Research on Mobile Machinery NOx Emission Control Based on a Physical Model and Closed-Loop Control
Abstract
:1. Introduction
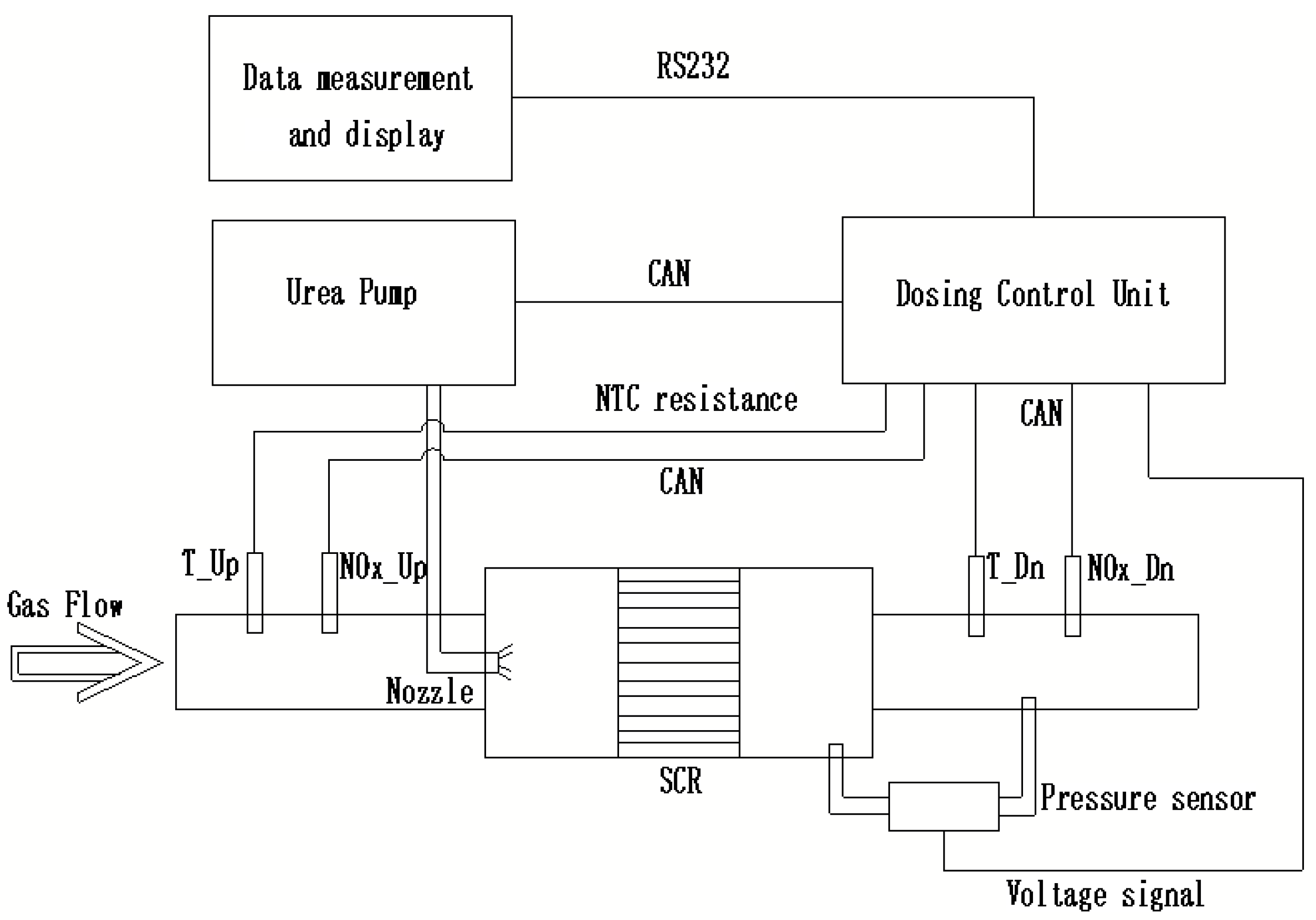
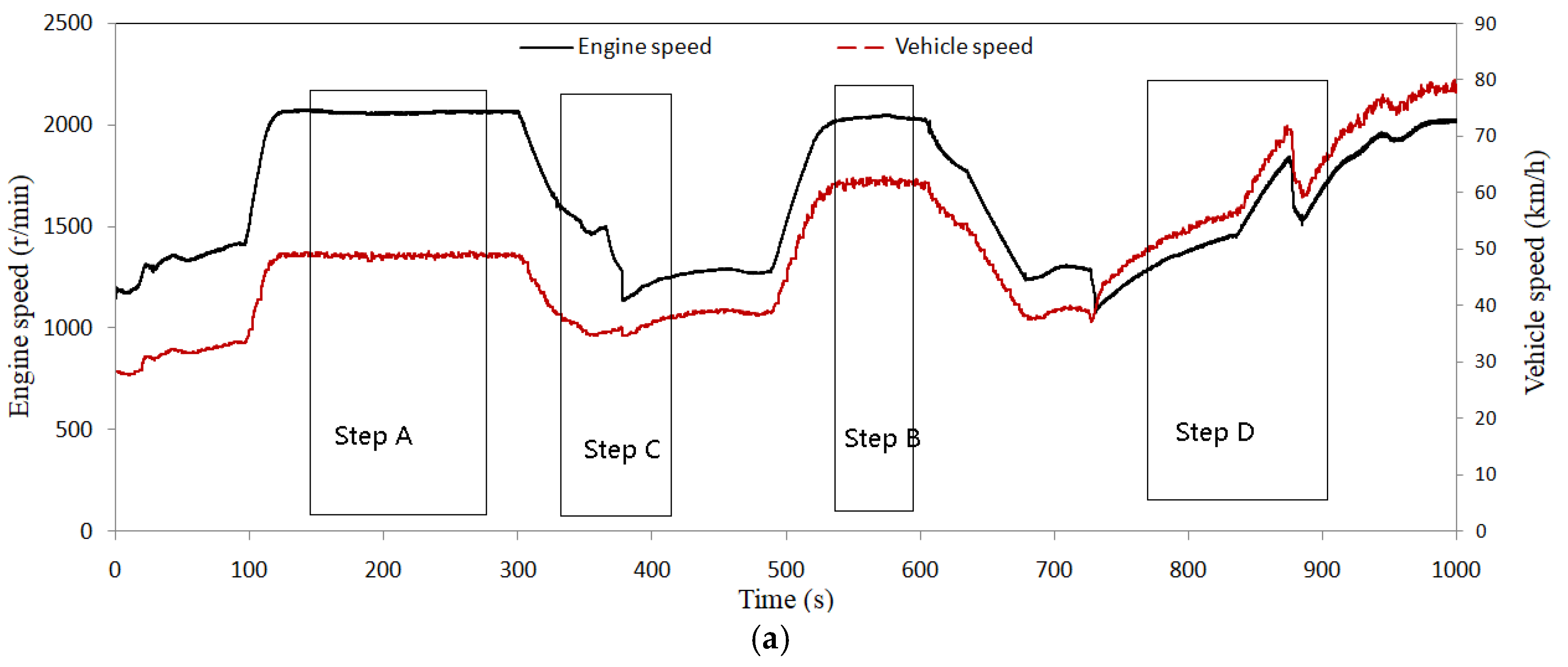
2. Scheme and Experimental Design
3. Physical Model and Classical Control Algorithm
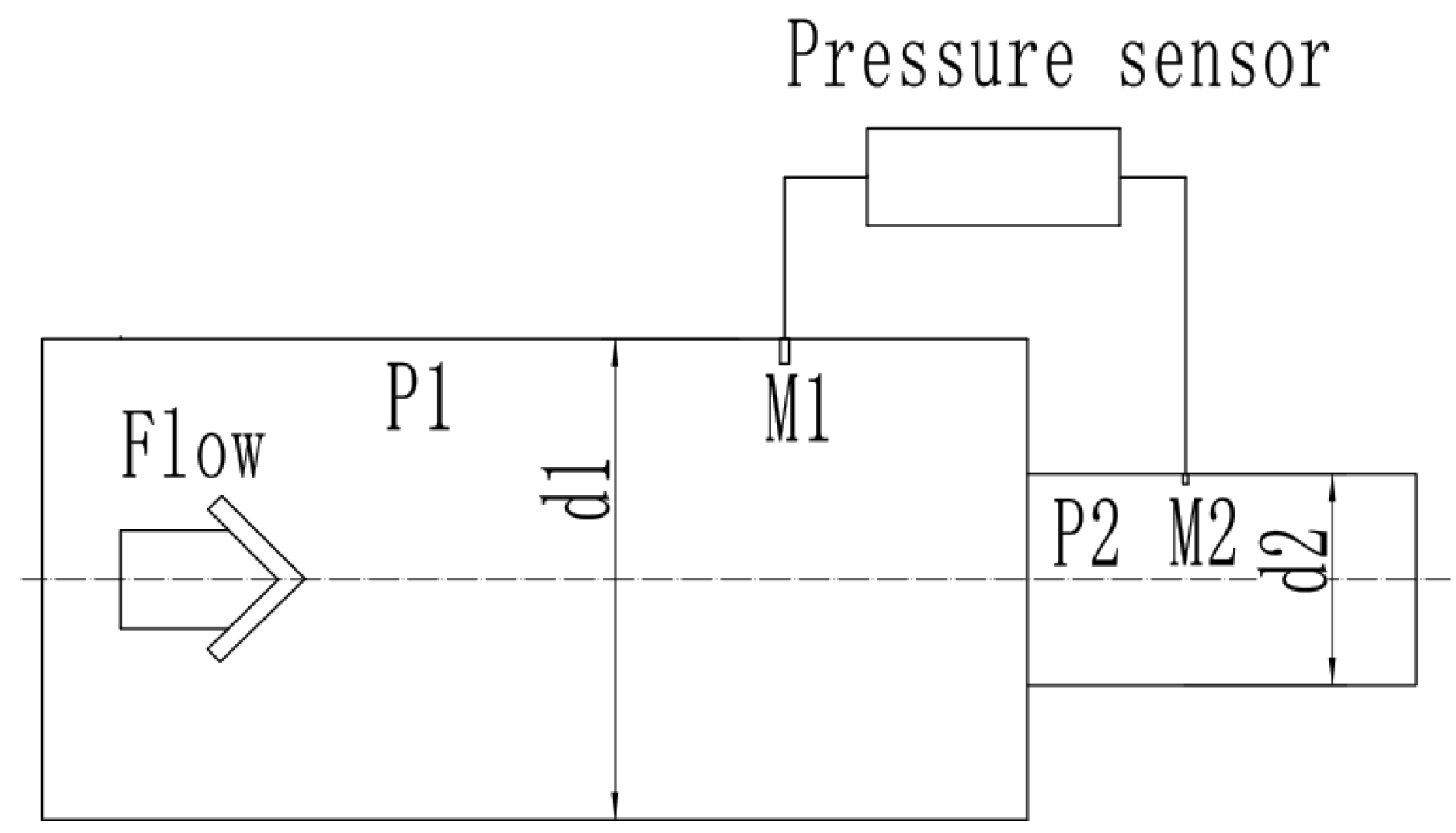
