Carbon Dioxide Reforming of Methane over Nickel-Supported Zeolites: A Screening Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation
2.2. Characterization Techniques
2.3. Catalytic Tests
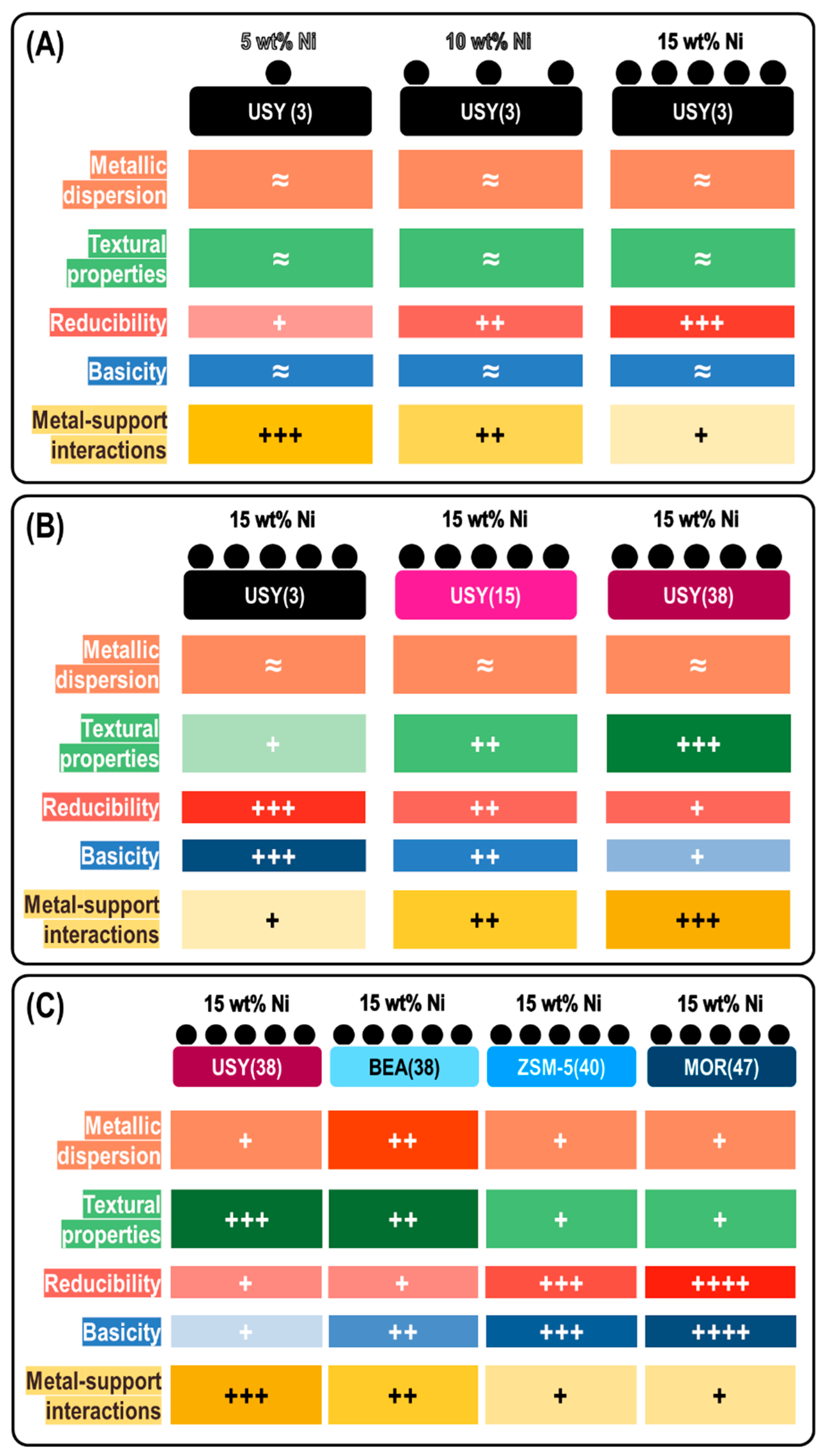
3. Results
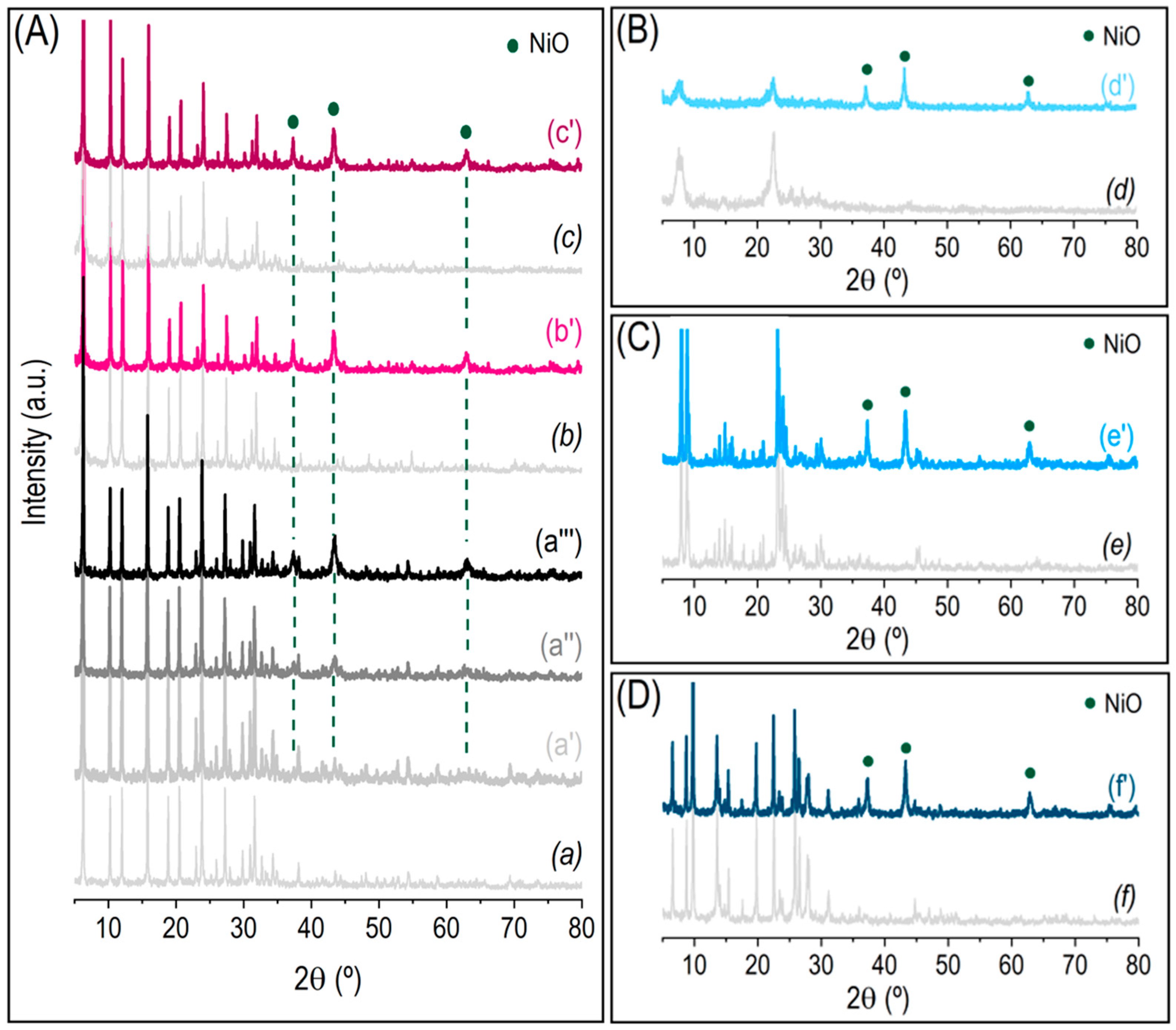
3.1. Structural and Textural Properties
3.2. Reducibility
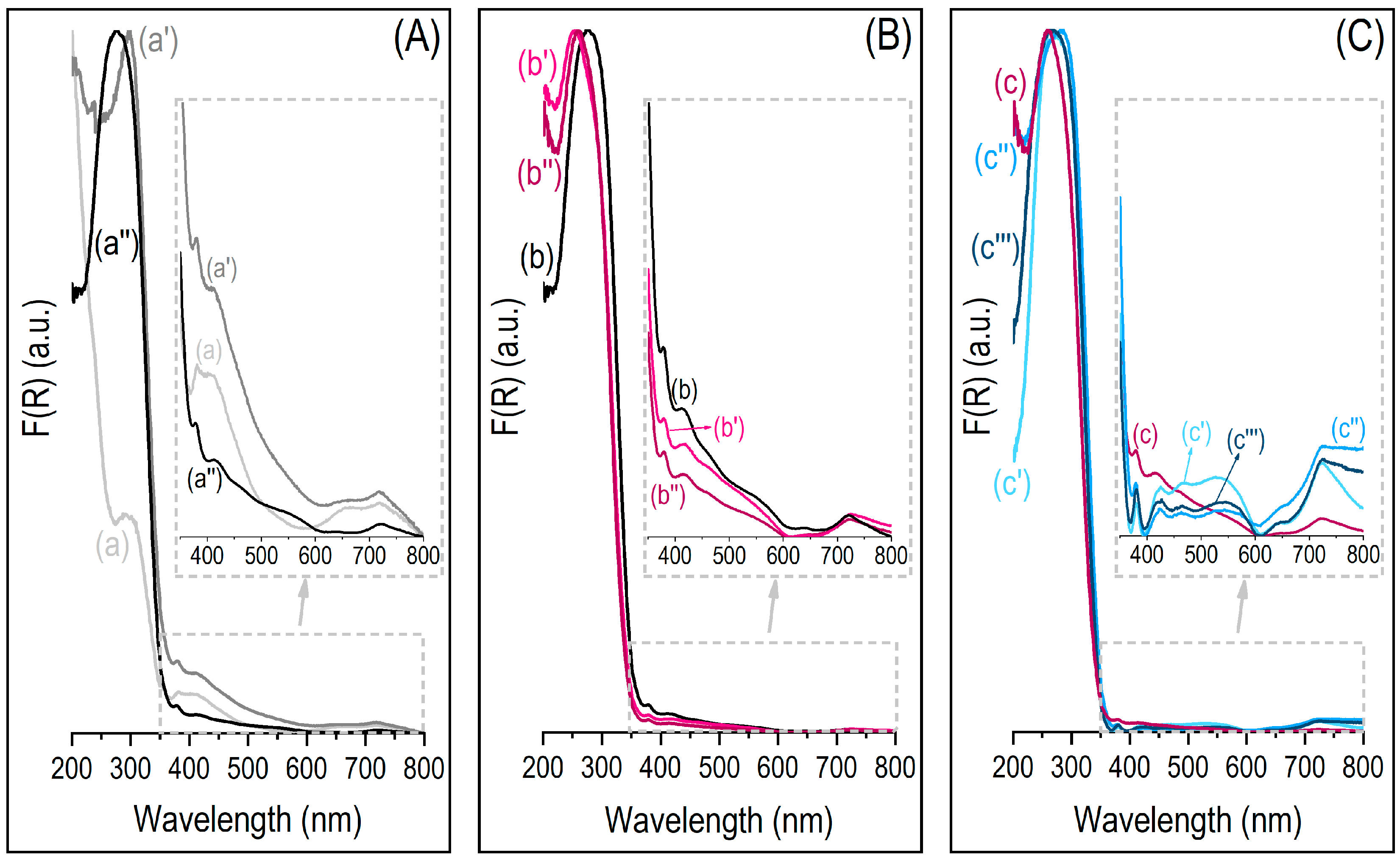
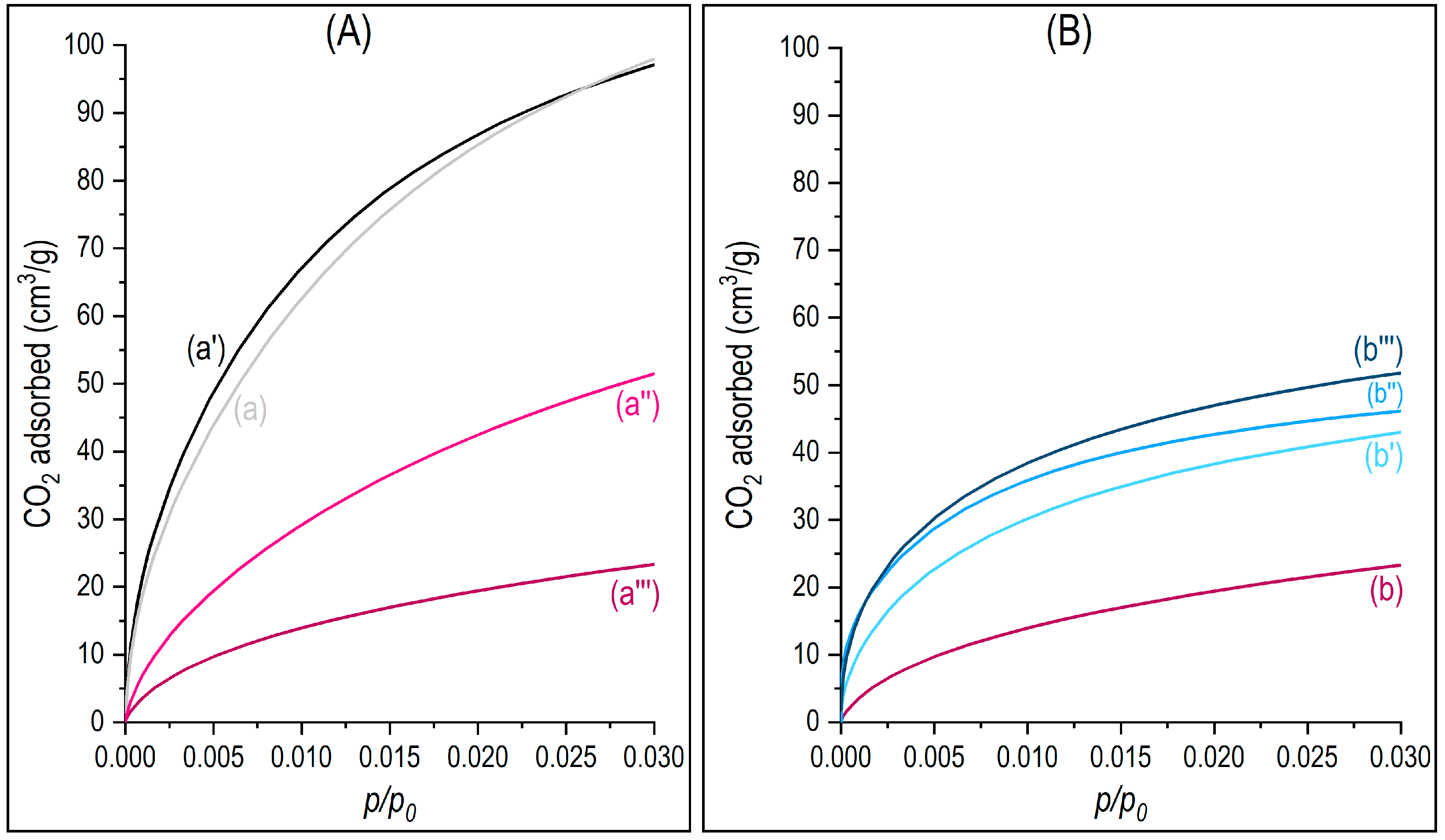
3.3. Surface Properties
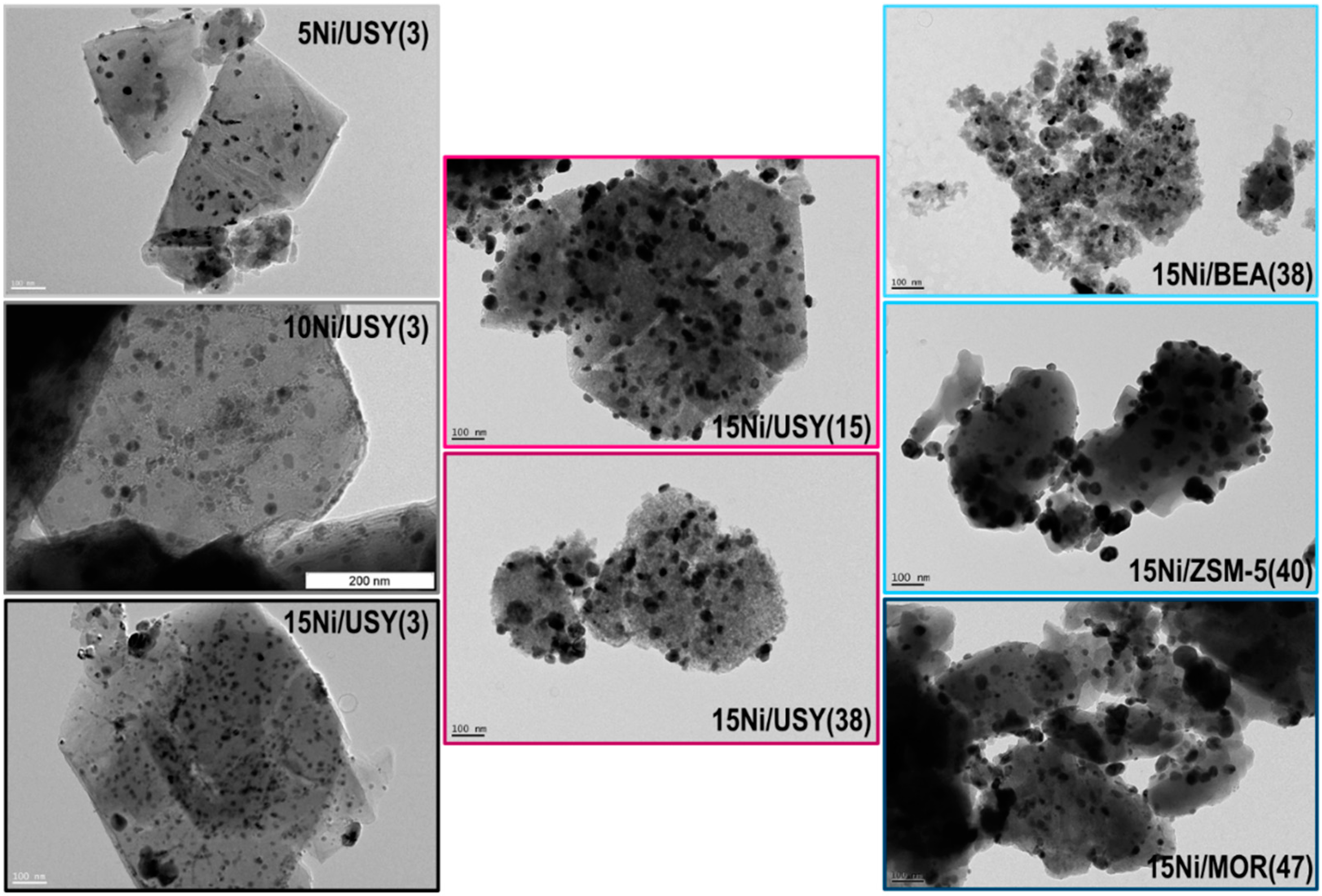
3.4. Metal Particles Size
3.5. Catalytic Performances towards DRM
3.5.1. Nickel Loading Effect
3.5.2. Si/Al Ratio Effect
3.5.3. Framework Type Effect
3.6. Comparison with Literature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kapoor, R.; Ghosh, P.; Kumar, M.; Vijay, V.K. Evaluation of Biogas Upgrading Technologies and Future Perspectives: A Review. Environ. Sci. Pollut. Res. 2019, 26, 11631–11661. [Google Scholar] [CrossRef]
- Minh, D.P.; Siang, T.J.; Vo, D.-V.N.; Phan, T.S.; Ridart, C.; Nzihou, A.; Grouset, D. Hydrogen Production from Biogas Reforming: An Overview of Steam Reforming, Dry Reforming, Dual Reforming, and Tri-Reforming of Methane; Elsevier: Amsterdam, The Netherlands, 2018; pp. 111–166. ISBN 978-0-12-811197-0. [Google Scholar]
- Singh, R.; Dhir, A.; Mohapatra, S.K.; Mahla, S.K. Dry Reforming of Methane Using Various Catalysts in the Process: Review. Biomass. Conv. Bioref. 2019, 10, 567–587. [Google Scholar] [CrossRef]
