Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Methods
2.2. Corrosion Inhibitor Preparation
2.3. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Techniques Used
2.4.1. Weight Loss (WL) Analysis
2.4.2. Electrochemical Measurements
2.5. Atomic Absorption Spectrophotometric Studies (AAS)
2.6. FT-IR and UV–Visible Analysis
2.7. Surface Morphology
3. Results and Discussion
3.1. TFC and TPC Analysis
3.2. Weight Loss Measurements
3.3. Atomic Absorption Spectroscopy
3.4. Electrochemical Studies
3.4.1. Potentiodynamic Polarization
3.4.2. Electrochemical Impedance Spectroscopy (EIS)
3.5. UV–Visible Spectroscopic Analysis
3.6. Fourier Transform-Infrared Spectroscopic Analysis
3.7. Surface Analysis
3.7.1. SEM and EDX
3.7.2. Atomic Force Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Experimental and theoretical evaluation of Acacia catechu extract as a natural, economical and effective corrosion inhibitor for mild steel in an acidic environment. J. Bio Tribo Corros. 2020, 6, 76. [Google Scholar] [CrossRef]
- Sedik, A.; Lerari, D.; Salci, A.; Athmani, S.; Bachari, K.; Gecibesler, I.H.; Solmaz, R. Dardagan fruit extract as eco-friendly corrosion inhibitor for mild steel in 1M HCl: Electrochemical and surface morphological studies. J. Taiwan Inst. Chem. Eng. 2020, 107, 189–200. [Google Scholar] [CrossRef]
- Kang, L.; Shi, L.; Zeng, Q.; Liao, B.; Wang, B.; Guo, X. Melamine resin-coated lignocellulose fibers with robust superhydrophobicity for highly effective oil/water separation. Sep. Purif. Technol. 2021, 279, 119737. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Bhardwaj, N. Extraction and experimental studies of Citrus aurantifolia as an economical and green corrosion inhibitor for mild steel in acidic media. J. Adhes. Sci. Technol. 2019, 33, 1169–1183. [Google Scholar] [CrossRef]
- Asadi, N.; Ramezanzadeh, M.; Bahlakeh, G.; Ramezanzadeh, B. Utilizing lemon balm extract as an effective green corrosion inhibitor for mild steel in 1M HCl solution: A detailed experimental, molecular dynamics, Monte Carlo and quantum mechanics study. J. Taiwan Inst. Chem. Eng. 2019, 95, 252–272. [Google Scholar] [CrossRef]
- Shahini, M.H.; Ramezanzadeh, B.; Mohammadloo, H.E. Recent advances in biopolymers/carbohydrate polymers as effective corrosion inhibitive macro-molecules: A review study from experimental and theoretical views. J. Mol. Liq. 2021, 325, 115110. [Google Scholar] [CrossRef]
- Bahlakeh, G.; Ramezanzadeh, B.; Dehghani, A.; Ramezanzadeh, M. Novel cost-effective and high-performance green inhibitor based on aqueous Peganum harmala (Esfand) seed extract for mild steel corrosion in HCl solution: Detailed experimental and electronic/atomic level computational explorations. J. Mol. Liq. 2019, 283, 174–195. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Myristica fragrans extract as an eco-friendly corrosion inhibitor for mild steel in 0.5M H2SO4 solution. J. Environ. Chem. Eng. 2018, 6, 2290–2301. [Google Scholar] [CrossRef]