3.1. Calculation of Exhaust Flow Based on Differential Pressure
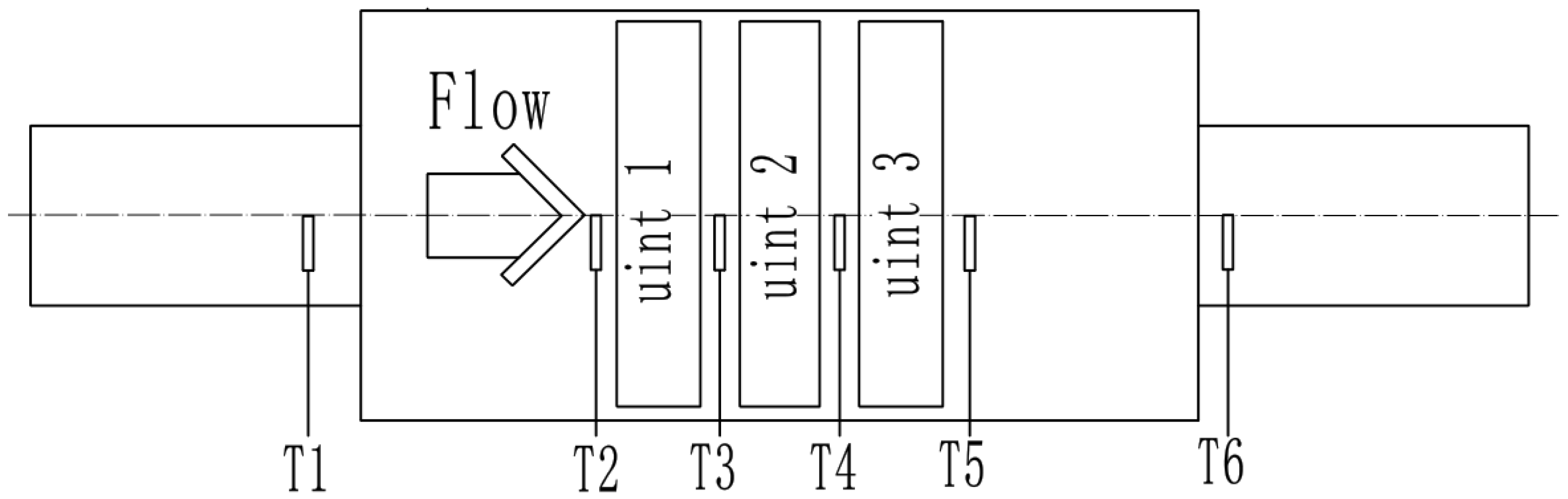
3.2. Calculation of the Internal Temperature of the Catalytic Converter
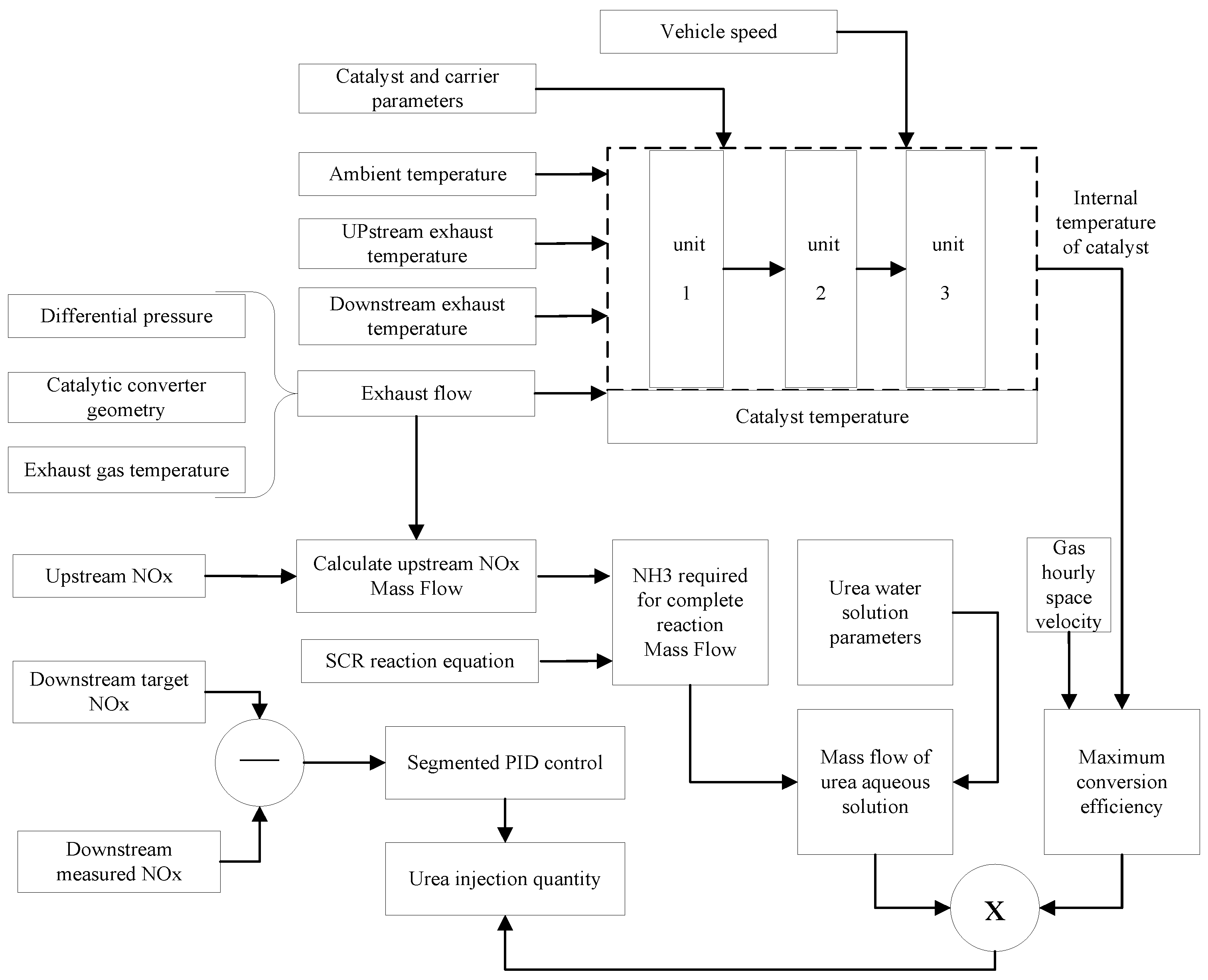
3.3. Closed-Loop Control Method
3.4. Closed-Loop Control Method
4. Discussion
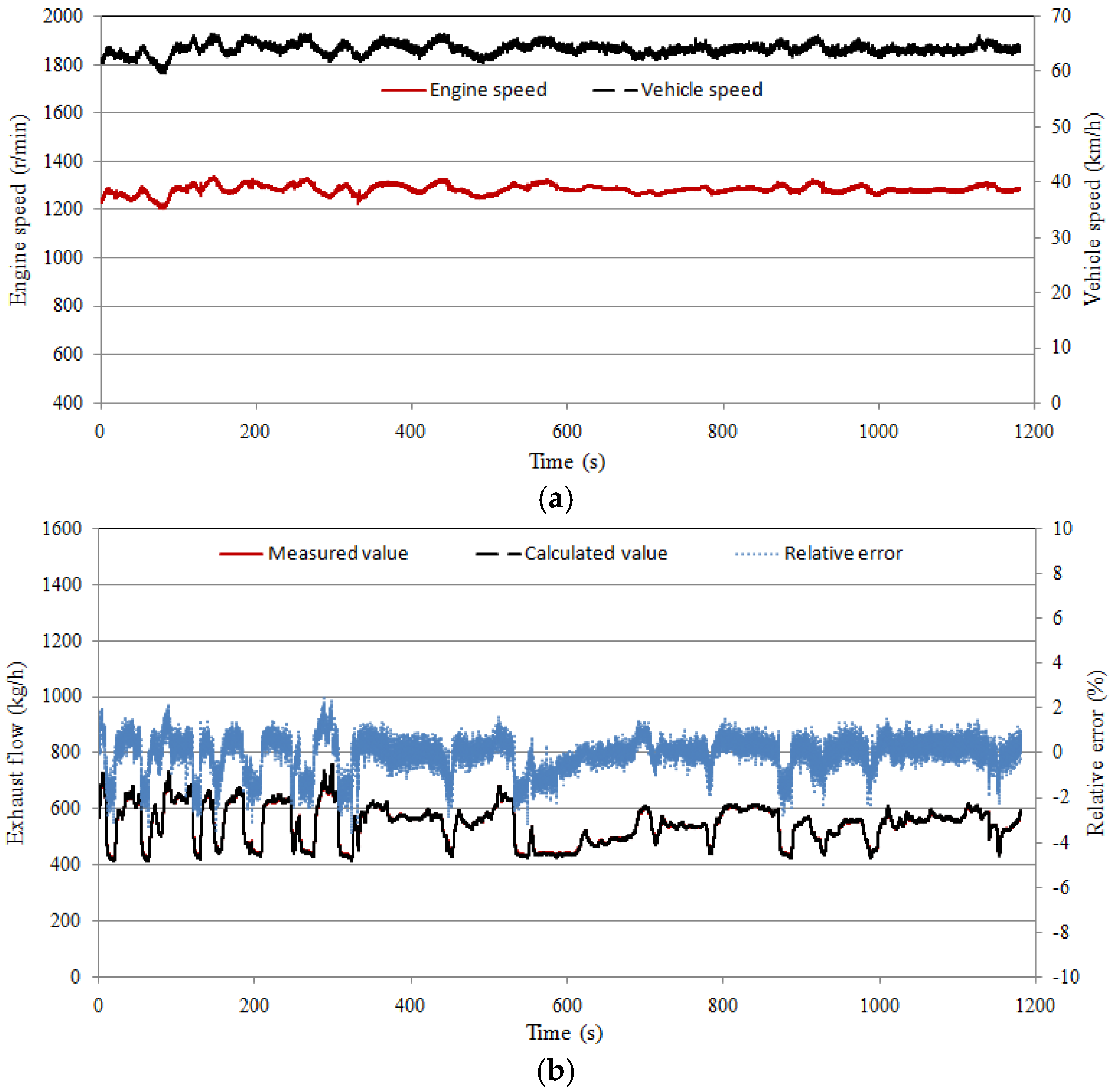