- Abdulrasheed, A.; Jalil, A.A.; Gambo, Y.; Ibrahim, M.; Hambali, H.U.; Shahul Hamid, M.Y. A Review on Catalyst Development for Dry Reforming of Methane to Syngas: Recent Advances. Renew. Sustain. Energy Rev. 2019, 108, 175–193. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A Review on Dry Reforming of Methane in Aspect of Catalytic Properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Karam, L.; Reboul, J.; Casale, S.; Massiani, P.; Hassan, N.E. Porous Nickel-Alumina Derived from Metal-Organic Framework (MIL-53): A New Approach to Achieve Active and Stable Catalysts in Methane Dry Reforming. ChemCatChem 2020, 12, 373–385. [Google Scholar] [CrossRef] [Green Version]
- Karam, L.; Reboul, J.; El Hassan, N.; Nelayah, J.; Massiani, P. Nanostructured Nickel Aluminate as a Key Intermediate for the Production of Highly Dispersed and Stable Nickel Nanoparticles Supported within Mesoporous Alumina for Dry Reforming of Methane. Molecules 2019, 24, 4107. [Google Scholar] [CrossRef] [Green Version]
- Kaydouh, M.-N.; El Hassan, N.; Davidson, A.; Casale, S.; El Zakhem, H.; Massiani, P. Effect of the Order of Ni and Ce Addition in SBA-15 on the Activity in Dry Reforming of Methane. Comptes Rendus Chim. 2015, 18, 293–301. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Chu, W.; Zhang, T.; Zhao, X.S. Synthesis, Characterization and Catalytic Performances of Ce-SBA-15 Supported Nickel Catalysts for Methane Dry Reforming to Hydrogen and Syngas. Int. J. Hydrogen Energy 2012, 37, 19–30. [Google Scholar] [CrossRef]
- Zhang, S.; Muratsugu, S.; Ishiguro, N.; Tada, M. Ceria-Doped Ni/SBA-16 Catalysts for Dry Reforming of Methane. ACS Catal. 2013, 3, 1855–1864. [Google Scholar] [CrossRef]