- Trindade, R.S.; Santos, M.R.; Cordeiro, R.F.B.; D’Elia, E. A study of the gorse aqueous extract as a green corrosion inhibitor for mild steel in HCl aqueous solution. Green Chem. Lett. Rev. 2017, 10, 444–454. [Google Scholar] [CrossRef] [Green Version]
- Saxena, A.; Prasad, D.; Haldhar, R. Investigation of Corrosion Inhibition Effect and Adsorption activities of Achyranthes aspera extract for mild steel in 0.5 M H2SO4. J. Fail. Anal. Preven. 2018, 18, 957–968. [Google Scholar] [CrossRef]
- Zaferani, S.H.; Sharifi, M.; Zaarei, D.; Shishesaz, M.R. Application of eco-friendly products as corrosion inhibitors for metals in acid pickling processes—A review. J. Env. Chem. Eng. 2013, 1, 652–657. [Google Scholar] [CrossRef]
- Hassannejad, H.; Nouri, A. Sunflower seed hull extract as a novel green corrosion inhibitor for mild steel in HCl solution. J. Mol. Liq. 2018, 254, 377–382. [Google Scholar] [CrossRef]
- Fernandes, C.M.; Fagundes, T.S.F.; Santos, N.E.; Rocha, T.S.M.; Garrett, R.; Borges, R.M.; Muricy, G.; Valverde, A.L.; Ponzio, E.A. Ircinia strobilina crude extract as corrosion inhibitor for mild steel in acid medium. J. Electrochim. Acta 2019, 312, 137–148. [Google Scholar] [CrossRef]
- Sharma, S.K.; Mudhoo, A.; Jain, G.; Sharma, J. Corrosion inhibition and adsorption properties of Azadirachta indica mature leaves extract as green inhibitor for mild steel in HNO3. Green Chem. Lett. Rev. 2010, 3, 7–15. [Google Scholar] [CrossRef]
- Fouda, A.S.; Awady, G.Y.E.; Behairy, W.T.E. Prosopis juliflora plant extract as potential corrosion inhibitor for low carbon steel in 1M HCl solution. J. Bio Tribo Corros. 2018, 4, 8. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Khayatkashani, M.; Jalali, M.R.; Mosavizade, A. Green approach to corrosion inhibition of 304 stainless steel in hydrochloric acid solution by the extract of Salvia officinalis leaves. Corros. Sci. 2012, 62, 122–135. [Google Scholar] [CrossRef]
- Li, X.; Deng, S. Inhibition effect of Dendrocalamus brandisii leaves extract on aluminum in HCl, H3PO4 solutions. Corros. Sci. 2012, 65, 299–308. [Google Scholar] [CrossRef]
- Muthukrishnan, P.; Jeyaprabha, B.; Prakash, P. Mild steel corrosion inhibition by aqueous extract of Hyptis suaveolens leaves. Int. J. Ind. Chem. 2014, 5, 5. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Zhang, T.; Chen, H.; Liao, B.; Guo, X. Kapok leaves extract and synergistic iodide as novel effective corrosion inhibitors for Q235 carbon steel in H2SO4 medium. Ind. Crops Prod. 2022, 178, 114649. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Bahlakeh, G.; Sanaei, Z.; Ramezanzadeh, B. Corrosion inhibition of mild steel in 1 M HCl solution by ethanolic extract of eco-friendly Mangifera indica (mango) leaves: Electrochemical, molecular dynamics, Monte Carlo and ab initio study. J. Appl. Surf. Sci. 2019, 463, 1058–1077. [Google Scholar] [CrossRef]