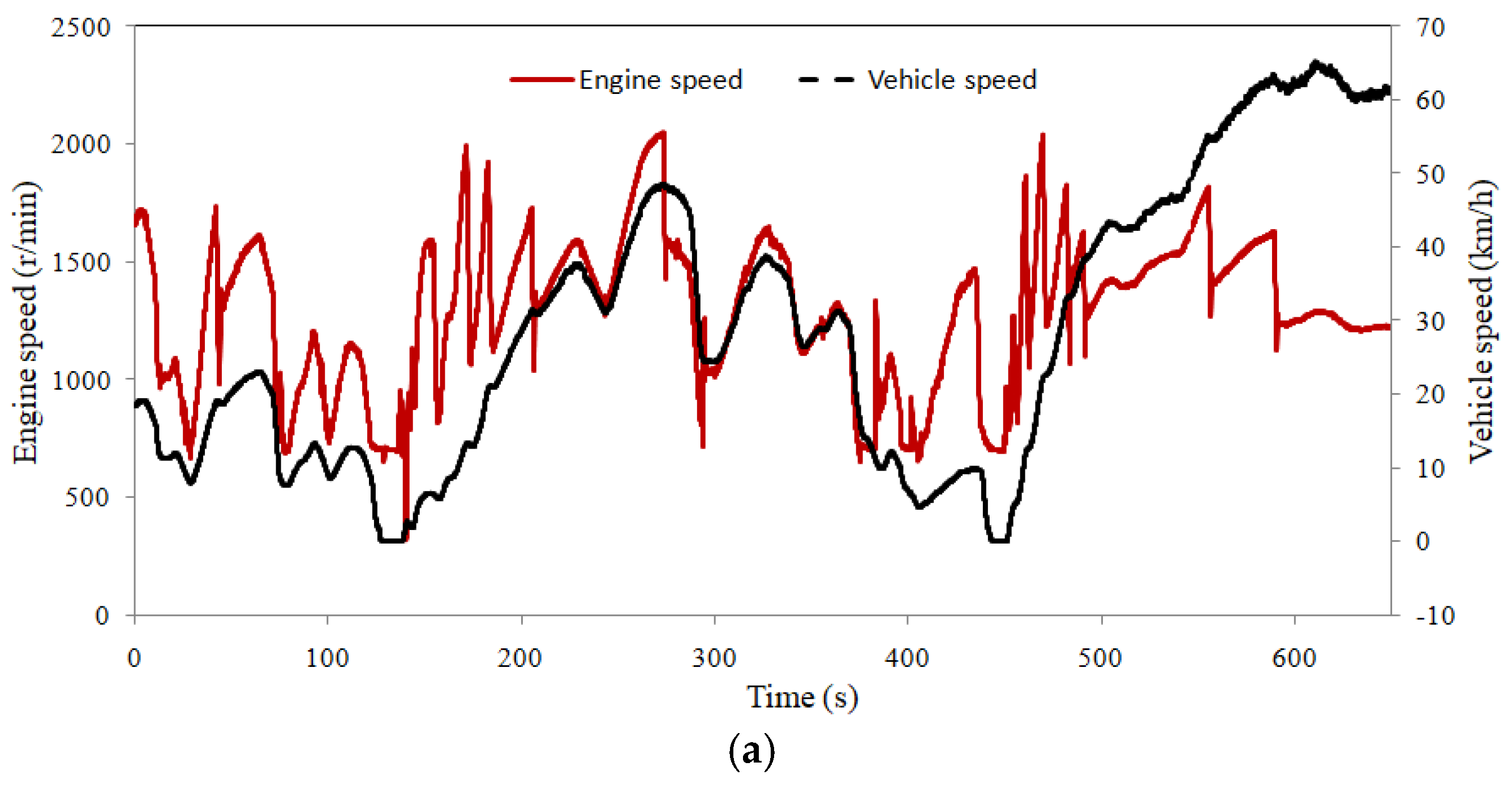
4.1. Exhaust Gas Flow Test
4.2. Exhaust Gas Temperature Test
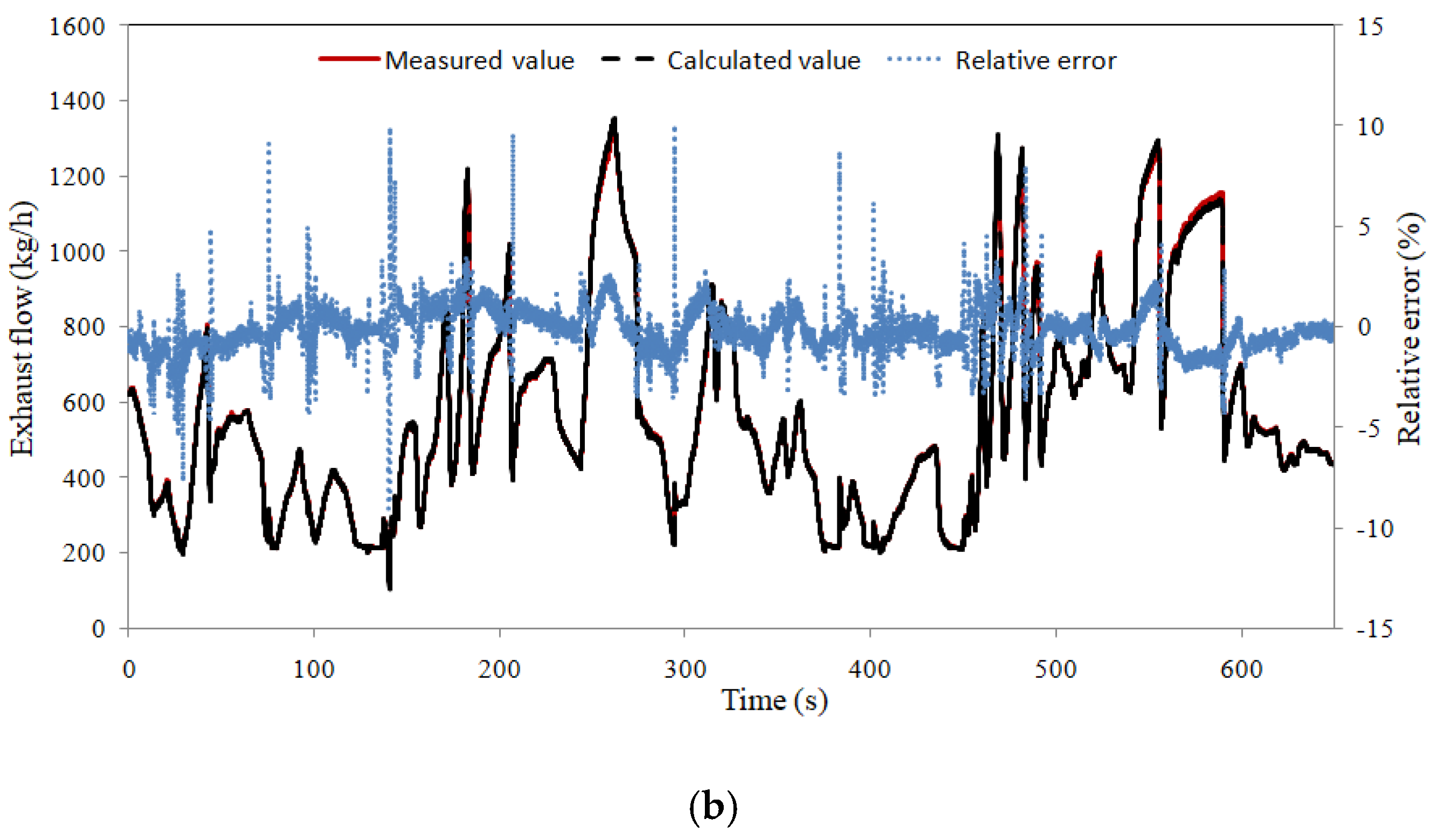
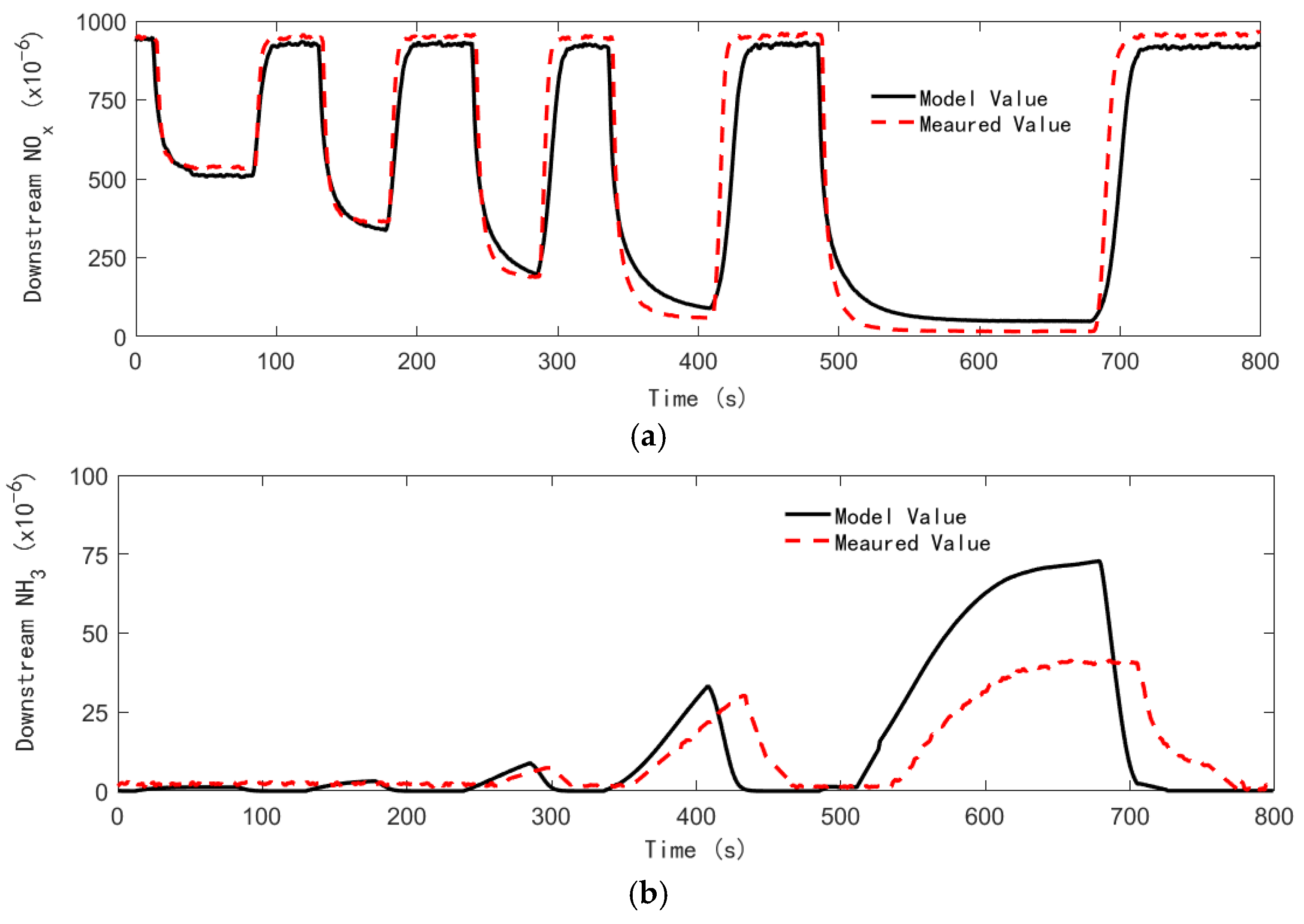
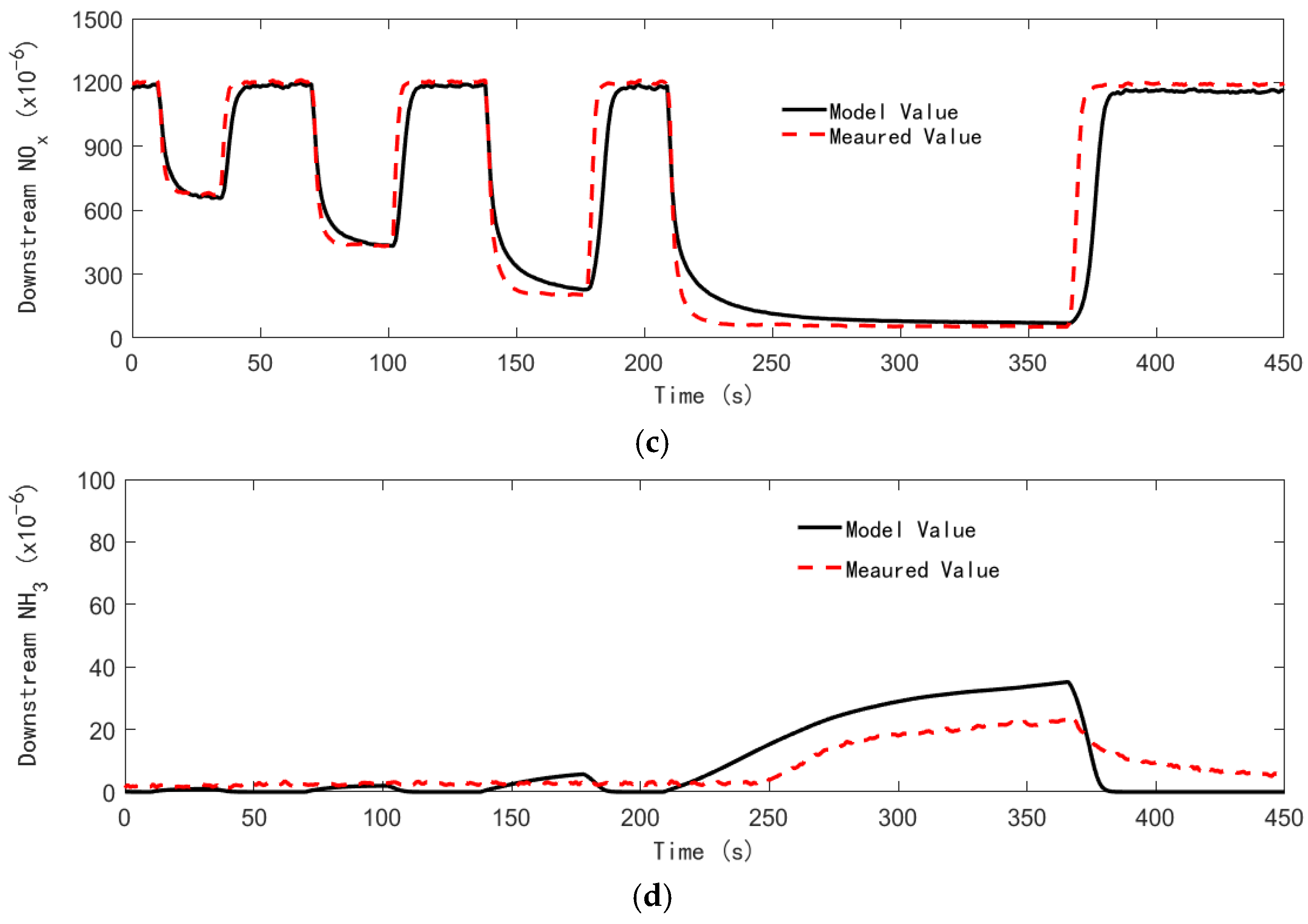
4.3. Control Strategy Test
5. Conclusions
- (1)
- According to the characteristics of the mobile source, a pressure difference-based flow calculation model was established to analyse the heat and mass transfer process of the SCR catalyst and establish a temperature model. Based on the downstream NOx signal and the SCR reaction characteristics, a segmented PID control strategy was established. The control strategy was constructed using MATLAB/Simulink, and the executable code of the embedded system was generated through RTW.
- (2)
- The test results of the whole vehicle show that the flow calculation model and the temperature model have good accuracy. The errors increase when the load step increases.
- (3)
- According to the differences in the internal temperature of the catalyst, downstream nitrogen–oxygen-based segmented PID closed-loop control was adopted. The model can better predict downstream nitrogen oxides and ammonia leakage, providing conditions for closed-loop control.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.J.; Cao, Y.; Li, R.K.; Ma, X.; Chen, J.; Wu, Z.; Xu, Q. The spatial variation in the effects of air pollution on cardiovascular mortality in Beijing, China. J. Expo. Sci. Environ. Epidemiol. 2018, 28, 297–304. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Ecology and Environment Issued the Annual Report of China Mobile Source Environmental Management, Highlighting the Urgency of Mobile Source Pollution Control. China Environ. Superv. 2020, 8, 9.