- Karam, L.; Casale, S.; El Zakhem, H.; El Hassan, N. Tuning the Properties of Nickel Nanoparticles inside SBA-15 Mesopores for Enhanced Stability in Methane Reforming. J. CO2 Util. 2017, 17, 119–124. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.L.; Bizkarra, K.; Gutierrez, A.M.; Arraibi, J.R.; Gartzia, L.; Bañuelos, J.; Lopez-Arbeloa, I.; Cambra, J.F. Ni and RhNi Catalysts Supported on Zeolites L for Hydrogen and Syngas Production by Biogas Reforming Processes. Chem. Eng. J. 2014, 238, 178–188. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Aloise, A.; Crea, F.; Antonucci, P.L.; Nagy, J.B.; Frusteri, F.; Giordano, G. Catalytic Dry-Reforming on Ni–Zeolite Supported Catalyst. Catal. Today 2012, 179, 52–60. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Khan, W.U.; Al-fatesh, A.S.; Abasaeed, A.E. Stabilities of Zeolite-Supported Ni Catalysts for Dry Reforming of Methane. Chin. J. Catal. 2013, 34, 764–768. [Google Scholar] [CrossRef]
- Kaengsilalai, A.; Luengnaruemitchai, A.; Jitkarnka, S.; Wongkasemjit, S. Potential of Ni Supported on KH Zeolite Catalysts for Carbon Dioxide Reforming of Methane. J. Power Sources 2007, 165, 347–352. [Google Scholar] [CrossRef]
- Nimwattanakul, W.; Luengnaruemitchai, A.; Jitkarnka, S. Potential of Ni Supported on Clinoptilolite Catalysts for Carbon Dioxide Reforming of Methane. Int. J. Hydrogen Energy 2006, 31, 93–100. [Google Scholar] [CrossRef]
- Chang, J.-S.; Park, S.-E.; Chon, H. Catalytic Activity and Coke Resistance in the Carbon Dioxide Reforming of Methane to Synthesis Gas over Zeolite-Supported Ni Catalysts. Appl. Catal. A Gen. 1996, 145, 111–124. [Google Scholar] [CrossRef]
- Crisafulli, C.; Scirè, S.; Minicò, S.; Solarino, L. Ni–Ru Bimetallic Catalysts for the CO2 Reforming of Methane. Appl. Catal. A Gen. 2002, 225, 1–9. [Google Scholar] [CrossRef]
