A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes
Abstract
:1. Introduction
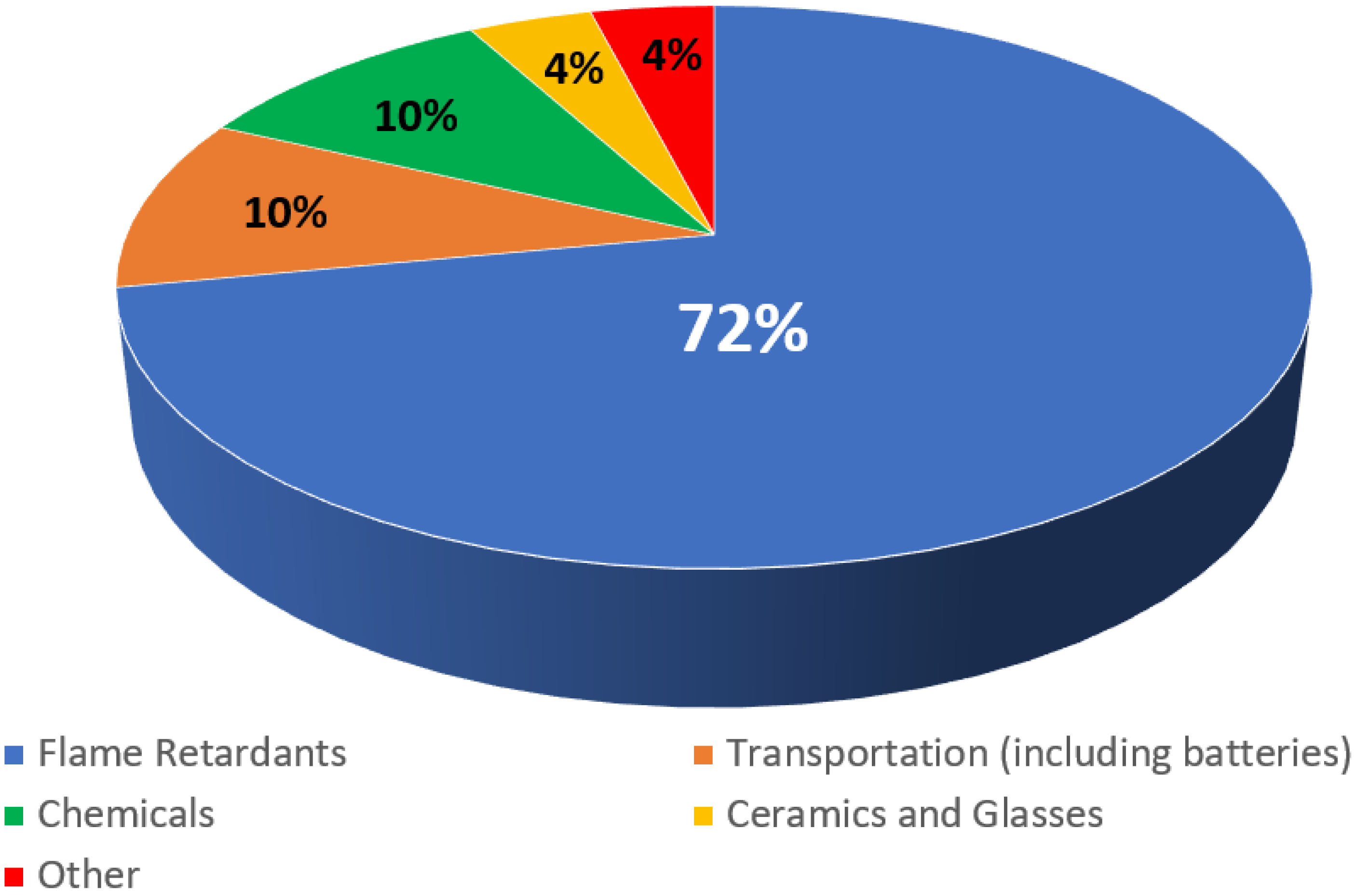
1.1. Applications
1.2. Mineralogy of Antimony Ores
1.3. Antimony Production
| Country | Mine Product. (t) [21] | Reserves (×1000 t) [21] | Main Producers [4] | Product [4] | Total Production Capacity (×1000 t/y Sb) [4] |
|---|---|---|---|---|---|
| USA | NA | 60 | Amspec Chemical Corporation Laurel Industries Inc. Great Lakes Chemical (Anzon) United States Antimony Corporation Sunshine Mining and Refining | Trioxide