- Shahini, M.H.; Ramezanzadeh, M.; Ramezanzadeh, B.; Bahlakeh, G. The role of ethanolic extract of Stachys byzantina’s leaves for effective decreasing the mild-steel (MS) degradation in the acidic solution; coupled theoretical/experimental assessments. J. Mol. Liq. 2021, 329, 115571. [Google Scholar] [CrossRef]
- Parthipan, P.; Cheng, L.; Rajasekar, A. Glycyrrhiza glabra extract as an eco-friendly inhibitor for microbiologically influenced corrosion of API 5LX carbon steel in oil well produced water environments. J. Mol. Liq. 2021, 333, 115952. [Google Scholar] [CrossRef]
- Fouda, A.S.; Rashwan, S.M.; Kamel, M.M.; Haleem, E.A. Inhibitive influence of cumin (Cuminum cyminum) seed extract on the dissolution of Al in 2M HCl acid medium. J. Bio. Tribo. Corros. 2021, 7, 55. [Google Scholar] [CrossRef]
- Li, H.; Qiang, Y.; Zhao, W.; Zhang, S. A green Brassica oleracea L extract as a novel corrosion inhibitor for Q235 steel in two typical acid media. Collo. Surf. A Physiochem. Eng. Asp. 2021, 616, 126077. [Google Scholar] [CrossRef]
- Fouda, A.S.; Shalabi, K.; Idress, A.A. Ceratonia siliqua extract as a green corrosion inhibitor for copper and brass in nitric acid solutions. Green Chem. Lett. Rev. 2015, 8, 17–29. [Google Scholar] [CrossRef]
- Aralu, C.C.; Okorie, H.O.C.; Akpomie, G.K. Inhibition and adsorption potentials of mild steel corrosion using methanol extract of Gongronema latifoliuim. Appl. Water Sci. 2021, 11, 22. [Google Scholar] [CrossRef]
- Kouache, A.; Khelifa, A.; Boutoumi, H.; Moulay, S.; Feghoul, A.; Idir, B.; Aoudj, S. Experimental and theoretical studies of Inula viscosa extract as a novel eco-friendly corrosion inhibitor for carbon steel in 1M HCl. J. Adhes. Sci. Technol. 2022, 36, 988–1016. [Google Scholar] [CrossRef]
- Haldhar, R.; Prasad, D.; Saxena, A. Armoracia rusticana as sustainable and eco-friendly corrosion inhibitor for mild steel in 0.5M sulphuric acid: Experimental and theoretical Investigations. J. Environ. Chem. Eng. 2018, 6, 5230–5238. [Google Scholar] [CrossRef]
- Ji, G.; Anjum, S.; Sundaram, S.; Prakash, R. Musa paradisica peel extract as green corrosion inhibitor for mild steel in HCl solution. Corros. Sci. 2018, 90, 107–117. [Google Scholar] [CrossRef]