- Halliche, D.; Cherifi, O.; Auroux, A. Microcalorimetric Studies and Methane Reforming by CO2 on Ni-Based Zeolite Catalysts. Thermochim. Acta 2005, 434, 125–131. [Google Scholar] [CrossRef]
- Pinheiro, A.N.; Valentini, A.; Sasaki, J.M.; Oliveira, A.C. Highly Stable Dealuminated Zeolite Support for the Production of Hydrogen by Dry Reforming of Methane. Appl. Catal. A Gen. 2009, 355, 156–168. [Google Scholar] [CrossRef]
- de Jesus, A.S.; Maloncy, M.L.; Batista, M.S. Effect of MgO Loading over Zeolite-Supported Ni Catalysts in Methane Reforming with Carbon Dioxide for Synthesis Gas Production. Reac. Kinet. Mech. Cat. 2017, 122, 501–511. [Google Scholar] [CrossRef]
- Moradi, G.; Khezeli, F.; Hemmati, H. Syngas Production with Dry Reforming of Methane over Ni/ZSM-5 Catalysts. J. Nat. Gas Sci. Eng. 2016, 33, 657–665. [Google Scholar] [CrossRef]
- Inoue, H.; Hatanaka, N.; Kidena, K.; Murata, S.; Nomura, M. Reforming of Methane with Carbon Dioxide over Nickel-Loaded Zeolite Catalysts. J. Jpn. Petrol. Inst. 2002, 45, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Alotaibi, R.; Alenazey, F.; Alotaibi, F.; Wei, N.; Al-Fatesh, A.; Fakeeha, A. Ni Catalysts with Different Promoters Supported on Zeolite for Dry Reforming of Methane. Appl. Petrochem. Res. 2015, 5, 329–337. [Google Scholar] [CrossRef] [Green Version]
- Frontera, P.; Macario, A.; Candamano, S.; Crea, F.; Barberio, M.; Antonucci, P.L. Alkaline-Promoted Zeolites for Methane Dry-Reforming Catalyst Preparation. Available online: https://www.ingentaconnect.com/content/asp/asl/2017/00000023/00000006/art00213;jsessionid=2ges69wt5j9w6.x-ic-live-02 (accessed on 9 April 2020).