Trioxide Trioxide Metal, Trioxide, Na-antimonate Metal, Na-antimonate | 15 12.5 6 1.5 1.5 |
| Australia | 3400 | 100 | |||
| Bolivia | 2700 | 310 | Enal | Trioxide | 9.3 |
| Burma | 2000 | 140 | |||
| Canada | 2 | 78 | |||
| China | 60,000 | 480 | Hsikwangshan Mining Administration Dachang Mining Administration Guzhou Dushan Dongfeng Hubei Chongyang Hunan Chenzhou Mining Co, Ltd., Guangxi China Tin Group Limited Yunnan Muli antimony industry Co., Ltd. Xikuangshan Flash-Antimony Industry Limited | Metal, Oxides, Na-antimonate Metal Metal, Trioxide Metal, Trioxide N/A N/A N/A Metal [25] | 30 N/A 10 4 4 N/A N/A N/A 40 |
| Guatemala | 80 | NA | |||
| Iran | 400 | NA | |||
| Kazakhstan | 100 | NA | |||
| Kyrgyzstan | NA | 260 | Kadamjaisk Antimony Combine | Metal, Trioxide | 20 |
| Mexico | 700 | 18 | |||
| Pakistan | 20 | 26 | |||
| Russia (Recoverable) | 25,000 | 350 | |||
| Tajikistan | 13,000 | 50 | |||
| Turkey | 1300 | 100 | |||
| Vietnam | 400 | NA | |||
| France | NA | Société Industrielle et Chimique de L’Aisne Mines de la Lucette AMG antimony [26] | Metal, Trioxide Metal Trioxide | 12 9.5 10 | |
