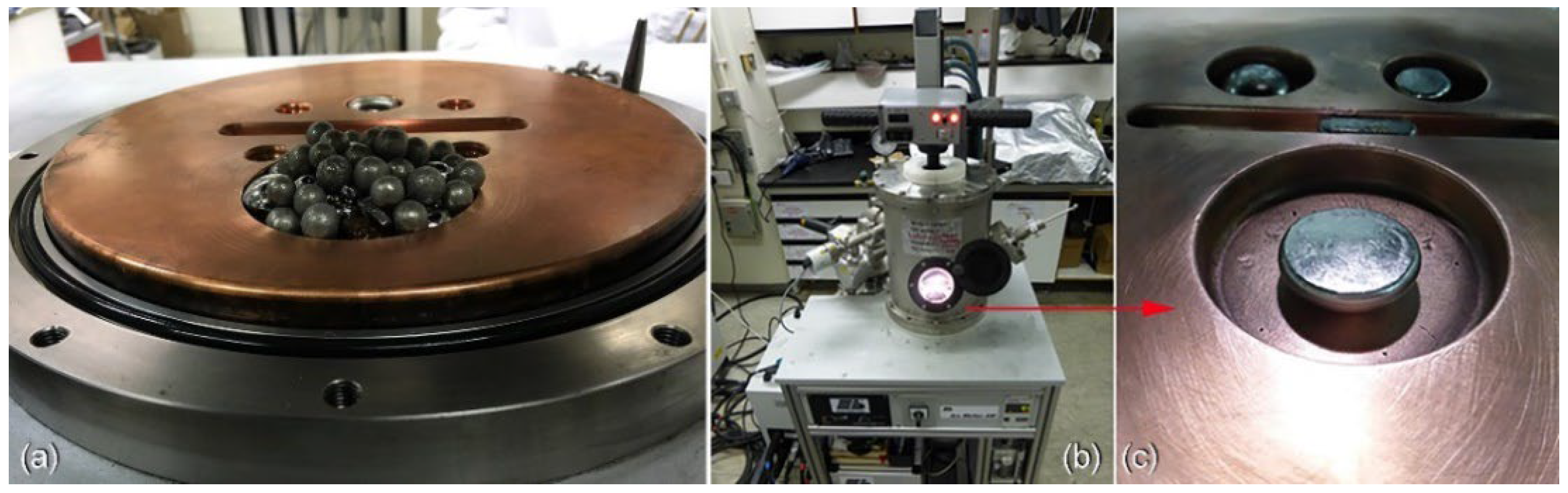
2.3. Fabrication of Metallic Glassy Powders Coated/SUS304 Composites by Cold Spray Process
2.4. Materials Characterizations
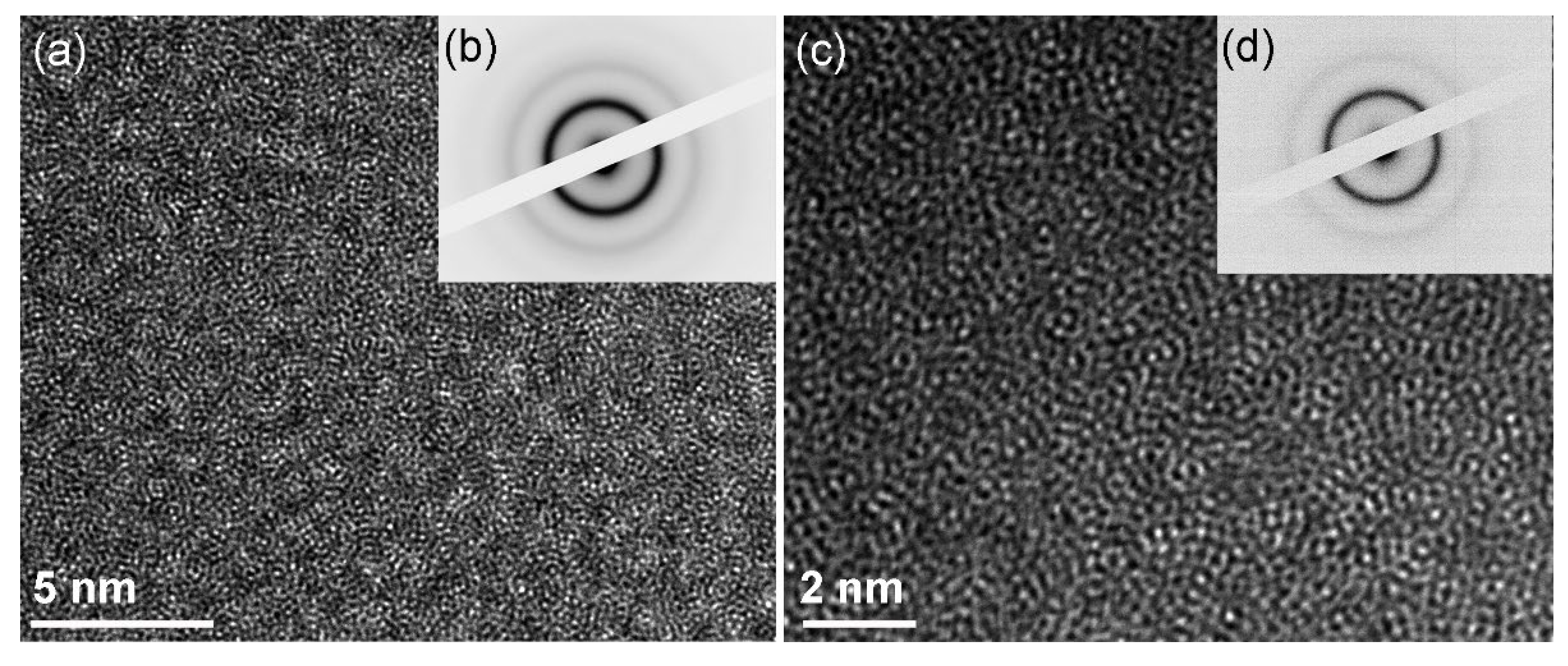
2.4.1. Structural Characterizations
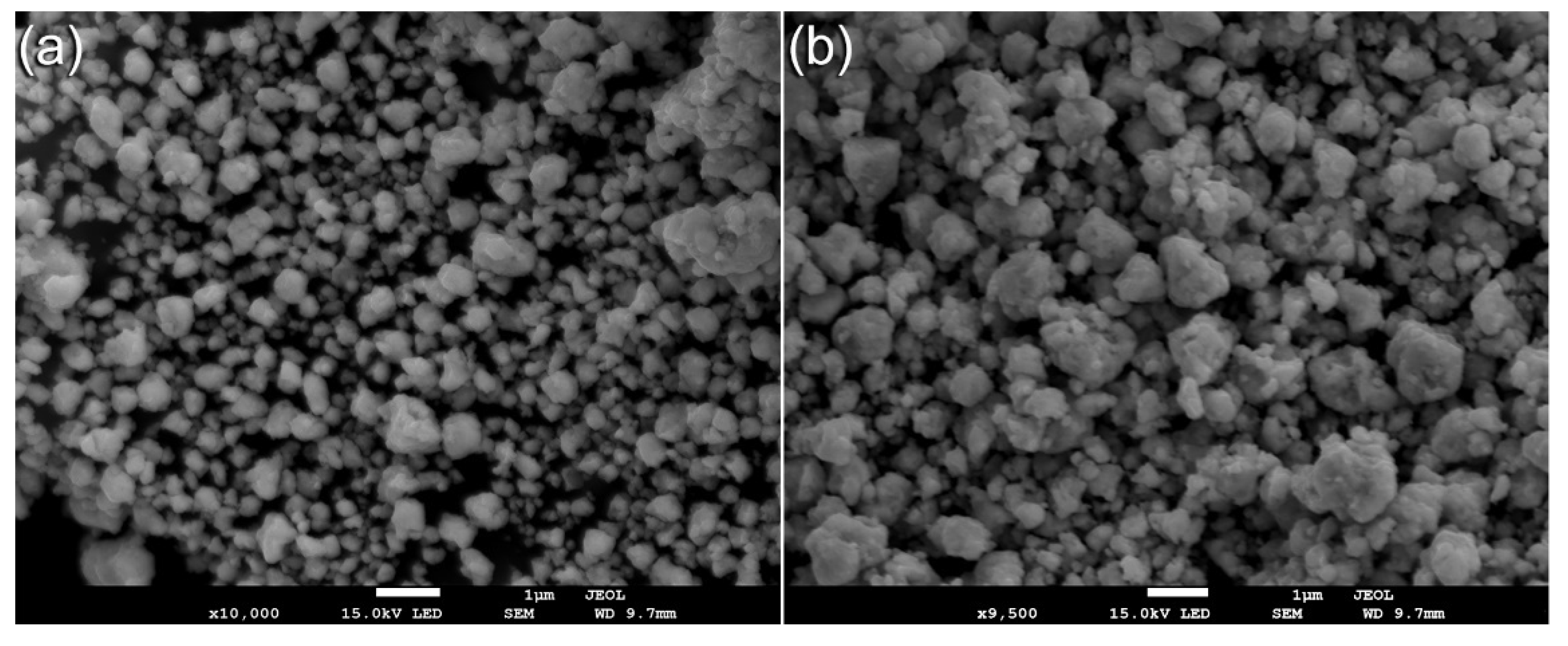
2.4.2. Morphological Characterizations and Elemental Analysis
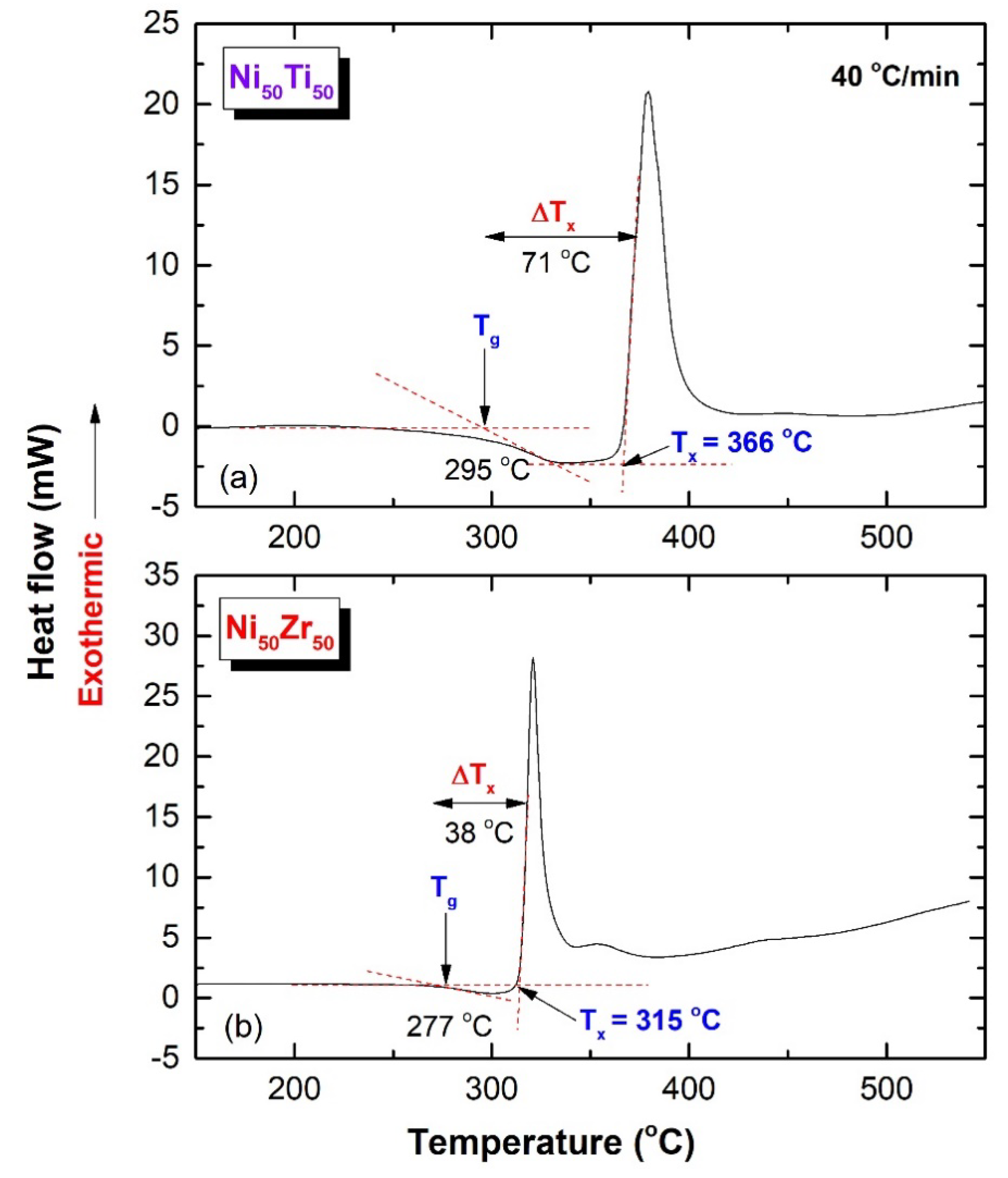
2.4.3. Thermal Stabilities
2.5. Bacterial Strain and Biofilm Growth Conditions
3. Results and Discussion
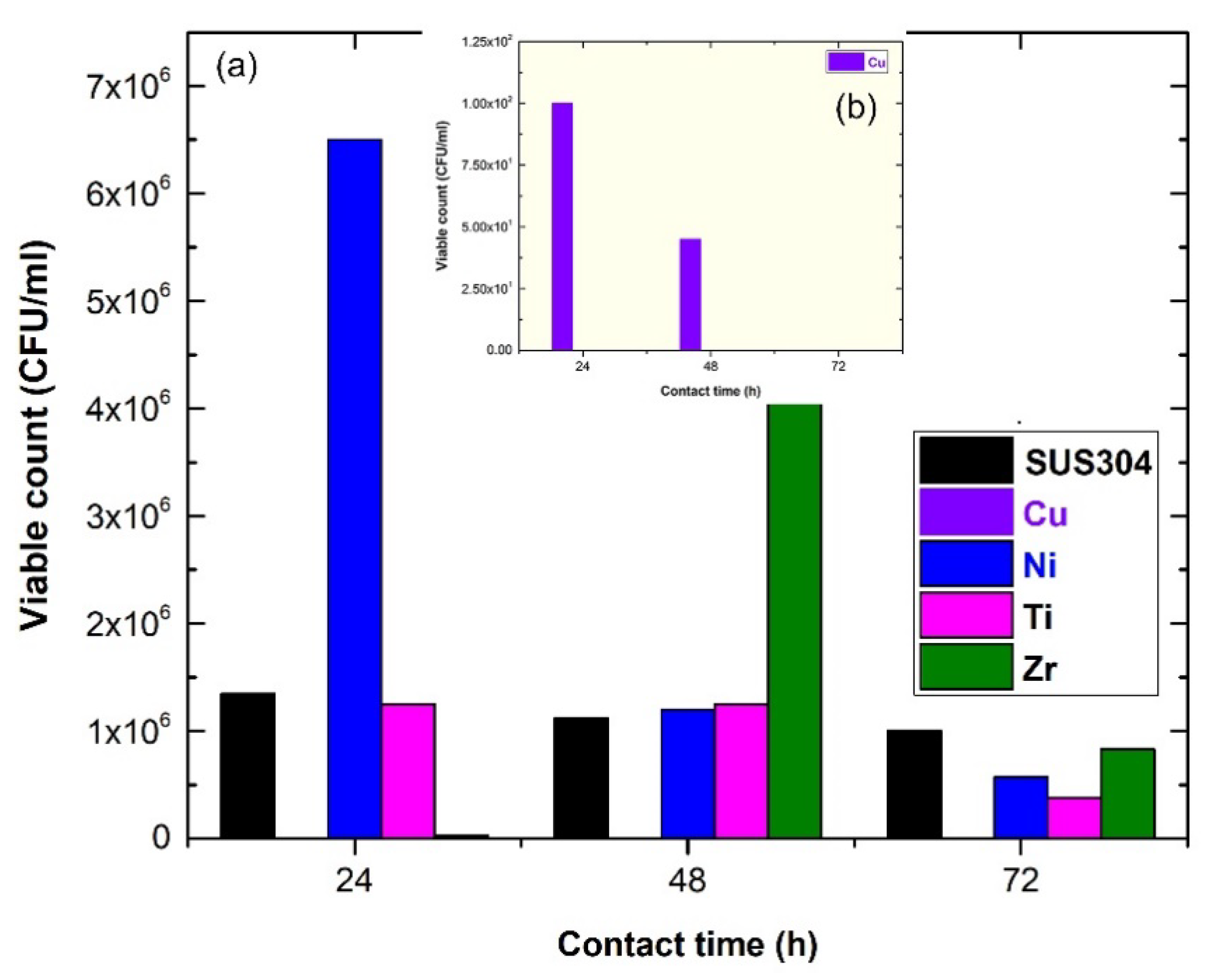
3.1. Elemental Metal Coated/SUS 304 Substrate
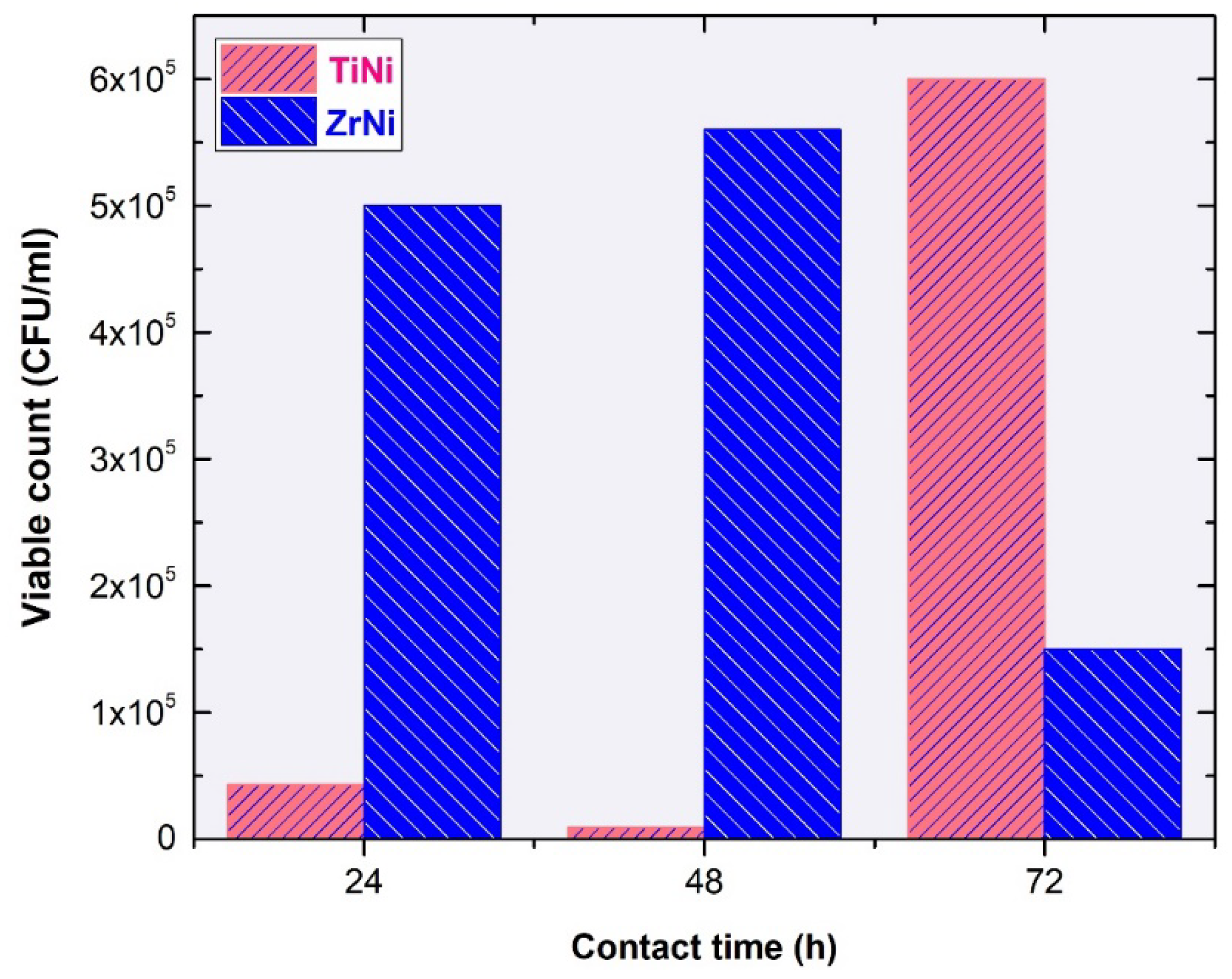
3.2. Metallic Glassy Ni50TM50 (TM; Ti, and Zr) Coated/SUS 304 Substrate
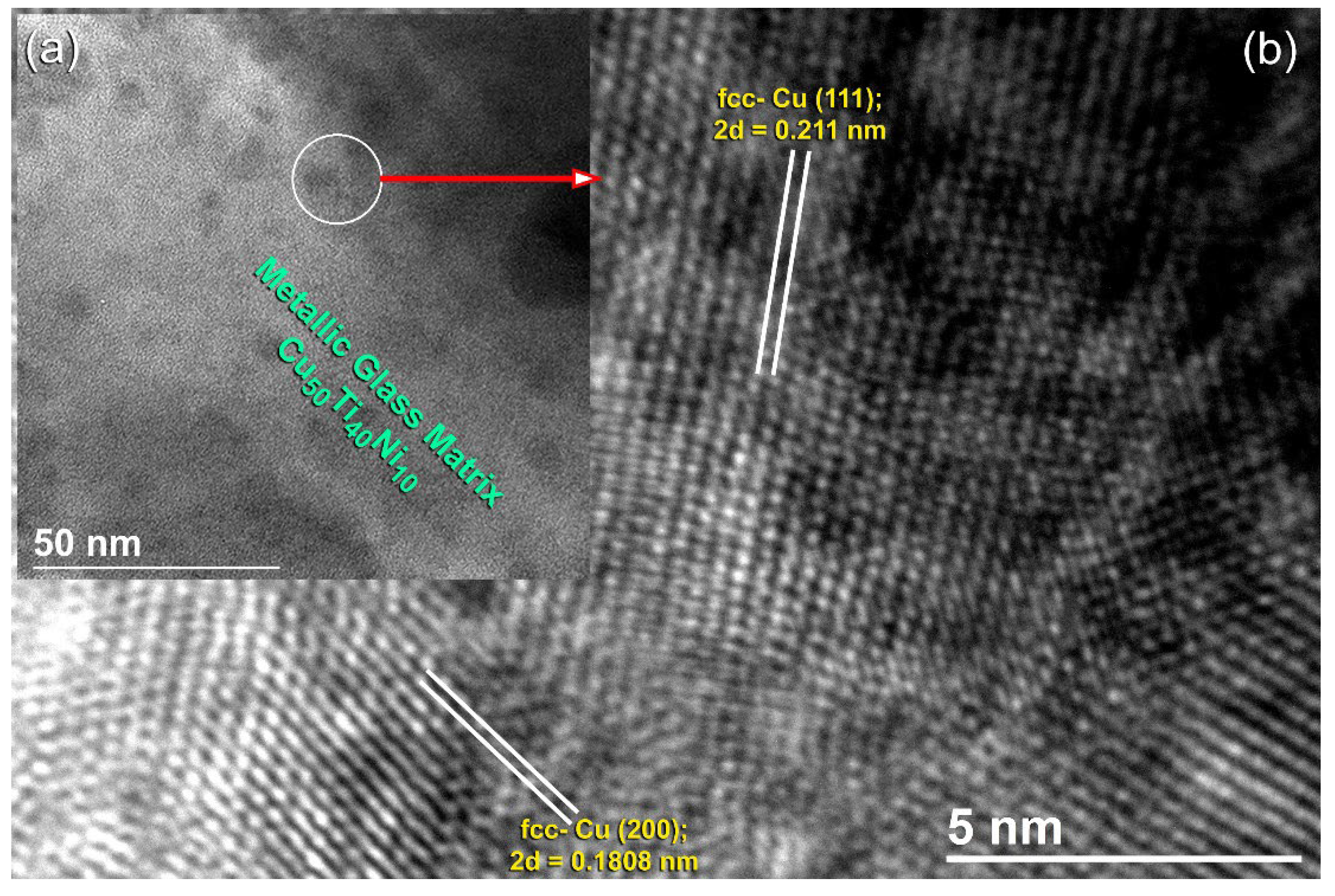
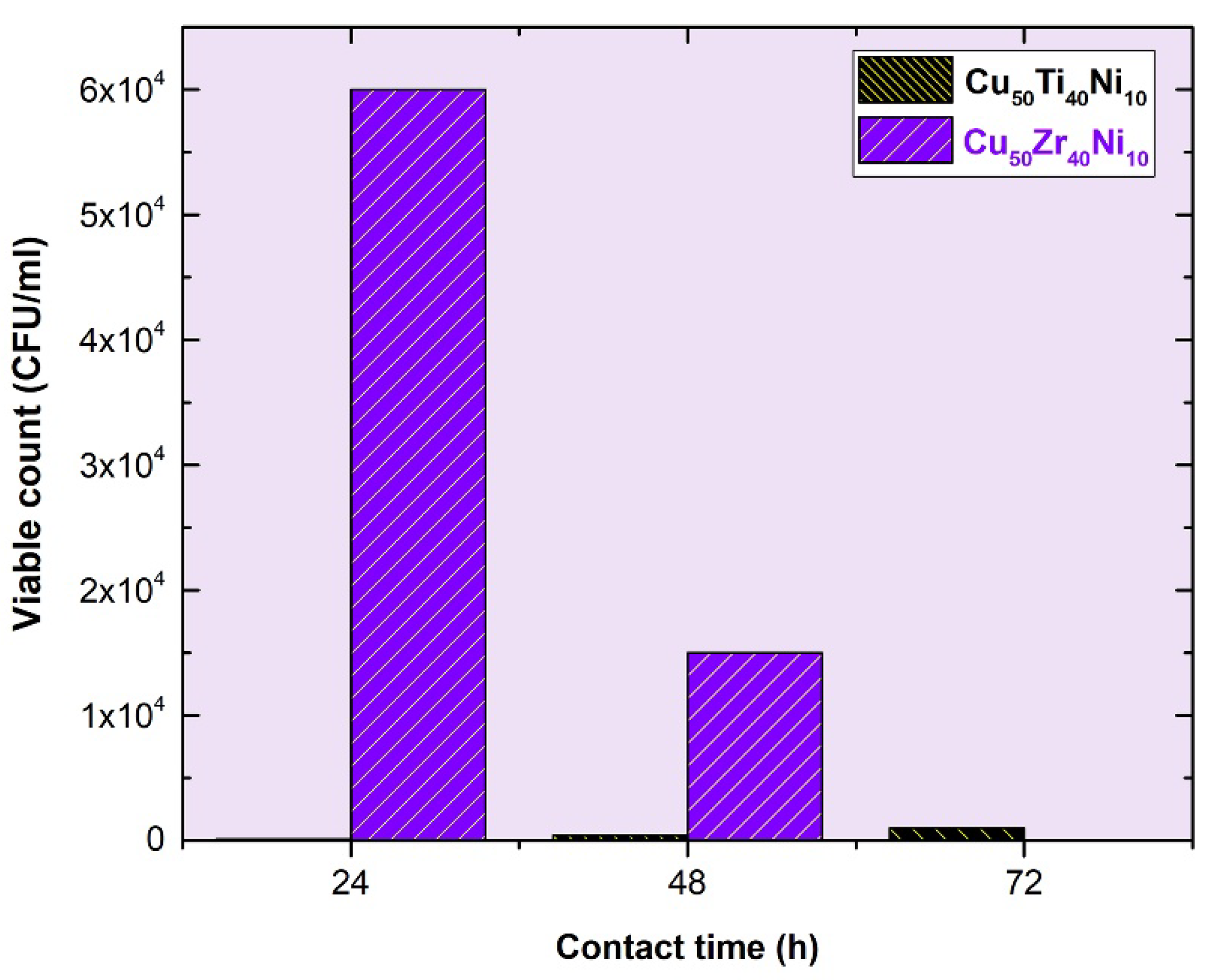