- Dai, C.; Zhang, S.; Zhang, A.; Song, C.; Shi, C.; Guo, X. Hollow Zeolite Encapsulated Ni–Pt Bimetals for Sintering and Coking Resistant Dry Reforming of Methane. J. Mater. Chem. A 2015, 3, 16461–16468. [Google Scholar] [CrossRef]
- Frontera, P.; Aloise, A.; Macario, A.; Antonucci, P.L.; Crea, F.; Giordano, G.; Nagy, J.B. Bimetallic Zeolite Catalyst for CO2 Reforming of Methane. Top. Catal. 2010, 53, 265–272. [Google Scholar] [CrossRef]
- Frontera, P.; Aloise, A.; Macario, A.; Crea, F.; Antonucci, P.L.; Giordano, G.; Nagy, J.B. Zeolite-Supported Ni Catalyst for Methane Reforming with Carbon Dioxide. Res. Chem. Intermed. 2011, 37, 267–279. [Google Scholar] [CrossRef]
- Frontera, P.; Macario, A.; Aloise, A.; Antonucci, P.L.; Giordano, G.; Nagy, J.B. Effect of Support Surface on Methane Dry-Reforming Catalyst Preparation. Catal. Today 2013, 218–219, 18–29. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Bértolo, R.; Graça, I.; Lopes, J.M.; Henriques, C. The Effect of the Compensating Cation on the Catalytic Performances of Ni/USY Zeolites towards CO2 Methanation. J. CO2 Util. 2017, 21, 280–291. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Lopes, J.M.; Henriques, C. Enhanced Activity of CO2 Hydrogenation to CH4 over Ni Based Zeolites through the Optimization of the Si/Al Ratio. Microporous Mesoporous Mater. 2018, 267, 9–19. [Google Scholar] [CrossRef]
- Anderson, M.W.; Klinowski, J. Zeolites Treated with Silicon Tetrachloride Vapour. Part 1.—Preparation and Characterisation. J. Chem. Soc. Faraday Trans. 1986, 82, 1449–1469. [Google Scholar] [CrossRef]
- Chayed, N.F.; Badar, N.; Rusdi, R.; Kamarudin, N.; Kamarulzaman, N. Optical Band Gap Energies of Magnesium Oxide (MgO) Thin Film and Spherical Nanostructures. AIP Conf. Proc. 2011, 1400, 328–332. [Google Scholar] [CrossRef]
- Nemade, K.R.; Waghuley, S.A. Synthesis of MgO Nanoparticles by Solvent Mixed Spray Pyrolysis Technique for Optical Investigation. Available online: https://www.hindawi.com/journals/ijmet/2014/389416/ (accessed on 5 February 2018).
- Palacio, L.A.; Silva, E.R.; Catalão, R.; Silva, J.M.; Hoyos, D.A.; Ribeiro, F.R.; Ribeiro, M.F. Performance of Supported Catalysts Based on a New Copper Vanadate-Type Precursor for Catalytic Oxidation of Toluene. J. Hazard. Mater. 2008, 153, 628–634. [Google Scholar] [CrossRef]
- Mohammadijoo, M.; Naderi Khorshidi, Z.; Sadrnezhaad, S.K.; Mazinani, V. Synthesis and Characterization of Nickel Oxide Nanoparticle with Wide Band Gap Energy Prepared via Thermochemical Processing. Nanosci. Nanotechnol. Int. J. 2014, 4, 6–9. [Google Scholar]
- Ning, X.; Lu, Y.; Fu, H.; Wan, H.; Xu, Z.; Zheng, S. Template Mediated Ni(II) Dispersion in Mesoporous SiO2 for Preparation of Highly Dispersed Ni Catalysts: Influence of Template Type. ACS Appl. Mater. Interfaces 2017. [Google Scholar] [CrossRef]
- Rossetti, I.; Compagnoni, M.; Finocchio, E.; Ramis, G.; Di Michele, A.; Zucchini, A.; Dzwigaj, S. Syngas Production via Steam Reforming of Bioethanol over Ni–BEA Catalysts: A BTL Strategy. Int. J. Hydrogen Energy 2016, 41, 16878–16889. [Google Scholar] [CrossRef]
- Miller, J.T.; Mojet, B.L.; Ramaker, D.E.; Koningsberger, D.C. A New Model for the Metal–Support Interaction. Catal. Today 2000, 62, 101–114. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, H.; Xu, J.; Shen, J. Effect of Surface Acidic and Basic Properties of the Supported Nickel Catalysts on the Hydrogenation of Pyridine to Piperidine. J. Phys. Chem. C 2013, 117, 10573–10580. [Google Scholar] [CrossRef]
- Graça, I.; González, L.V.; Bacariza, M.C.; Fernandes, A.; Henriques, C.; Lopes, J.M.; Ribeiro, M.F. CO2 Hydrogenation into CH4 on NiHNaUSY Zeolites. Appl. Catal. B Environ. 2014, 147, 101–110. [Google Scholar] [CrossRef]
- Treacy, M.M.J.; Higgins, J.B. Collection of Simulated XRD Powder Patterns for Zeolites; Elsevier: Amsterdam, The Netherlands, 2007; ISBN 978-0-444-53067-7. [Google Scholar]
- Levecque, P.; Gammon, D.W.; Jacobs, P.; Vos, D.D.; Sels, B. The Use of Ultrastable Y Zeolites in the Ferrier Rearrangement of Acetylated and Benzylated Glycals. Green Chem. 2010, 12, 828–835. [Google Scholar] [CrossRef]
- Santos Marques, J.P. Preparação de Amostras Ácidas de Zeólito BEA por Modificação Pós-Síntese. Caracterização Físico-Química e Catalítica. Ph.D. Thesis, Universidade de Lisboa, Lisboa, Portugal, 2005. [Google Scholar]