| Belgium | NA | Campine Union Minière/Umicore [5] | Trioxide Na-antimonate | 10 6 | |
| Oman | Strategic and Precious Metals Processing (SPMP) | Metal, Trioxide | 20 [27] | ||
| World Total (Rounded) | 110,000 | >2000 |
1.4. Objectives of the Current Review
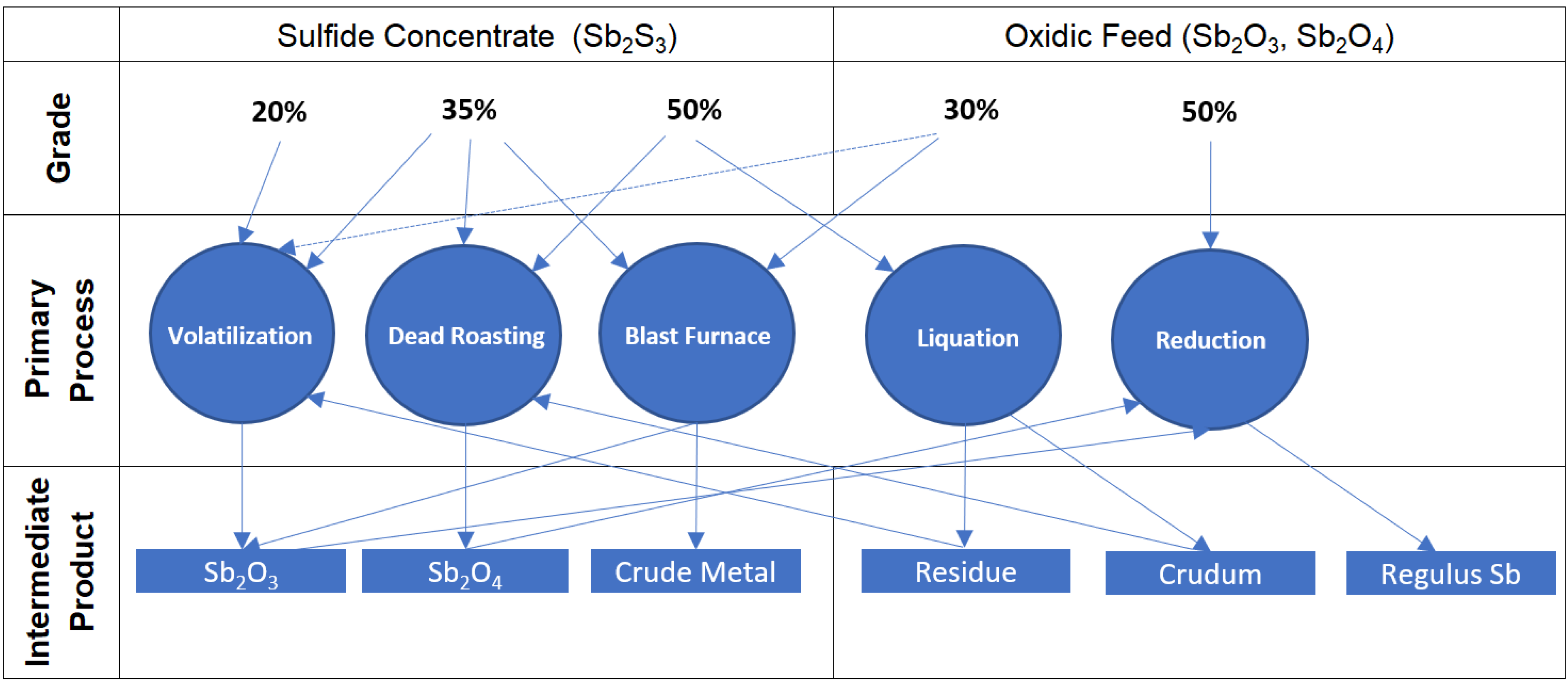
2. Pyrometallurgical Treatment of Antimony Concentrates
2.1. Volatilization—Reduction Smelting
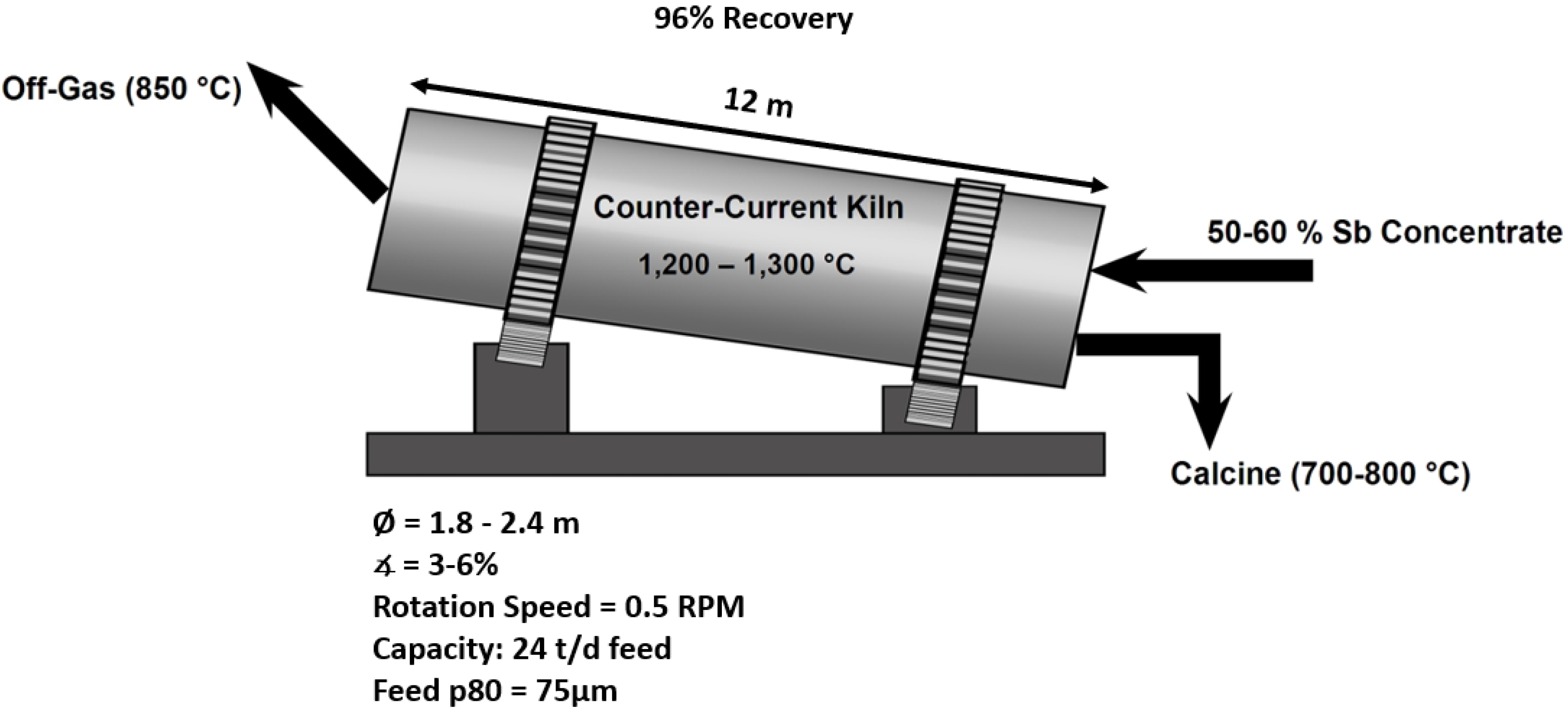
2.1.1. Conventional Volatilization Roasting
2.1.2. Flash Volatilization