- Strijk, J.S.; Hinsinger, D.D.; Zhang, F.; Cao, K. Trochodendron aralioides the first chromosome-level draft genome in Trochodendrales and a valuable resource for basal eudicot research. Gigascience 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yagishita, K. Isolation and identification of betulin, lupeol, and β-amyrin from the Bird-lime of Trochodendron aralioides Siebold et Zuccarini. Bull. Agric. Chem. Soc. Jpn. 1957, 21, 77–81. [Google Scholar] [CrossRef]
- Wu, H.C.; Su, H.J.; Hu, J.M. The identification of A-, B-, C-, and e-class mads-box genes and implications for perianth evolution in the basal eudicot Trochodendron aralioides (Trochodendraceae). Int. J. Plant Sci. 2007, 168, 775–799. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Tsai, W.T.; Chen, C.K.; Chen, C.H.; Lee, S.S. Diastereomeric identification of neolignan rhamnosides from Trochodendron aralioides leaves by LC-SPE-NMR and circular dichroism. Fitoterapia 2020, 144, 104455. [Google Scholar] [CrossRef] [PubMed]
- Prabakaran, M.; Kim, S.H.; Hemapriya, V.; Chung, I.M. Tragia plukenetii extract as an eco-friendly inhibitor for mild steel corrosion in HCl 1M acidic medium. Res. Chem. Intermed. 2016, 42, 3703–3719. [Google Scholar] [CrossRef]
- Manokaran, G.; Prabakaran, M. Evaluation of antioxidant and anticorrosion activities of Ligularia fischeri plant extract. Chem. Sci. Eng. Res. 2019, 1, 16–24. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar] [CrossRef]
- Jessima, S.J.H.M.; Subhashini, S.; Arulraj, J. Sunova spirulina powder as an effective environmentally friendly corrosion inhibitor for mild steel in acid medium. J. Bio Tribo Corros. 2020, 6, 71. [Google Scholar] [CrossRef]
- Kurniawan, F.; Madurani, K.A. Electrochemical and optical microscopy study of red pepper seed oil corrosion inhibition by self-assembled monolayers (SAM) on 304 SS. Prog. Org. Coat. 2015, 88, 256–262. [Google Scholar] [CrossRef]
- Kalaiselvi, K.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Corrosion resistance of mild steel in sulphuric acid solution by Coreopsis tinctoria extract: Electrochemical and surface studies. Anti-Corros. Methods Mater. 2018, 65, 408–416. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Hemapriya, V.; Chung, I.M. Evaluation of polyphenol composition and anti-corrosion properties of Cryptostegia grandiflora plant extract on mild steel in acidic medium. J. Ind. Eng. Chem. 2016, 37, 47–56. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Mugila, N.; Hemapriya, V.; Parameswari, K.; Chitra, S.; Chung, I.M. Aster koraiensis as nontoxic corrosion inhibitor for mild steel in sulfuric acid. J. Ind. Eng. Chem. 2017, 52, 235–242. [Google Scholar] [CrossRef]
- El-Etre, A.Y. Inhibition of C-steel corrosion in acidic solution using the aqueous extract of zallouh root. J. Mater. Chem. Phys. 2008, 108, 278–282. [Google Scholar] [CrossRef]
- Satapathy, A.K.; Gunasekaran, G.; Sahoo, S.C.; Amit, K.; Rodrigues, P.V. Corrosion inhibition by Justicia gendarussa plant extract in hydrochloric acid solution. Corros. Sci. 2009, 51, 2848–2856. [Google Scholar] [CrossRef]