- Bacariza, M.C.; Graça, I.; Westermann, A.; Ribeiro, M.F.; Lopes, J.M.; Henriques, C. CO2 Hydrogenation Over Ni-Based Zeolites: Effect of Catalysts Preparation and Pre-Reduction Conditions on Methanation Performance. Top. Catal. 2015, 59, 314–325. [Google Scholar] [CrossRef]
- Wu, C.; Dong, L.; Onwudili, J.; Williams, P.T.; Huang, J. Effect of Ni Particle Location within the Mesoporous MCM-41 Support for Hydrogen Production from the Catalytic Gasification of Biomass. ACS Sustain. Chem. Eng. 2013, 1, 1083–1091. [Google Scholar] [CrossRef]
- Zhang, J.; Xin, Z.; Meng, X.; Tao, M. Synthesis, Characterization and Properties of Anti-Sintering Nickel Incorporated MCM-41 Methanation Catalysts. Fuel 2013, 109, 693–701. [Google Scholar] [CrossRef]
- Tao, M.; Meng, X.; Lv, Y.; Bian, Z.; Xin, Z. Effect of Impregnation Solvent on Ni Dispersion and Catalytic Properties of Ni/SBA-15 for CO Methanation Reaction. Fuel 2016, 165, 289–297. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Graça, I.; Bebiano, S.S.; Lopes, J.M.; Henriques, C. Micro- and Mesoporous Supports for CO2 Methanation Catalysts: A Comparison between SBA-15, MCM-41 and USY Zeolite. Chem. Eng. Sci. 2018, 175, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Mihályi, R.M.; Lónyi, F.; Beyer, H.K.; Szegedi, Á.; Kollár, M.; Pál-Borbély, G.; Valyon, J. N-Heptane Hydroconversion over Nickel-Loaded Aluminum- and/or Boron-Containing BEA Zeolites Prepared by Recrystallization of Magadiite Varieties. J. Mol. Catal. A Chem. 2013, 367, 77–88. [Google Scholar] [CrossRef] [Green Version]
- Naresh, G.; Vijay Kumar, V.; Anjaneyulu, C.; Tardio, J.; Bhargava, S.K.; Patel, J.; Venugopal, A. Nano Size Hβ Zeolite as an Effective Support for Ni and NiCu for COx Free Hydrogen Production by Catalytic Decomposition of Methane. Int. J. Hydrogen Energy 2016, 41, 19855–19862. [Google Scholar] [CrossRef]
- Zhao, C.; Yu, Y.; Jentys, A.; Lercher, J.A. Understanding the Impact of Aluminum Oxide Binder on Ni/HZSM-5 for Phenol Hydrodeoxygenation. Appl. Catal. B Environ. 2013, 132, 282–292. [Google Scholar] [CrossRef]
- Bacariza, M.C.; Maleval, M.; Graça, I.; Lopes, J.M.; Henriques, C. Power-to-Methane over Ni/Zeolites: Influence of the Framework Type. Microporous Mesoporous Mater. 2019, 274, 102–112. [Google Scholar] [CrossRef]
- Remy, T.; Peter, S.A.; Van Tendeloo, L.; Van der Perre, S.; Lorgouilloux, Y.; Kirschhock, C.E.A.; Martens, J.A.; Xiong, Y.; Baron, G.V.; Denayer, J.F.M. Adsorption and Separation of CO2 on KFI Zeolites: Effect of Cation Type and Si/Al Ratio on Equilibrium and Kinetic Properties. Langmuir 2013, 29, 4998–5012. [Google Scholar] [CrossRef]
- Park, Y.; Moon, D.-K.; Kim, Y.-H.; Ahn, H.; Lee, C.-H. Adsorption Isotherms of CO2, CO, N2, CH4, Ar and H2 on Activated Carbon and Zeolite LiX up to 1.0 MPa. Adsorption 2014, 20, 631–647. [Google Scholar] [CrossRef]
- Prats, H.; Bahamon, D.; Alonso, G.; Giménez, X.; Gamallo, P.; Sayós, R. Optimal Faujasite Structures for Post Combustion CO2 Capture and Separation in Different Swing Adsorption Processes. J. CO2 Util. 2017, 19, 100–111. [Google Scholar] [CrossRef]
- Cheung, O.; Hedin, N. Zeolites and Related Sorbents with Narrow Pores for CO2 Separation from Flue Gas. RSC Adv. 2014, 4, 14480–14494. [Google Scholar] [CrossRef] [Green Version]
- Hedin, N.; Chen, L.; Laaksonen, A. Sorbents for CO2 Capture from Flue Gas—Aspects from Materials and Theoretical Chemistry. Nanoscale 2010, 2, 1819–1841. [Google Scholar] [CrossRef]
- Pirngruber, G.D.; Raybaud, P.; Belmabkhout, Y.; Čejka, J.; Zukal, A. The Role of the Extra-Framework Cations in the Adsorption of CO2 on Faujasite, Y. Phys. Chem. Chem. Phys. 2010, 12, 13534. [Google Scholar] [CrossRef]
- Pham, T.D.; Hudson, M.R.; Brown, C.M.; Lobo, R.F. Molecular Basis for the High CO2 Adsorption Capacity of Chabazite Zeolites. ChemSusChem 2014, 7, 3031–3038. [Google Scholar] [CrossRef]
- Alonso, G.; Prats, H.; Bahamón, D.; Keshavarz, F.; Gamallo, P.; Giménez, X.; Sayós, R. Nous mètodes de captura i separació de CO2 aplicables a processos industrials. Rev. Soc. Catalana Química 2016, 15, 21–30. [Google Scholar]
- Radlik, M.; Adamowska-Teyssier, M.; Krztoń, A.; Kozieł, K.; Krajewski, W.; Turek, W.; Da Costa, P. Dry Reforming of Methane over Ni/Ce0.62Zr0.38O2 Catalysts: Effect of Ni Loading on the Catalytic Activity and on H2/CO Production. Comptes Rendus Chim. 2015, 18, 1242–1249. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of Ni Loadings on the Activity and Coke Formation of MgO-Modified Ni/Al2O3 Nanocatalyst in Dry Reforming of Methane. J. Energy Chem. 2014, 23, 633–638. [Google Scholar] [CrossRef]