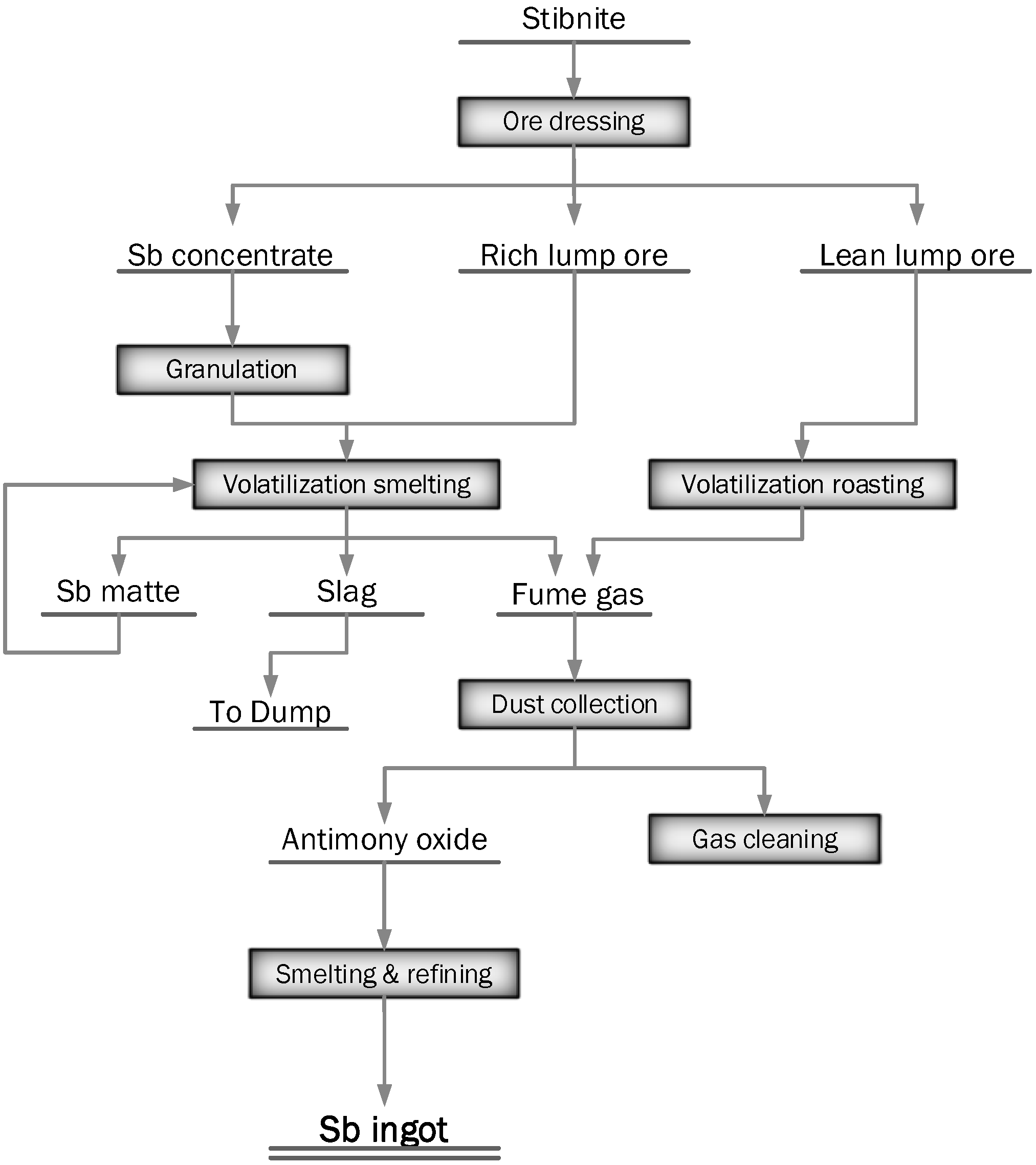
2.1.3. Volatilization Smelting in Blast Furnace (BF)
Stibnite Smelting in BF
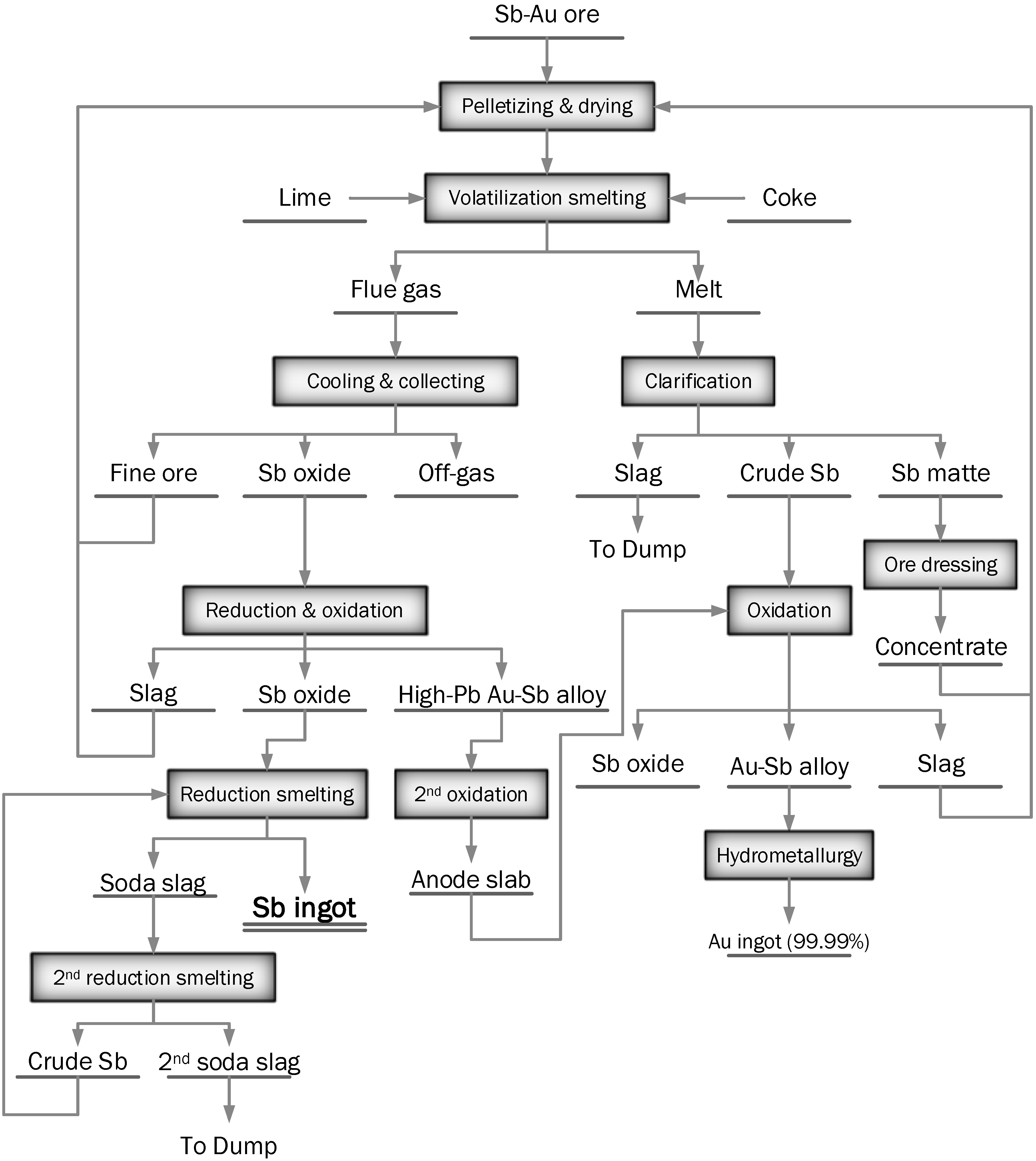
Gold-Antimony Smelting in BF
Copper-Antimony Smelting in BF
2.1.4. Volatilization Smelting in a Side-Blown Vessel
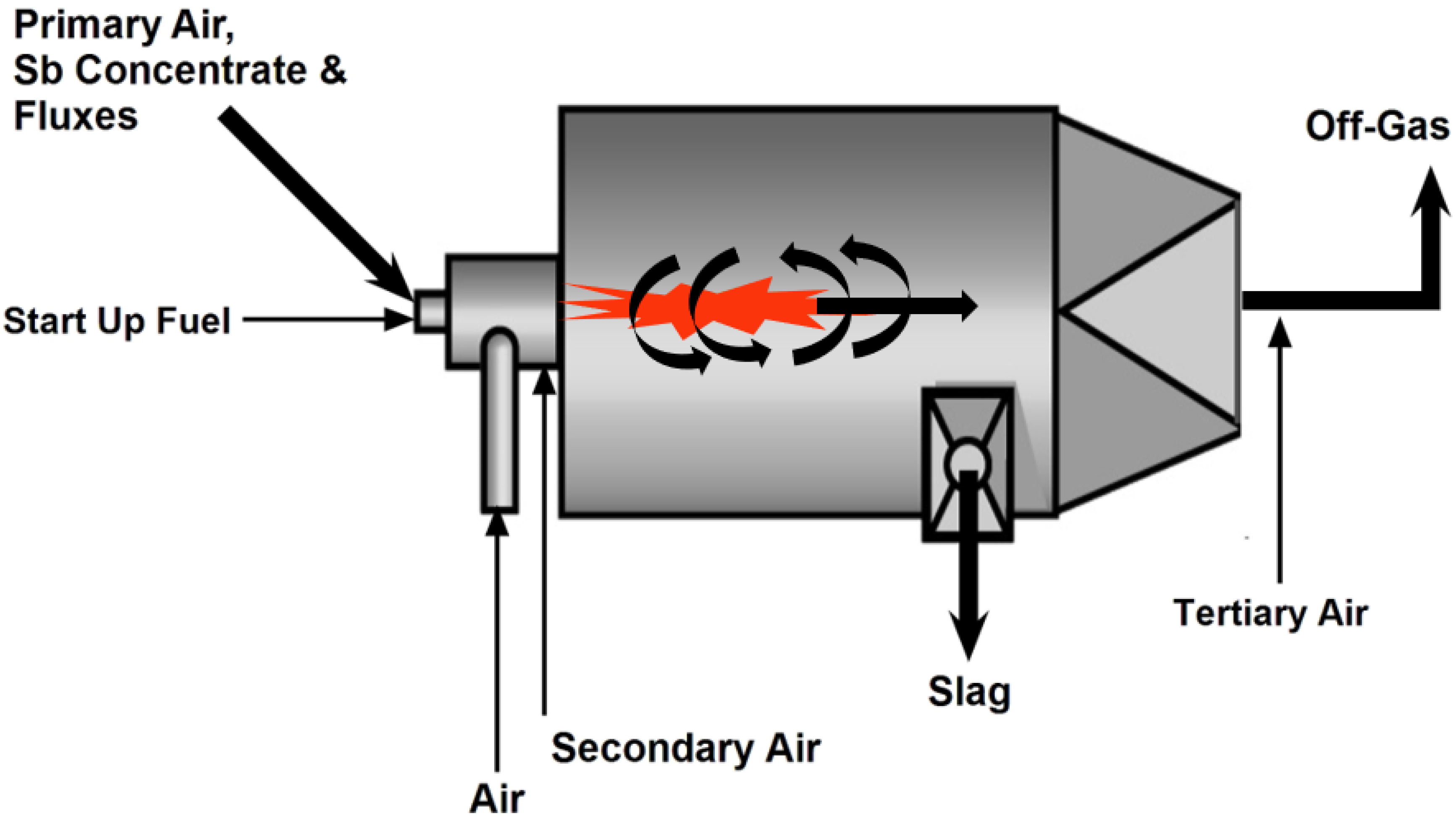
2.1.5. Cyclone Smelting
2.1.6. Vacuum Distillation Process
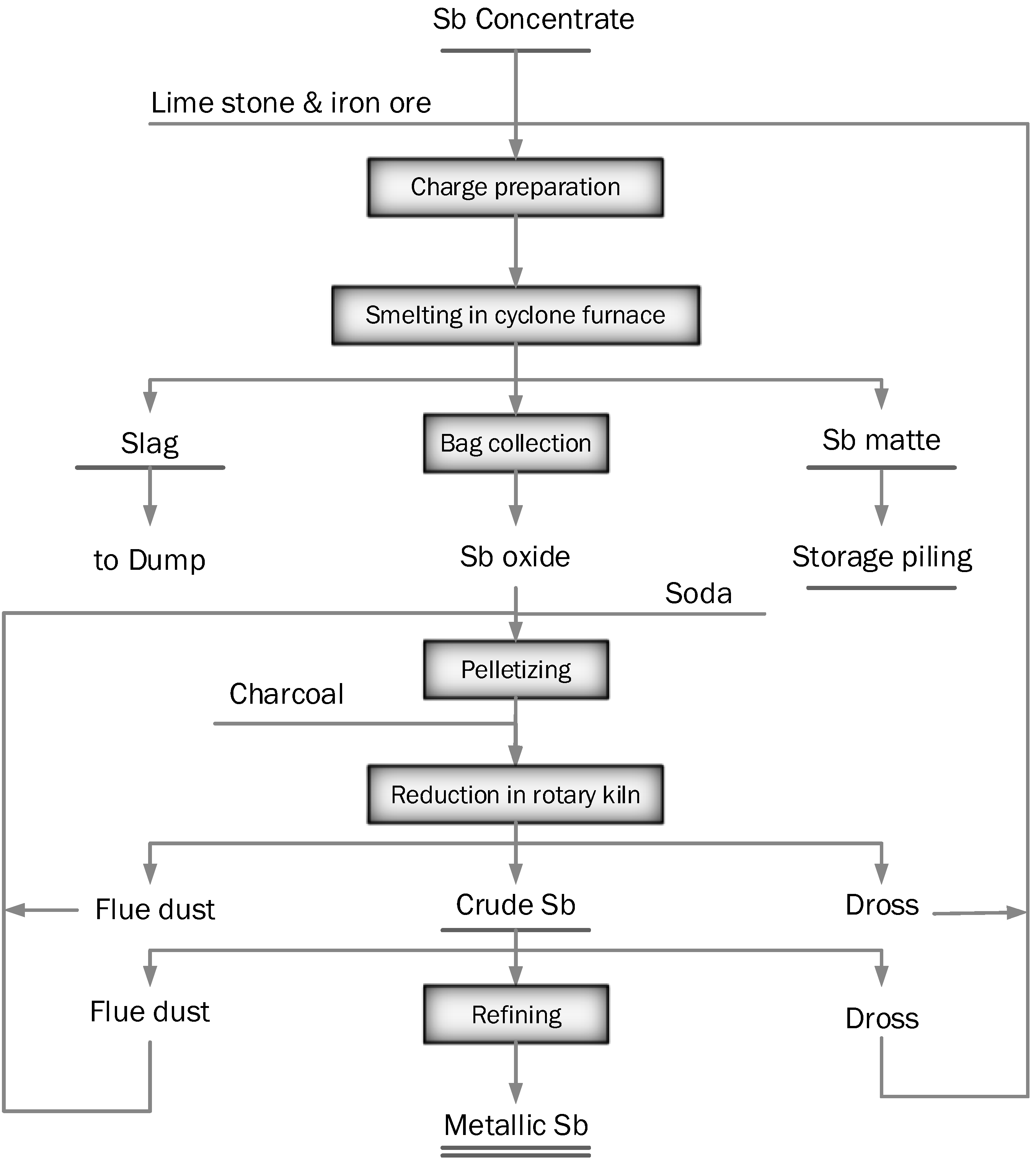
2.1.7. Reduction Smelting or Reverberatory Smelting of Oxides
2.2. Precipitation Processes
2.2.1. Iron Precipitation or English Precipitation