- Johnson, T.V. Review of Vehicular Emissions Trends. SAE Int. J. Engines 2015, 8, 1152–1167. [Google Scholar] [CrossRef] [Green Version]
- Shuai, S.J.; Tang, T.; Zhao, Y.; Hua, L. State of the art and outlook of diesel emission regulations and aftertreatment technologies. Automot. Saf. Energy 2012, 3, 200–217. [Google Scholar]
- Shijin, S.H.U.A.I.; Shiyu, L.I.U.; Xiao, M.A. Technology analysis of heavy-duty diesel vehicles to meet near-zero emission regulations. J. Automot. Saf. Energy 2019, 10, 16. [Google Scholar]
- Sampath, M.K.; Lacin, F. CFD Study of Sensitivity Parameters in SCR NOx Reduction Modeling; 2014-04-2346; SAE: Detroit, MI, USA, 2014. [Google Scholar]
- Suarez-Bertoa, R.; Valverde, V.; Clairotte, M.; Pavlovic, J.; Giechaskiel, B.; Franco, V.; Kregar, Z.; Astorga, C. On-road emissions of passenger cars beyond the boundary conditions of the real-driving emissions test. Environ. Res. 2019, 176, 108572. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.Z.; Franco, V.; Mock, P.; Kolke, R.; Zhang, S.; Wu, Y.; Germa, J. Experimental Assessment of NOx Emissions from 73 Euro 6 Diesel Passenger Cars. Environ. Sci. Technol. 2015, 49, 14409–14415. [Google Scholar] [CrossRef] [PubMed]
- Kun, W.; Yi, L.; Wei, W. Study on factors affecting conversion efficiency of double SCR system of heavy duty diesel engine. J. Heifei Univ. Technol. 2021, 44, 743–747. [Google Scholar]
- Tan, P.; Wang, D.; Lou, D.; Hu, Z. Progress of control technologies on exhaust emissions for agricultural machinery. Trans. Chin. Soc. Agric. Eng. 2018, 34, 1–14. [Google Scholar]
- Chatterjee, D.; Koci, P.; Schmeißer, V.; Marek, M.; Weibel, M.; Krutzsch, B. Modeling of a combined NOx storage and NH3-SCR catalytic system for diesel exhaust gas after treatment. Catal. Today 2010, 151, 395–409. [Google Scholar] [CrossRef]
- Mráček, D.; Kočí, P.; Choi, J.-S.; Partridge, W.P. New operation strategy for driving the selectivity of NOx reduction to N-2, NH3 or N2O during lean/rich cycling of a lean NOx trap catalyst. Appl. Catal. B Environ. Int. J. Devot. Catal. Sci. Its Appl. 2016, 182, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Hommen, G.; Kupper, F.; Seykens, X. Model-Based Urea Dosing Control for SCR Aftertreatment Systems Using a Cross-Sensitive Tailpipe NOx Sensor; SAE Paper 2017-01-0938; SAE: Detroit, MI, USA, 2017. [Google Scholar]
- Wang, Y.; Zhang, H.; Wang, J. NOx sensor reading correction in diesel engine selective catalytic reduction system applications. IEEE/ASME Trans. Mechatron. 2016, 21, 460–471. [Google Scholar]
- Aliramezani, M.; Koch, C.R.; Hayes, R.E. Estimating tailpipe NOx concentration using a dynamic NOx/ammonia cross sensitivity model coupled to a three state control oriented SCR model. IFACPapersOnLine 2016, 49, 8–13. [Google Scholar] [CrossRef]
- Stadlbauer, S.; Waschl, H.; del Re, L. SCR ammonia dosing control by a nonlinear model predictive controller. IFAC Proc. Vol. 2014, 47, 3018–3023. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Lan, X.; Xu, X.; Liang, H. Numerical model and control strategies for the advanced thermal management system of diesel engine. Appl. Therm. Eng. 2016, 82, 368–379. [Google Scholar] [CrossRef]
- Ke, H.; Liu, H.M.; Tian, G.D. An Uncertain Random Programming Model for Project Scheduling Problem. Int. J. Intell. Syst. 2015, 30, 66–79. [Google Scholar] [CrossRef]
- Yue, G.; Qiu, T.; Lei, Y. Experimental demonstration of NOx reduction and ammonia slip for diesel engine SCR system. Environ. Sci. Pollut. Res. 2022, 29, 1118–1133. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, G.; Wu, H.; Zhang, T.; Liu, R.; Sun, J. Research on Mobile Machinery NOx Emission Control Based on a Physical Model and Closed-Loop Control. Processes 2022, 10, 1374. https://doi.org/10.3390/pr10071374
Yue G, Wu H, Zhang T, Liu R, Sun J. Research on Mobile Machinery NOx Emission Control Based on a Physical Model and Closed-Loop Control. Processes. 2022; 10(7):1374. https://doi.org/10.3390/pr10071374
Chicago/Turabian StyleYue, Guangzhao, Hanming Wu, Tiezhu Zhang, Ruijun Liu, and Jianxin Sun. 2022. "Research on Mobile Machinery NOx Emission Control Based on a Physical Model and Closed-Loop Control" Processes 10, no. 7: 1374. https://doi.org/10.3390/pr10071374
APA StyleYue, G., Wu, H., Zhang, T., Liu, R., & Sun, J. (2022). Research on Mobile Machinery NOx Emission Control Based on a Physical Model and Closed-Loop Control. Processes, 10(7), 1374. https://doi.org/10.3390/pr10071374