- Khadom, A.A.; Abd, A.N.; Ahmed, N.A. Xanthium strumarium leaves extracts as a friendly corrosion inhibitor of low carbon steel in hydrochloric acid: Kinetics and mathematical studies. S. Afr. J. Chem. Eng. 2018, 25, 13–21. [Google Scholar] [CrossRef]
- Fouda, A.S.; Abousalem, A.S.; El-Ewady, G.Y. Mitigation of corrosion of carbon steel in acidic solutions using an aqueous extract of Tilia cordata as green corrosion inhibitor. Int. J. Ind. Chem. 2017, 8, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, P.E.; Fiori-Bimbi, M.V.; Neske, A.; Brand’an, S.A.; Gervasi, C.A. Rollinia occidentalis extract as green corrosion inhibitor for carbon steel in HCl solution. J. Ind. Eng. Chem. 2018, 58, 92–99. [Google Scholar] [CrossRef]
- Hussin, M.H.; Kassim, M.J. The corrosion inhibition and adsorption behavior of Uncaria gambir extract on mild steel in 1M HCl. Mater. Chem. Phys. 2011, 125, 461–468. [Google Scholar]
- Lebrini, M.; Robert, F.; Lecante, A.; Roos, C. Corrosion inhibition of C38 steel in 1M hydrochloric acid medium by alkaloids extract from Oxandra asbeckii plant. Corros. Sci. 2011, 53, 687–695. [Google Scholar] [CrossRef]
- Bammou, L.; Belkhaouda, M.; Salghi, R.; Benali, O.; Zarrouk, A.; Zarrok, H.; Hammouti, B. Corrosion inhibition of steel in sulfuric acidic solution by the Chenopodium ambrosioides extracts. J. Assoc. Arab. Univ. Basic Appl. Sci. 2014, 16, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Prabakaran, M.; Kim, S.H.; Oh, Y.T.; Raj, V.; Chung, I.M. Anticorrosion properties of momilactone A isolated from rich hulls. J. Ind. Eng. Chem. 2017, 45, 380–386. [Google Scholar] [CrossRef]
- Jyothi, S.; Ravichandran, J. Inhibitive action of the acid extract of Luffa aegyptiaca leaves on the corrosion of mild steel in acidic medium. J. Adhes. Sci. Technol. 2015, 29, 207–231. [Google Scholar] [CrossRef]
- Fouda, A.S.; Mohamed, O.A.; Elabbasy, H.M. Ferula hermonis plant extract as safe corrosion inhibitor for zinc in hydrochloric acid solution. J. Bio Tribo Corros. 2021, 7, 135. [Google Scholar] [CrossRef]
- Chung, I.M.; Malathy, R.; Priyadharshini, R.; Hemapriya, V.; Kim, S.H.; Prabakaran, M. Inhibition of mild steel corrosion using Magnolia kobus extract in sulphuric acid medium. Mater. Today Commun. 2020, 25, 101687. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Lei, J.; He, J.; Zhang, S.; Pan, F. Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel. Corros. Sci. 2012, 63, 82–90. [Google Scholar] [CrossRef]
- Saxena, A.; Prasad, D.; Haldhar, R.; Singh, G.; Kumar, A. Use of Sida cordifolia extract as green inhibitor for mild steel in 0.5M H2SO4. J. Environ. Chem. Eng. 2018, 6, 694–700. [Google Scholar] [CrossRef]