2.2.2. Liquation Followed by Iron-Precipitation
Alkaline Smelting/Reduction Smelting of Sulfides
2.2.3. Sulfur Fixing Methods
Sulfur-Fixing with Iron-Oxide as an Agent
Sulfur-Fixing with ZnO as an Agent
Carbothermic Reduction-Sulfur Fixation Using Lime
2.2.4. Decomposition of Stibnite
2.3. Direct Reduction to Metal
2.3.1. Blast Furnace Direct Reduction to Metal
2.3.2. Jamesonite Smelting in BF
2.3.3. Reaction Smelting (Mutual Reduction)
2.3.4. Hydrogen Reduction
2.4. Oxidation Smelting-Reduction Smelting
2.4.1. Dead Roasting Followed by Reduction Smelting
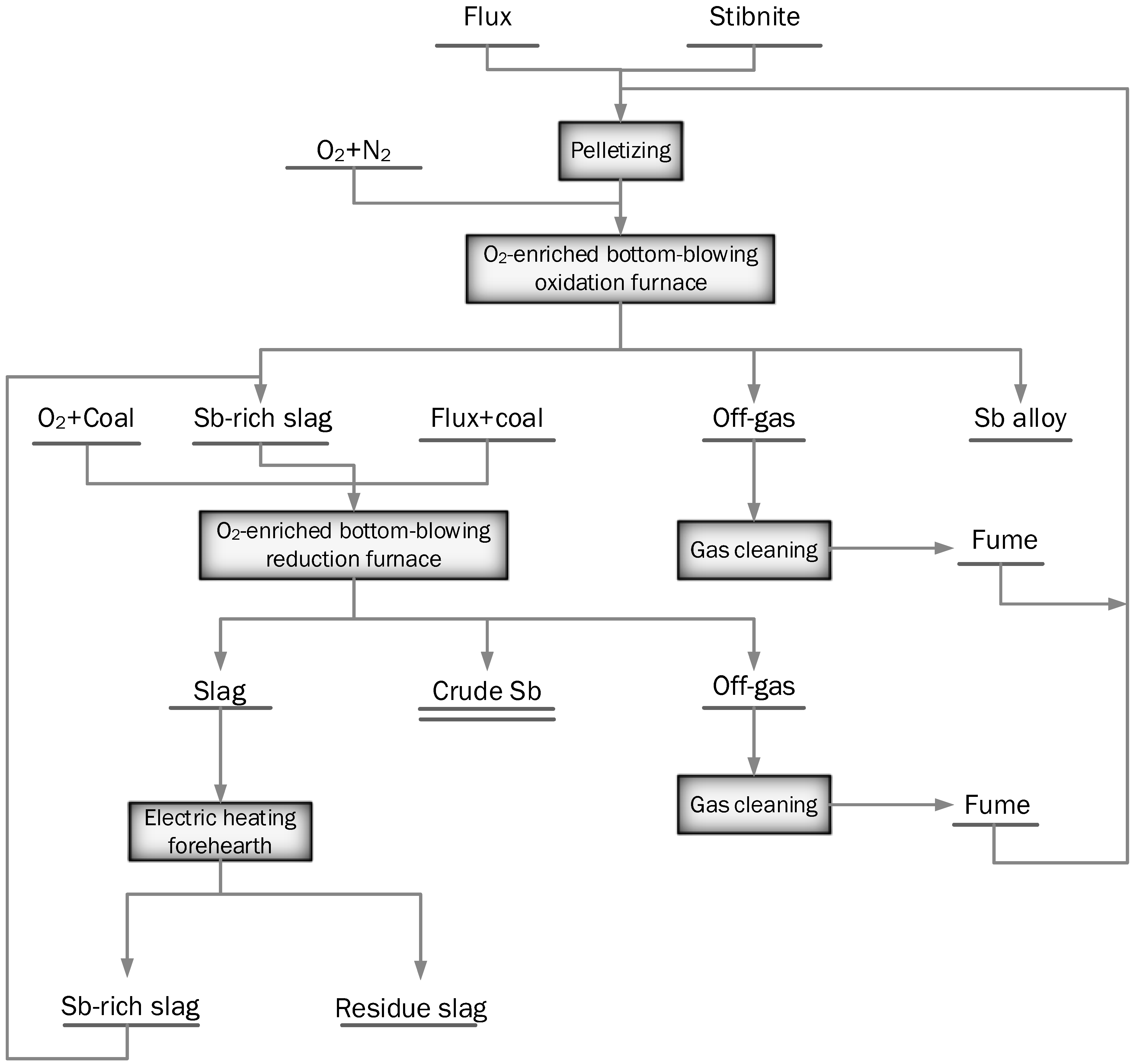
2.4.2. Oxygen-Enriched Bottom-Blown Vessels
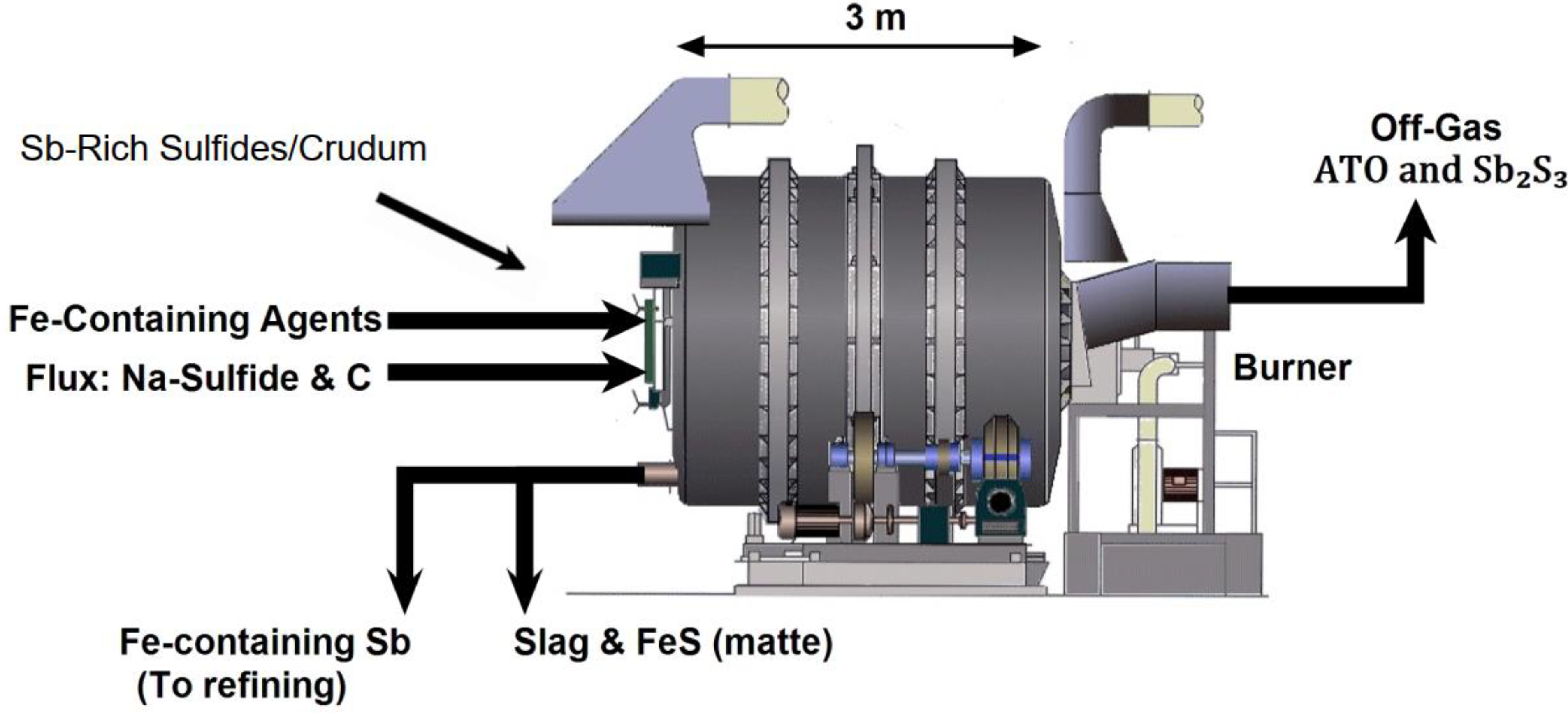
2.5. Matte Smelting
3. Technology Selection
4. Refining
4.1. Removal of Sulfur
4.2. Removal of Arsenic
4.3. Removal of Lead
4.4. Removal of Iron
4.5. Removal of Copper
5. Conclusions
- (1)
- Blast furnace volatilization—reduction smelting is currently the most commonly used method for treatment of concentrates; capable of smelting a wide range of antimony concentration in the feed.