- Luengnaruemitchai, A.; Kaengsilalai, A. Activity of Different Zeolite-Supported Ni Catalysts for Methane Reforming with Carbon Dioxide. Chem. Eng. J. 2008, 144, 96–102. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Owen, R.E.; Shearing, P.R.; Maskell, W.C.; Brett, D.J.L.; Manos, G. A Study of Coke Formed by Heavy Oil Volatilization/Decomposition on Y-Zeolite. J. Anal. Appl. Pyrolysis 2019, 141, 104630. [Google Scholar] [CrossRef]
- Lu, H.; Yang, X.; Gao, G.; Wang, K.; Shi, Q.; Wang, J.; Han, C.; Liu, J.; Tong, M.; Liang, X.; et al. Mesoporous Zirconia-Modified Clays Supported Nickel Catalysts for CO and CO2 Methanation. Int. J. Hydrogen Energy 2014, 39, 18894–18907. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Guan, Y.; Xu, H.; Zhang, L.; Wu, P. Nickel/USY Catalyst Derived from a Layered Double Hydroxide/Zeolite Hybrid Structure with a High Hydrogenation Efficiency. ChemCatChem 2017, 9, 4552–4561. [Google Scholar] [CrossRef]
- Musyoka, N.M.; Petrik, L.F.; Hums, E.; Kuhnt, A.; Schwieger, W. Thermal Stability Studies of Zeolites A and X Synthesized from South African Coal Fly Ash. Res. Chem. Intermed. 2015, 41, 575–582. [Google Scholar] [CrossRef]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-Based Micro- and Mesoporous Catalysts for Dry Reforming of Methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Karam, L.; Armandi, M.; Casale, S.; El Khoury, V.; Bonelli, B.; Massiani, P.; El Hassan, N. Comprehensive Study on the Effect of Magnesium Loading over Nickel-Ordered Mesoporous Alumina for Dry Reforming of Methane. Energy Convers. Manag. 2020, 225, 113470. [Google Scholar] [CrossRef]
- Pastor-Pérez, L.; Baibars, F.; Le Sache, E.; Arellano-García, H.; Gu, S.; Reina, T.R. CO2 Valorisation via Reverse Water-Gas Shift Reaction Using Advanced Cs Doped Fe-Cu/Al2O3 Catalysts. J. CO2 Util. 2017, 21, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Mfoumou, C.M.; Mignard, S.; Belin, T. The Preferential Adsorption Sites of H2O on Adsorption Sites of CO2 at Low Temperature onto NaX and BaX Zeolites. Adsorpt. Sci. Technol. 2018, 0263617418762494. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Sun, Y.; Idem, R.O. Nickel-Based Ceria, Zirconia, and Ceria–Zirconia Catalytic Systems for Low-Temperature Carbon Dioxide Reforming of Methane. Energy Fuels 2007, 21, 3113–3123. [Google Scholar] [CrossRef]
- Gallego, G.S.; Mondragón, F.; Barrault, J.; Tatibouët, J.-M.; Batiot-Dupeyrat, C. CO2 Reforming of CH4 over La–Ni Based Perovskite Precursors. Appl. Catal. A Gen. 2006, 311, 164–171. [Google Scholar] [CrossRef]
- de Miguel, S.R.; Vilella, I.M.J.; Maina, S.P.; San José-Alonso, D.; Román-Martínez, M.C.; Illán-Gómez, M.J. Influence of Pt Addition to Ni Catalysts on the Catalytic Performance for Long Term Dry Reforming of Methane. Appl. Catal. A Gen. 2012, 435–436, 10–18. [Google Scholar] [CrossRef]
- Guo, J.; Lou, H.; Zhao, H.; Chai, D.; Zheng, X. Dry Reforming of Methane over Nickel Catalysts Supported on Magnesium Aluminate Spinels. Appl. Catal. A Gen. 2004, 273, 75–82. [Google Scholar] [CrossRef]
- Yang, R.; Xing, C.; Lv, C.; Shi, L.; Tsubaki, N. Promotional Effect of La2O3 and CeO2 on Ni/γ-Al2O3 Catalysts for CO2 Reforming of CH4. Appl. Catal. A Gen. 2010, 385, 92–100. [Google Scholar] [CrossRef]
- Roh, H.-S.; Jun, K.-W.; Baek, S.-C.; Park, S.-E. A Highly Active and Stable Catalyst for Carbon Dioxide Reforming of Methane: Ni/Ce–ZrO2/θ-Al2O3. Catal. Lett. 2002, 81, 147–151. [Google Scholar] [CrossRef]
- Özkara-Aydınoğlu, Ş.; Aksoylu, A.E. CO2 Reforming of Methane over Pt–Ni/Al2O3 Catalysts: Effects of Catalyst Composition, and Water and Oxygen Addition to the Feed. Int. J. Hydrogen Energy 2011, 36, 2950–2959. [Google Scholar] [CrossRef]
- Arbag, H.; Yasyerli, S.; Yasyerli, N.; Dogu, G. Activity and Stability Enhancement of Ni-MCM-41 Catalysts by Rh Incorporation for Hydrogen from Dry Reforming of Methane. Int. J. Hydrogen Energy 2010, 35, 2296–2304. [Google Scholar] [CrossRef]
- Yu, M.; Zhu, Y.-A.; Lu, Y.; Tong, G.; Zhu, K.; Zhou, X. The Promoting Role of Ag in Ni-CeO2 Catalyzed CH4-CO2 Dry Reforming Reaction. Appl. Catal. B Environ. 2015, 165, 43–56. [Google Scholar] [CrossRef]
- Liu, D.; Quek, X.Y.; Cheo, W.N.E.; Lau, R.; Borgna, A.; Yang, Y. MCM-41 Supported Nickel-Based Bimetallic Catalysts with Superior Stability during Carbon Dioxide Reforming of Methane: Effect of Strong Metal–Support Interaction. J. Catal. 2009, 266, 380–390. [Google Scholar] [CrossRef]