- Prabakaran, M.; Kim, S.H.; Kalaiselvi, K.; Hemapriya, V.; Chung, I.M. Highly efficient Ligularia fischeri green extract for the protection against corrosion of mild steel in acidic medium: Electrochemical and spectroscopic investigations. J. Taiwan Inst. Chem. Eng. 2016, 59, 553–562. [Google Scholar] [CrossRef]
- Hemapriya, V.; Prabakaran, M.; Parameswari, K.; Chitra, S.; Kim, S.H.; Chung, I.M. Experimental and theoretical studies on inhibition of benzothiazines against corrosion of mild steel in acidic medium. Anti-Corros. Methods Mater. 2017, 64, 306–314. [Google Scholar] [CrossRef]
- Chung, I.M.; Hemapriya, V.; Ponnusamy, K.; Arunadevi, N.; Chitra, S.; Chi, H.Y.; Kim, S.H.; Prabakaran, M. Assessment of low carbon steel corrosion inhibition by ecofriendly green Chaenomeles sinensis extract in acid medium. J. Electrochem. Sci. Technol. 2018, 9, 238–249. [Google Scholar] [CrossRef] [Green Version]
- Chung, I.M.; Hemapriya, V.; Kim, S.H.; Ponnusamy, K.; Arunadevi, N.; Chitra, S.; Prabakaran, M.; Gopiraman, M. Liriope platyphylla extract as a green inhibitor for mild steel corrosion in sulfuric acid medium. Chem. Eng. Commun. 2021, 208, 72–88. [Google Scholar] [CrossRef]
- Hemapriya, V.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Inhibitory effect of biowaste on copper corrosion in 1 M HCl solution. Mater. Today Commun. 2021, 27, 102249. [Google Scholar] [CrossRef]
- Zeng, Y.; Kang, L.; Wu, Y.; Wan, S.; Liao, B.; Li, N.; Guo, X. Melamine modified carbon dots as high effective corrosion inhibitor for Q235 carbon steel in neutral 3.5 wt% NaCl solution. J. Mol. Liq. 2022, 349, 118108. [Google Scholar] [CrossRef]
- Chitra, S.; Chung, I.M.; Kim, S.H.; Prabakaran, M. A study on anticorrosive property of phenolic components from Pachysandra terminalis against low carbon steel corrosion in acidic medium. Pigment. Resin Technol. 2019, 48, 389–396. [Google Scholar] [CrossRef]
- Shamsuzzaman, M.; Kalaiselvi, K.; Prabakaran, M. Evaluation of antioxidant and anticorrosive activities of Ceriops tagal plant extract. Appl. Sci. 2021, 11, 10150. [Google Scholar] [CrossRef]
- Chung, I.M.; Kalaiselvi, K.; Sasireka, A.; Kim, S.H.; Prabakaran, M. Anticorrosive property of Spiraea cantoniensisis extract as an eco-friendly inhibitor on mild steel surface in acid medium. J. Dispers. Sci. Technol. 2019, 40, 1326–1337. [Google Scholar] [CrossRef]
- Devi, G.N.; Unnisa, C.B.N.; Roopan, S.M.; Hemapriya, V.; Chitra, S.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Floxacins: As Mediators in enhancing the corrosion inhibition efficiency of natural polymer dextrin. Macromol. Res. 2020, 28, 558–566. [Google Scholar] [CrossRef]