3.3. Metallic Glassy Cu50TM40Ni10 (TM; Ti, and Zr) Coated/SUS 304 Substrate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cahn, R.W.; Greer, A.L. (Eds.) Metastable states of alloys. In Physical Metallurgy, 4th ed.; Elsevier: Amsterdam, The Netherlands, 1996; pp. 1723–1830. [Google Scholar]
- Greer, A.L. New horizons for glass formation and stability. Nat. Mater. 2015, 14, 542–546. [Google Scholar] [CrossRef] [PubMed]
- El-Eskandarany, M.S. Mechanical Alloying: Energy Storage, Protective Coatings, and Medical Applications, 3rd ed.; Elsevier: Oxford, UK, 2020. [Google Scholar]
- Zhang, M.; Sun, J.; Wang, Y.; Yu, M.; Liu, F.; Ding, G.; Zhao, X.; Liu, L. Preparation of stable and durable superhydrophobic surface on Zr-based bulk metallic glass. Colloids Surf. A Physicochem. Eng. Asp. 2021, 631, 127654. [Google Scholar] [CrossRef]
- Louzguine-Luzgin, D.V.; Seki, I.; Yamamoto, T.; Kawaji, H.; Suryanarayana, C.; Inoue, A. Double-stage glass transition in a metallic glass. Phys. Rev. B 2010, 81, 144202. [Google Scholar] [CrossRef] [Green Version]
- Escobar, A.; Muzzio, N.; Moya, S.E. Antibacterial Layer-by-Layer Coatings for Medical Implants. Pharmaceutics 2020, 13, 16. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Srinivasan, R.; Santhakumari, S.; Poonguzhali, P.; Geetha, M.; Dyavaiah, M.; Xiangmin, L. Bacterial Biofilm Inhibition: A Focused Review on Recent Therapeutic Strategies for Combating the Biofilm Mediated Infections. Front. Microbiol. 2021, 12, 676458. [Google Scholar] [CrossRef]
- Bharadishettar, N.; Bhat, K.U.; Bhat Panemangalore, D. Coating technologies for copper based antimicrobial active surfaces: A perspective review. Metals 2021, 11, 711. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilm Formation: A Clinically Relevant Microbiological Process. Clin. Infect. Dis. 2001, 33, 1387–1392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol 2006, 201, 3642–3652. [Google Scholar] [CrossRef] [Green Version]
- Hickok, N.J.; Shapiro, I.M. Immobilized Antibiotics to Prevent Orthopaedic Implant infections. Adv. Drug Deliv. Rev. 2012, 64, 1165–1176. [Google Scholar] [CrossRef] [Green Version]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial Activity of Metals: Mechanisms, Molecular Targets and Applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef]
- Ren, J.; Han, P.; Wei, H.; Jia, L. Fouling-resistant behavior of silver nanoparticle-modified surfaces against the bioadhesion of microalgae. ACS Appl. Mater. Interfaces 2014, 6, 3829–3838. [Google Scholar] [CrossRef]
- Wang, X.; Ye, X.; Zhang, L.; Shao, Y.; Zhou, X.; Lu, M.; Chu, C.; Xue, F.; Bai, J. Corrosion and Antimicrobial Behavior of Stainless Steel Prepared by One-Step Electrodeposition of Silver at The Grain Boundaries. Surf. Coat. Technol 2022, 439, 128428. [Google Scholar] [CrossRef]
- Raletz, F.; Vardelle, M.; Ezo’o, G. Critical particle velocity under cold spray conditions. Surf. Coat. Technol. 2006, 201, 1942–1947. [Google Scholar] [CrossRef]
- Pan, C.H.; Zhou, Z.B.; Yu, X.W. Coatings as the useful drug delivery system for the prevention of implant-related infections. J. Orthop. Surg. Res. 2018, 13, 220. [Google Scholar] [CrossRef] [Green Version]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [Green Version]
- Wei, T.; Yu, Q.; Chen, H. Responsive and synergistic antibacterial coatings: Fighting against bacteria in a smart and effective way. Adv. Healthc. Mater. 