- Albarazi, A.; Beaunier, P.; Da Costa, P. Hydrogen and Syngas Production by Methane Dry Reforming on SBA-15 Supported Nickel Catalysts: On the Effect of Promotion by Ce0.75Zr0.25O2 Mixed Oxide. Int. J. Hydrogen Energy 2013, 38, 127–139. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Tsubaki, N.; Tan, Y.; Han, Y. Carbon Dioxide Reforming of Methane over Ni Nanoparticles Incorporated into Mesoporous Amorphous ZrO2 Matrix. Fuel 2015, 147, 243–252. [Google Scholar] [CrossRef]




| Code | Preparation Method | Na Loading 1 (wt.%) | Si/Alglobal 1 | Ni 1 (wt.%) |
|---|---|---|---|---|
| USY(3) | IE 2 | 2.7 | 3 | - |
| 5Ni/USY(3) | IWI 3 | 2.7 | 3 | 5 |
| 10Ni/USY(3) | IWI 3 | 2.7 | 3 | 10 |
| 15Ni/USY(3) | IWI 3 | 2.7 | 3 | 15 |
| USY(15) | IE 2 | 0.8 | 15 | - |
| 15Ni/USY(15) | IWI 3 | 0.8 | 15 | 15 |
| USY(38) | IE 2 | 0.3 | 38 | - |
| 15Ni/USY(38) | IWI 3 | 0.3 | 38 | 15 |
| BEA(38) | IE 2 | 0.3 | 38 | - |
| 15Ni/BEA(38) | IWI 3 | 0.3 | 38 | 15 |
| ZSM-5(40) | IE 2 | 0.7 | 40 | - |
| 15Ni/ZSM-5(40) | IWI 3 | 0.7 | 40 | 15 |
| MOR(47) | IE 2 | 0.7 | 47 | - |
| 15Ni/MOR(47) | IWI 3 | 0.7 | 47 | 15 |
| Catalyst | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Sext (m2 g−1) |
|---|---|---|---|
| USY(3) | 0.30 | 0.04 | 17 |
| 5Ni/USY(3) | 0.28 | 0.03 | 13 |
| 10Ni/USY(3) | 0.21 | 0.03 | 14 |
| 15Ni/USY(3) | 0.22 | 0.03 | 14 |
| USY(15) | 0.25 | 0.27 | 250 |
| 15Ni/USY(15) | 0.19 | 0.19 | 159 |
| USY(38) | 0.20 | 0.29 | 325 |
| 15Ni/USY(38) | 0.19 | 0.19 | 165 |
| BEA(38) | 0.15 | 0.59 | 203 |
| 15Ni/BEA(38) | 0.11 | 0.36 | 51 |
| ZSM-5(40) | 0.11 | 0.10 | 140 |
| 15Ni/ZSM-5(40) | 0.09 | 0.07 | 99 |
| MOR(47) | 0.17 | 0.11 | 77 |
| 15Ni/MOR(47) | 0.14 | 0.08 | 57 |
| Catalyst | Calcined | Reduced at 470 °C | |||
|---|---|---|---|---|---|
| h Index | BGNiO (eV) | Ni0 size (nm) | Standard Deviation (nm) | Dispersion (%) | |
| 5Ni/USY(3) | 0.52 | 3.7 | 19 | 7 | 5 |
| 10Ni/USY(3) | 0.60 | 3.7 | 18 | 7 | 5 |
| 15Ni/USY(3) | 0.61 | 3.7 | 18 | 7 | 5 |
| 15Ni/USY(15) | 0.86 | 3.8 | 28 | 11 | 3 |
| 15Ni/USY(38) | 0.91 | 3.8 | 25 | 9 | 3 |
| 15Ni/BEA(38) | 0.78 | 3.7 | 18 | 6 | 5 |
| 15Ni/ZSM-5(40) | 0.76 | 3.7 | 24 | 10 | 4 |
| 15Ni/MOR(47) | 0.84 | 3.7 | 23 | 12 | 4 |
| Catalyst | Ni (wt.%) | Temperature (°C) | GHSV (L·g−1·h−1) | Time (h) | Initial X (%) | Final X (%) | H2:CO | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| CH4 | CO2 | CH4 | CO2 | |||||||
| Ni/ZrO2 | 13 | 700 | 38 | n.a. | 50 | n.a. | 30 | n.a. | n.a. | [73] |
| Ni/La2O3 | 5 | 700 | 300 | 15 | n.a. | n.a. | 59 | 73 | 0.66 | [74] |
| Ni/Al2O3 | 10 | 700 | n.a. | 108 | 68 | 92 | 68 | 90 | 0.6 | [75] |
| Ni/γ-Al2O3 | 10 | 750 | 50 | 10 | 61 | 63 | 32 | 29 | 0.88 | [76] |
| Ni/γ-Al2O3 | 15 | 700 | 12 | 8 | 65 | 82 | n.a. | n.a. | 0.92 | [77] |
| Ni/θ-Al2O3 | 3 | 800 | 60 | 20 | 98 | n.a. | 62 | n.a. | n.a. | [78] |
| Ni/Al2O3 | 10 | 650 | 16 | 4 | 40 | n.a. | 0 | n.a. | n.a. | [79] |
| Ni/Zeolite A | 7 | 700 | 30 | 5 | 70 | 80 | 13 | 20 | 0.31 | [64] |
| Ni/Zeolite X | 7 | 85 | 85 | 72 | 80 | 1.27 | ||||
| Ni/ZSM-5 | 7 | 90 | 92 | 58 | 70 | 1.03 | ||||
| Ni/MCM-41 | n.a. | 600 | 36 | 14 | 29 | 42 | 20 | 33 | n.a. | [80] |
| Ni/CeO2 | 10 | 760 | 14 | 100 | 82 | 90 | 65 | 80 | 0.9 | [81] |
| Ni/MCM-41 | 2 | 750 | 50 | 72 | 94 | n.a. | 50 | n.a. | 0.9 | [82] |
| Ni/SBA-15 | 10 | 600 | 20 | 30 | 52 | 87 | 39 | 70 | 0.8 | [83] |
| Ni/ZrO2 | 15 | 750 | 24 | 10 | 90 | 98 | 69 | 70 | n.a. | [84] |
| Ni/MgAl2O4 | 10 | 750 | 50 | 10 | 84 | 92 | 84 | 92 | 0.98 | [76] |
| Ni/USY | 15 | 650 | 72 | 12 | 66 | 67 | 51 | 54 | 1.0 | This work |
| Ni/MOR | 15 | 69 | 69 | 61 | 58 | 1.1 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bacariza, C.; Karam, L.; El Hassan, N.; Lopes, J.M.; Henriques, C. Carbon Dioxide Reforming of Methane over Nickel-Supported Zeolites: A Screening Study. Processes 2022, 10, 1331. https://doi.org/10.3390/pr10071331
Bacariza C, Karam L, El Hassan N, Lopes JM, Henriques C. Carbon Dioxide Reforming of Methane over Nickel-Supported Zeolites: A Screening Study. Processes. 2022; 10(7):1331. https://doi.org/10.3390/pr10071331
Chicago/Turabian StyleBacariza, Carmen, Leila Karam, Nissrine El Hassan, José M. Lopes, and Carlos Henriques. 2022. "Carbon Dioxide Reforming of Methane over Nickel-Supported Zeolites: A Screening Study" Processes 10, no. 7: 1331. https://doi.org/10.3390/pr10071331