- Hemapriya, V.; Chung, I.M.; Parameswari, K.; Chitra, S.; Kim, S.H.; Prabakaran, M. Corrosion inhibition behavior of benzothiazine derivative on low carbon steel in acid medium: Adsorption and quantum chemical investigations. Surf. Rev. Lett. 2019, 26, 1950066. [Google Scholar] [CrossRef]
- Chung, I.M.; Kim, S.H.; Prabakaran, M. Evaluation of phytochemical, polyphenol composition and anti-corrosion capacity of Cucumis anguria L. leaf extract on metal surface in sulfuric acid medium. Prot. Met. Phys. Chem. Surf. 2020, 56, 214–224. [Google Scholar] [CrossRef]
- Unnisa, C.B.N.; Chitra, S.; Devi, G.N.; Kiruthika, A.; Roopan, S.M.; Hemapriya, V.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Electrochemical and nonelectrochemical analyses of cardo polyesters at the metal/0.5 M H2SO4 interface for corrosion protection. Res. Chem. Intermed. 2019, 45, 5425–5449. [Google Scholar] [CrossRef]
- Chung, I.M.; Hemapriya, V.; Kanchana, P.; Arunadevi, N.; Chitra, S.; Kim, S.H.; Prabakaran, M. Active-polyphenolic-compounds-rich green inhibitor for the surface protection of low carbon steel in acidic medium. Surf. Rev. Lett. 2020, 27, 1950154. [Google Scholar] [CrossRef]
- Malathy, R.; Prabakaran, M.; Kalaiselvi, K.; Chung, I.M.; Kim, S.H. Comparative polyphenol composition, antioxidant and anticorrosion properties in various parts of Panax ginseng extracted in different solvents. Appl. Sci. 2021, 11, 93. [Google Scholar] [CrossRef]
- Mahalakshmi, D.; Unnisa, C.B.N.; Hemapriya, V.; Subramaniam, E.P.; Roopan, S.M.; Chitra, S.; Chung, I.M.; Kim, S.H.; Prabakaran, M. Anticorrosive potential of ethanol extract of Delonix elata for mild steel in 0.5 M H2SO4—A green approach. Bulg. Chem. Commun. 2019, 51, 31–37. [Google Scholar]
- Manh, T.D.; Huynh, T.L.; Thi, B.V.; Lee, S.; Yi, J.; Dang, N.N. Corrosion inhibition of mild steel in hydrochloric acid environments containing Sonneratia caseolaris leaf extract. ACS Omega 2022, 7, 8874–8886. [Google Scholar] [CrossRef] [PubMed]
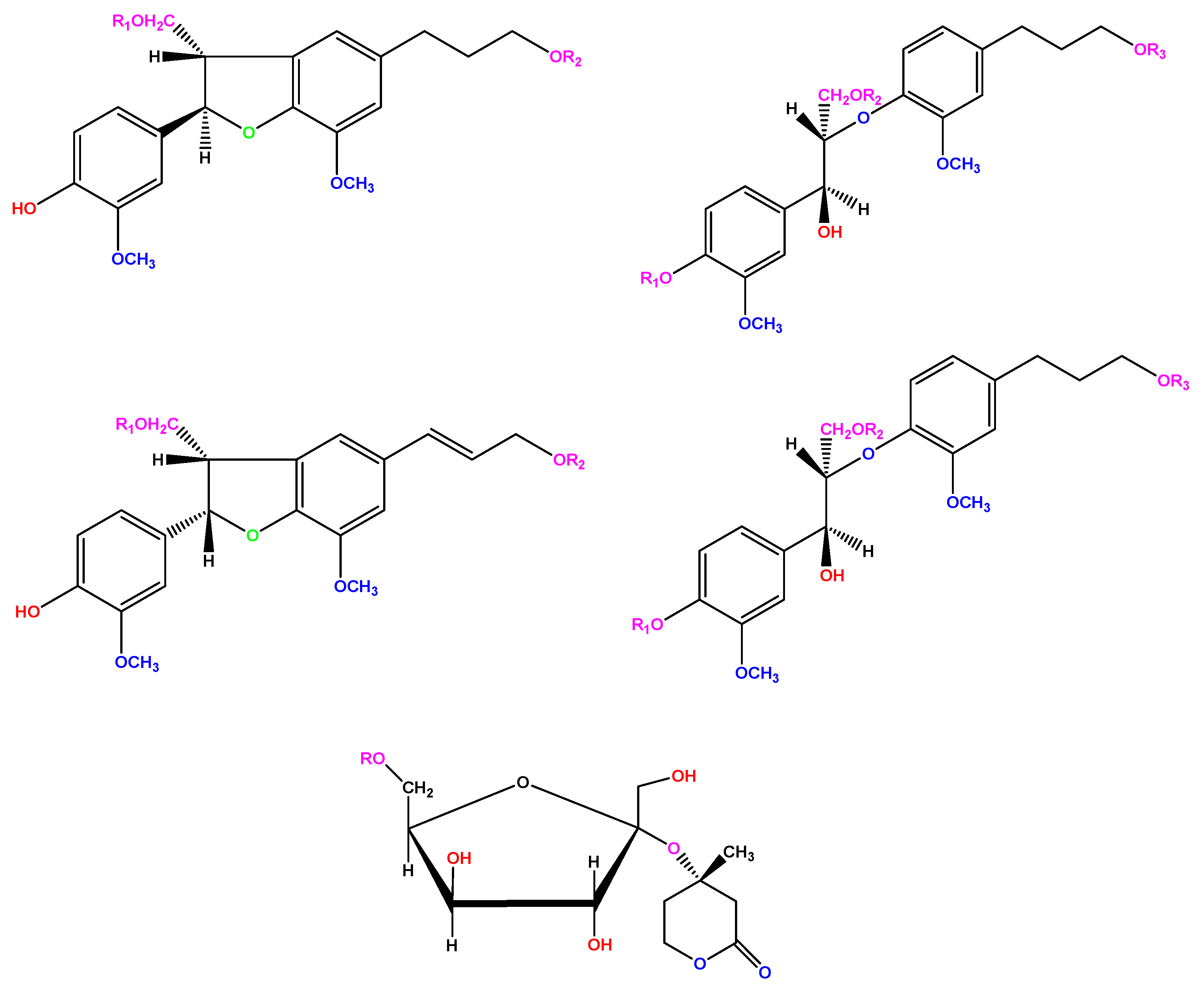



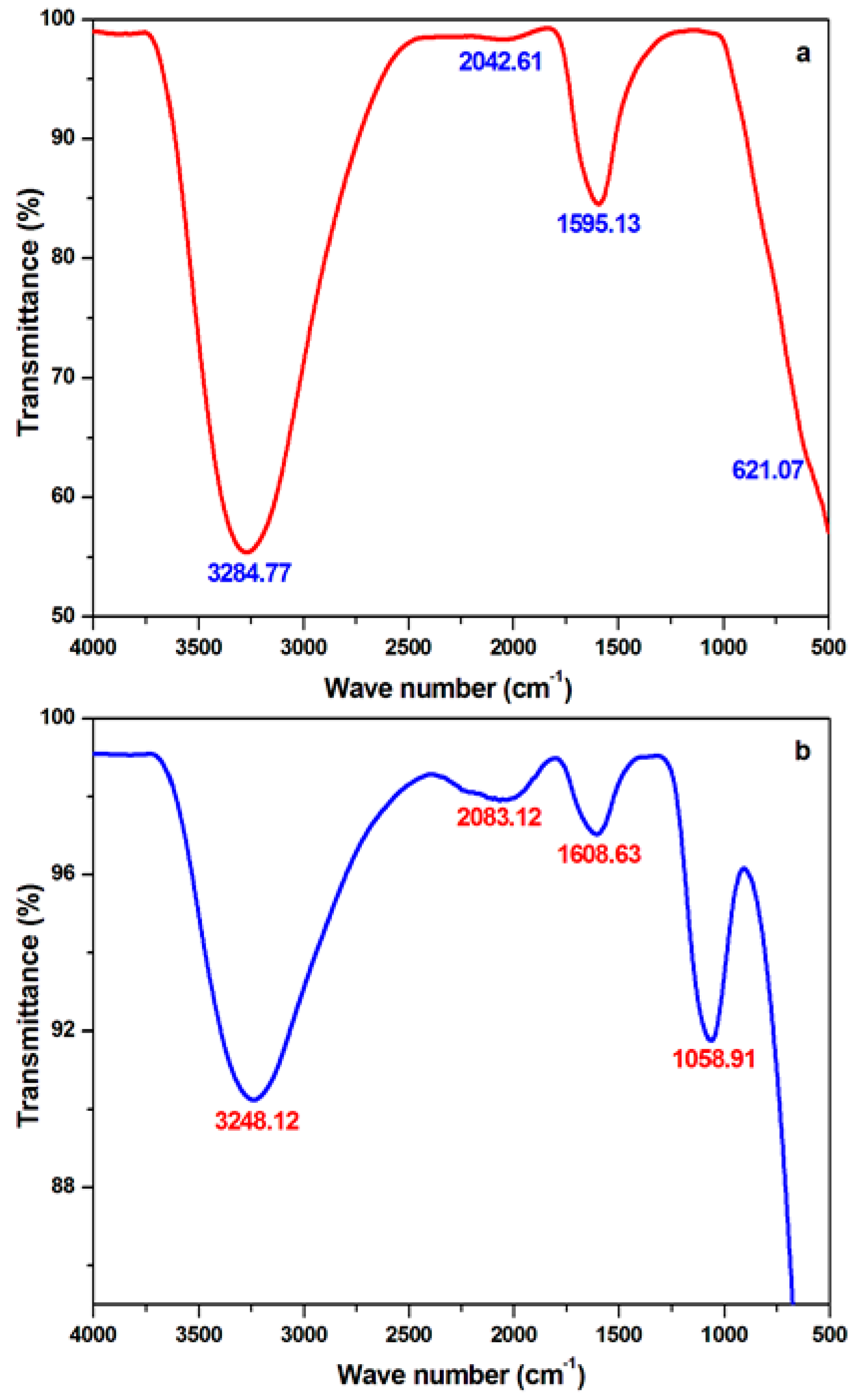
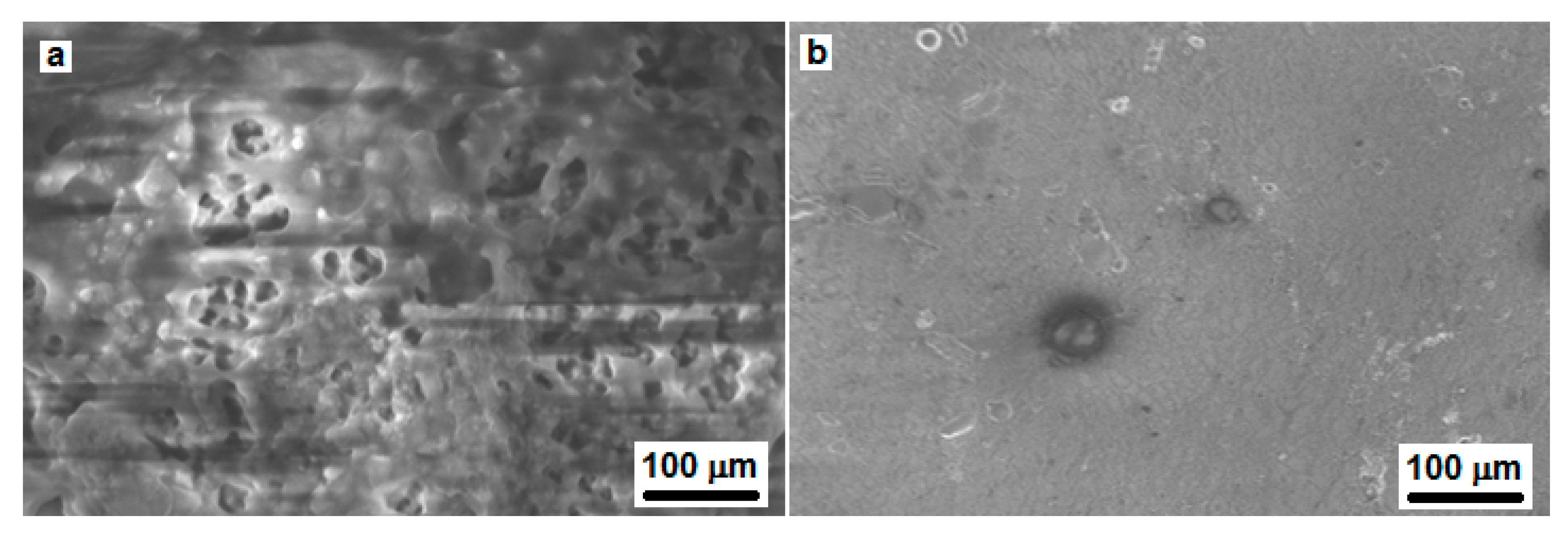

| Conc. (ppm) | W (mg·cm−2) | Cr (mg·cm−2 h−1) | θ | IE% | σa |
|---|---|---|---|---|---|
| Blank | 0.1231 | 0.0137 | - | - | - |
| 50 | 0.0595 | 0.0066 | 0.5166 | 51.66 | 0.05 |
| 100 | 0.0389 | 0.0043 | 0.6839 | 68.39 | 0.03 |
| 150 | 0.0256 | 0.0028 | 0.7920 | 79.20 | 0.02 |
| 200 | 0.0168 | 0.0019 | 0.8635 | 86.35 | 0.01 |
| 250 | 0.0044 | 0.0005 | 0.9642 | 96.42 | 0.01 |
| T (±1 K) | Conc. (ppm) | W (mg·cm−2) | Cr (mg·cm−2 h−1) | θ | IE% | σa |
|---|---|---|---|---|---|---|
| Blank | 0.1200 | 0.0133 | - | - | - | |
| 50 | 0.0650 | 0.0072 | 0.4583 | 45.83 | 0.04 | |
| 100 | 0.0468 | 0.0052 | 0.6100 | 61.00 | 0.06 | |
| 313 | 150 | 0.0304 | 0.0034 | 0.7466 | 74.66 | 0.01 |
| 200 | 0.0213 | 0.0024 | 0.8075 | 80.75 | 0.05 | |
| 250 | 0.0150 | 0.0017 | 0.8750 | 87.50 | 0.01 | |