2019, 8, 1801381. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.C.; Cavin, O.B.; McKamey, C.G.; Scarbrough, J.O. Preparation of Amorphous Ni60Nb40 by Mechanical Alloying. Appl. Phys. Lett. 1983, 43, 1017–1019. [Google Scholar] [CrossRef]
- El-Eskandarany, M.S.; Aoki, K.; Suzuki. Difference between mechanical alloying and mechanical disordering in the amorphization reaction of Al50Ta50 in a rod mill. J. Alloys Compd. 1991, 177, 229–244. [Google Scholar] [CrossRef]
- Lisoń, J.; Taratuta, A.; Paszenda, Z.; Szindler, M.; Basiaga, M. Perspectives in Prevention of Biofilm for Medical Applications. Coatings 2022, 12, 197. [Google Scholar] [CrossRef]
- Gomes, I.B.; Simões, M.; Simões, L.C. Copper Surfaces in Biofilm Control. Nanomaterials 2020, 10, 2491. [Google Scholar] [CrossRef] [PubMed]
- Mei, L.; Zheng, M.; Ye, Z.; Xia, H.; Yao, M.; Chu, X.; Wang, X.; Yang, K.; Yang, M.; Zhang, Y.; et al. Toward a molecular understanding of the antibacterial mechanism of copper-bearing titanium alloys against Staphylococcus aureus. Adv. Healthc. Mater. 2015, 5, 557–566. [Google Scholar]
- Hefeng, W.; Shu, X.; Guo, M.; Huang, D.; Li, Z.; Li, X.; Tang, B. Structural, tribological and antibacterial activities of Ti–Cu–N hard coatings prepared by plasma surface alloying technique. Surf. Coat. Technol. 2013, 235, 235–240. [Google Scholar]





| Composition (at. %) | ||
|---|---|---|
| Nominal | As-Arc-Melt | As 50 h Milling |
| Ni50Ti50 | Ni49Ti51 | Ni49.3Ti50.7 |
| Ni50Zr50 | Ni48.7Zr51.3 | Ni48.6Zr51.4 |
| Cu50Ti40Ni10 | Cu49.3Ti40.9Ni9.8 | Cu49.2Ti40.8Ni10 |
| Cu50Zr40Ni10 | Cu49.1Zr41.8Ni9.1 | Cu49.2Zr40.7Ni10.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldhameer, A.; El-Eskandarany, M.S.; Alajmi, F.; Kishk, M.; Banyan, M. The Effect of Cu Additions on the Antibacterial Properties of Metallic Glassy Ni50TM50 (TM; Ti, Zr) Binary Systems. Processes 2022, 10, 1279. https://doi.org/10.3390/pr10071279
Aldhameer A, El-Eskandarany MS, Alajmi F, Kishk M, Banyan M. The Effect of Cu Additions on the Antibacterial Properties of Metallic Glassy Ni50TM50 (TM; Ti, Zr) Binary Systems. Processes. 2022; 10(7):1279. https://doi.org/10.3390/pr10071279
Chicago/Turabian StyleAldhameer, Ahmad, Mohamed Sherif El-Eskandarany, Fahad Alajmi, Mohamed Kishk, and Mohmmad Banyan. 2022. "The Effect of Cu Additions on the Antibacterial Properties of Metallic Glassy Ni50TM50 (TM; Ti, Zr) Binary Systems" Processes 10, no. 7: 1279. https://doi.org/10.3390/pr10071279