| Blank | 0.1255 | 0.0139 | - | - | - | |
| 50 | 0.0751 | 0.0083 | 0.4015 | 40.15 | 0.05 | |
| 100 | 0.0535 | 0.0059 | 0.5737 | 57.37 | 0.02 | |
| 323 | 150 | 0.0393 | 0.0044 | 0.6868 | 68.68 | 0.03 |
| 200 | 0.0285 | 0.0032 | 0.7729 | 77.29 | 0.04 | |
| 250 | 0.0210 | 0.0023 | 0.8326 | 83.26 | 0.03 | |
| Blank | 0.1250 | 0.0139 | - | - | - | |
| 50 | 0.0802 | 0.0089 | 0.3584 | 35.84 | 0.01 | |
| 100 | 0.0631 | 0.0070 | 0.4952 | 49.52 | 0.03 | |
| 333 | 150 | 0.0459 | 0.0051 | 0.6328 | 63.28 | 0.02 |
| 200 | 0.0371 | 0.0041 | 0.7032 | 70.32 | 0.01 | |
| 250 | 0.0293 | 0.0033 | 0.7656 | 76.56 | 0.04 | |
| Blank | 0.1251 | 0.0139 | - | - | - | |
| 50 | 0.0901 | 0.0100 | 0.2797 | 27.97 | 0.03 | |
| 343 | 100 | 0.0711 | 0.0079 | 0.4316 | 43.16 | 0.01 |
| 150 | 0.0533 | 0.0059 | 0.5739 | 57.39 | 0.02 | |
| 200 | 0.0484 | 0.0054 | 0.6131 | 61.31 | 0.02 | |
| 250 | 0.0400 | 0.0044 | 0.6802 | 68.02 | 0.01 |
| Conc. (ppm) | Amount of MS Corrodant (mg/I) | IE% | σa |
|---|---|---|---|
| Blank | 36.87 | - | - |
| 50 | 22.95 | 37.75 | 0.02 |
| 100 | 18.22 | 50.58 | 0.04 |
| 150 | 12.88 | 65.06 | 0.03 |
| 200 | 10.21 | 72.30 | 0.01 |
| 250 | 07.01 | 80.98 | 0.02 |
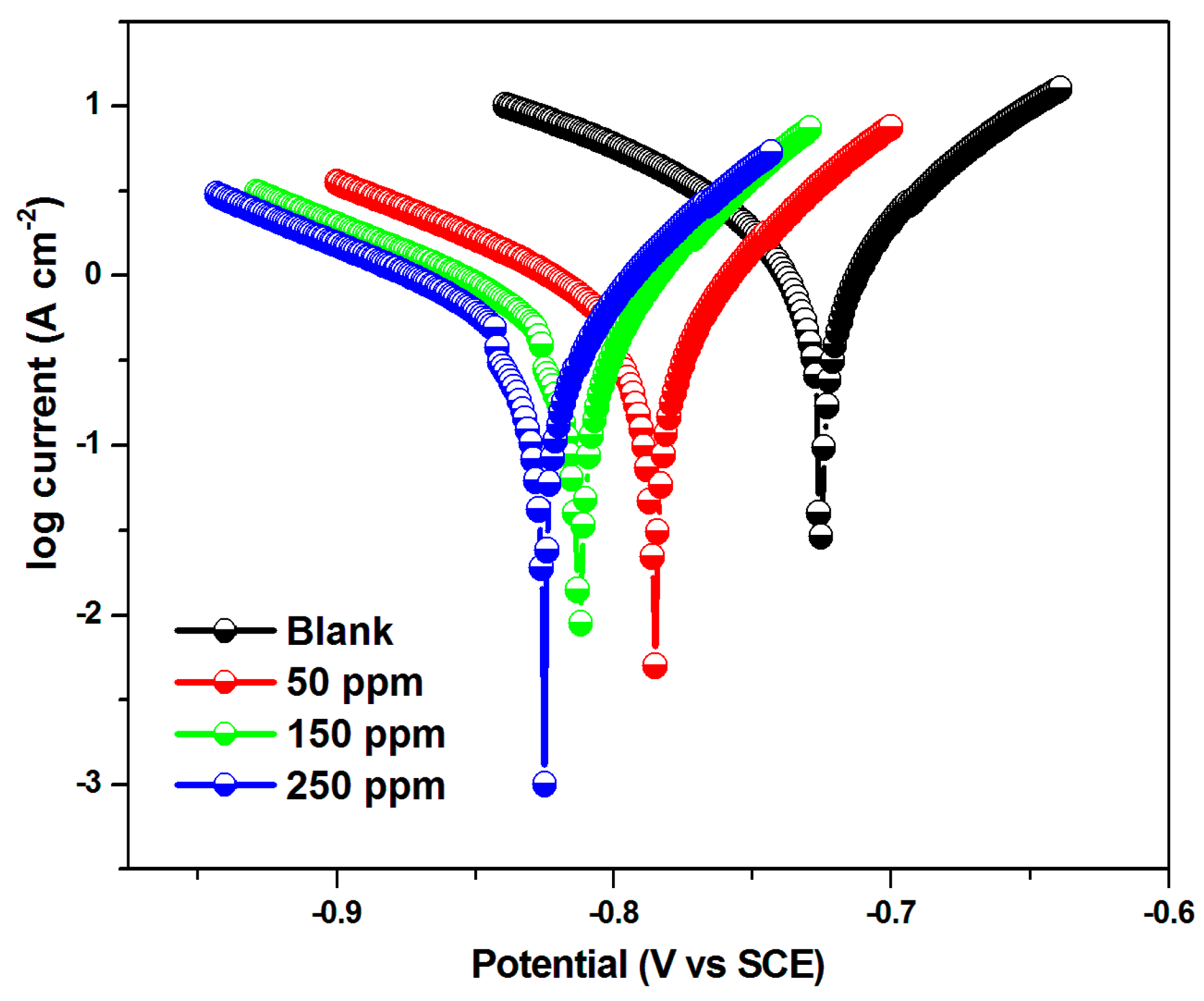
| Conc. | Tafel Slopes (mV vs. dec) | Ecorr (mV) | Icorr (µAcm−2) | IE (%) | |
|---|---|---|---|---|---|
| ba | bc | ||||
| Blank | 71 | 122 | −717.7 | 1452 | - |
| 50 | 62 | 135 | −778.0 | 464 | 68 |
| 150 | 60 | 148 | −805.5 | 327 | 77 |
| 250 | 61 | 144 | −816.8 | 188 | 87 |
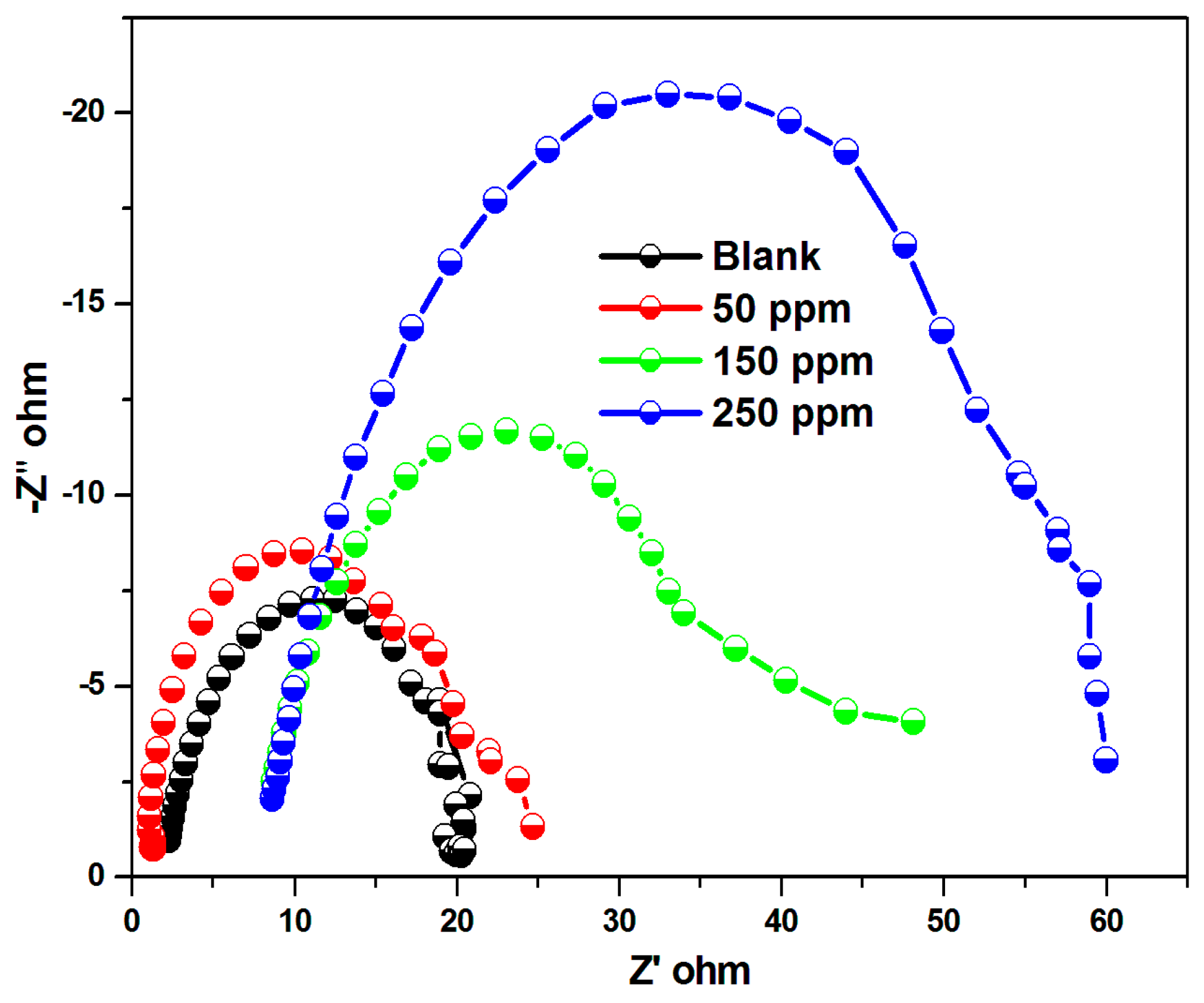
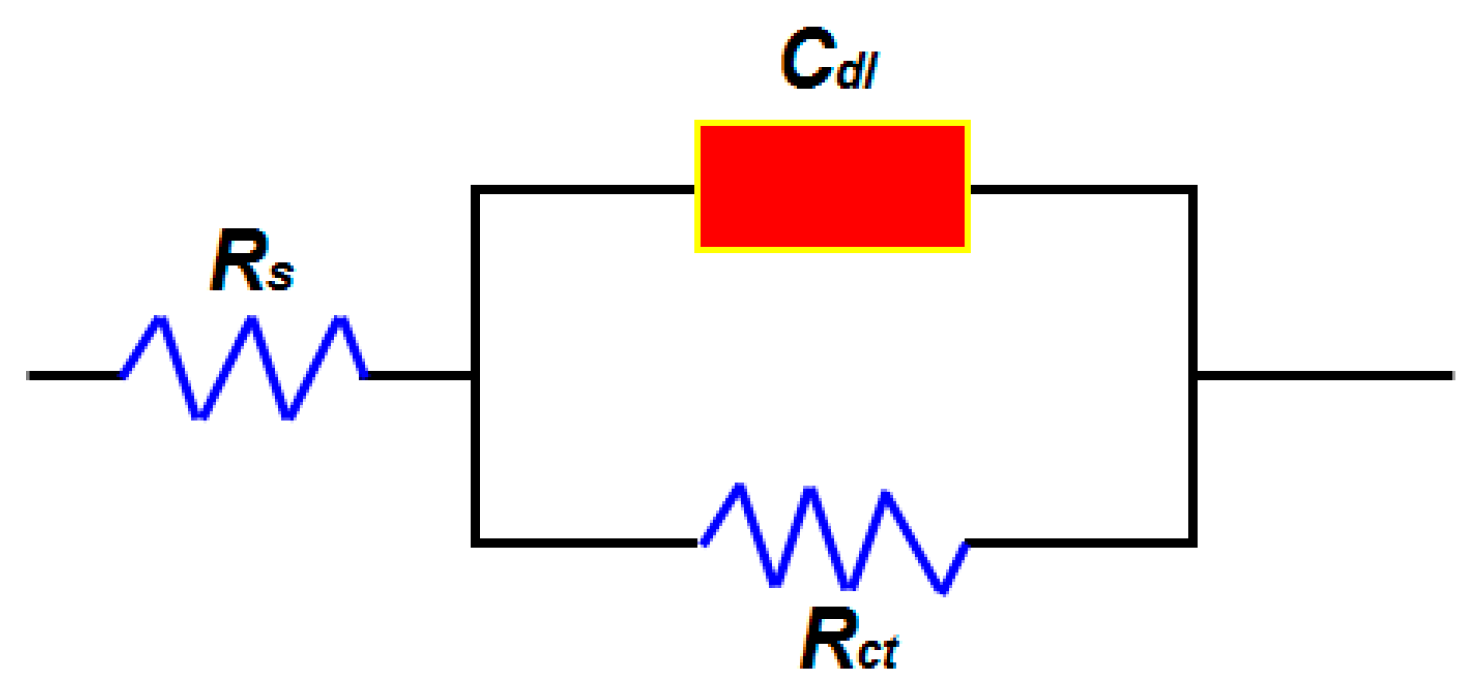
| Conc. | Rct (Ω cm2) | Cdl (Ω cm2) | IE (%) |
|---|---|---|---|
| Blank | 11.12 | 50.6 | - |
| 50 | 23.51 | 44.7 | 52 |
| 150 | 38.12 | 20.8 | 71 |
| 250 | 65.26 | 16.8 | 83 |
| Elements | Absence of Inhibitor (wt%) | Presence of Inhibitor (wt%) |
|---|---|---|
| C | 1.320 | 1.680 |
| O | 15.59 | 10.25 |
| Mg | 0.550 | 0.750 |
| Fe | 82.54 | 87.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baskar, P.; Rathinapriya, P.; Prabakaran, M. Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions. Processes 2022, 10, 1480. https://doi.org/10.3390/pr10081480
Baskar P, Rathinapriya P, Prabakaran M. Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions. Processes. 2022; 10(8):1480. https://doi.org/10.3390/pr10081480
Chicago/Turabian StyleBaskar, Prabu, Periyasamy Rathinapriya, and Mayakrishnan Prabakaran. 2022. "Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions" Processes 10, no. 8: 1480. https://doi.org/10.3390/pr10081480
APA StyleBaskar, P., Rathinapriya, P., & Prabakaran, M. (2022). Use of Trochodendron Aralioides Extract as Green Corrosion Inhibitor for Mild Steel in 1M HCl Solutions. Processes, 10(8), 1480. https://doi.org/10.3390/pr10081480