- (2)
- Although smelting technology selection is highly dependent on antimony grade of the feed, modern technologies such as flash volatilization or side blown volatilization followed by reduction smelting are very promising and if proven at full-scale can be potential candidates for custom smelters. Molten salt electrolysis of antimony smelting is also a promising new technology of low carbon and green antimony smelting.
- (3)
- Promising environmentally friendly processes such as use of sulfur fixing agents have been recently developed but have yet to be proven at pilot- and full-scales.
- (4)
- Use of hydrogen in smelting of antimony sulfide and reduction of antimony oxides can be another green pyro-route for antimony production, which can contribute to deep decarbonization of the industry. However, more research and experimental work needs to be performed before the process can be used at industrial scale.
- (5)
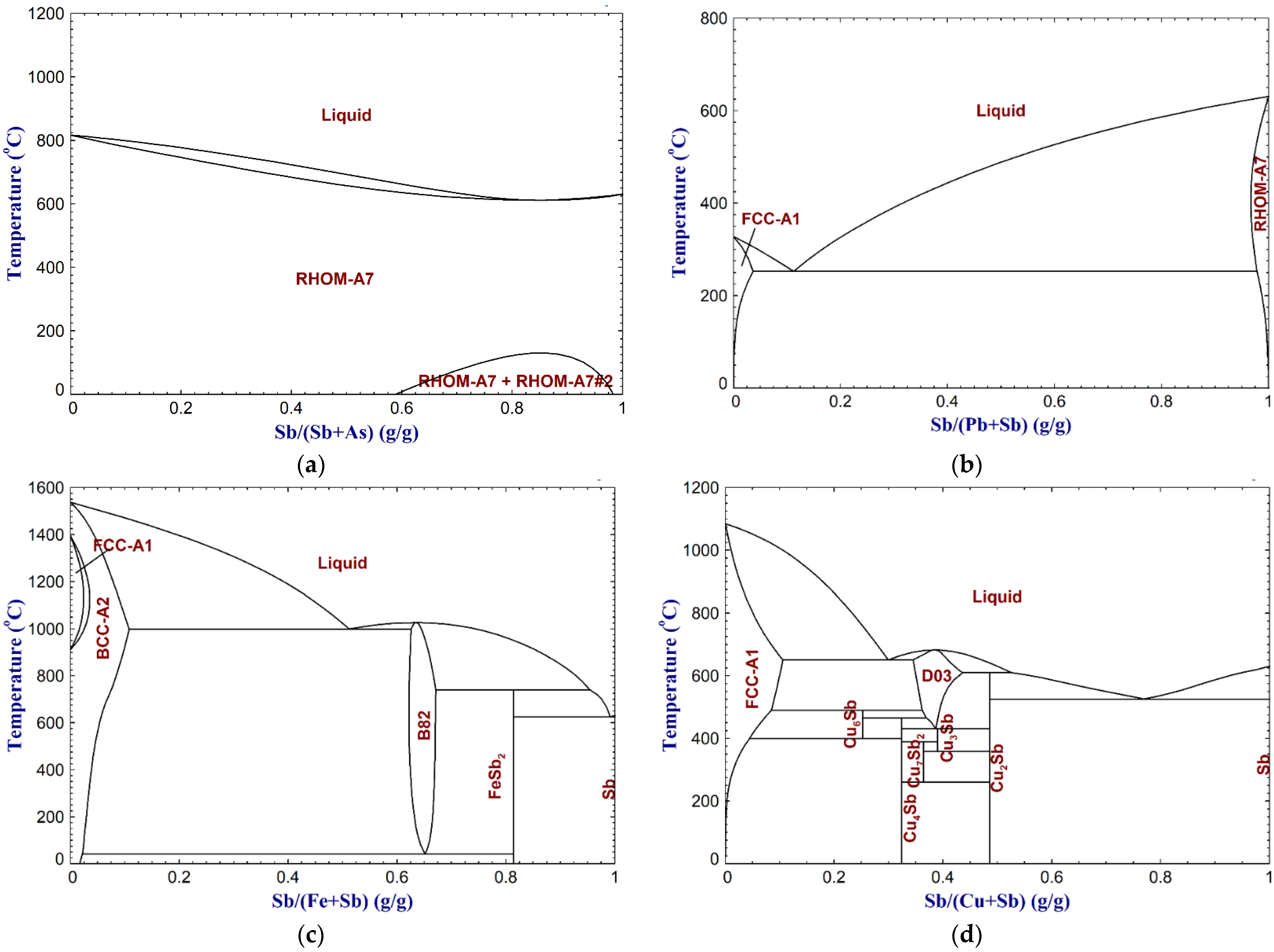
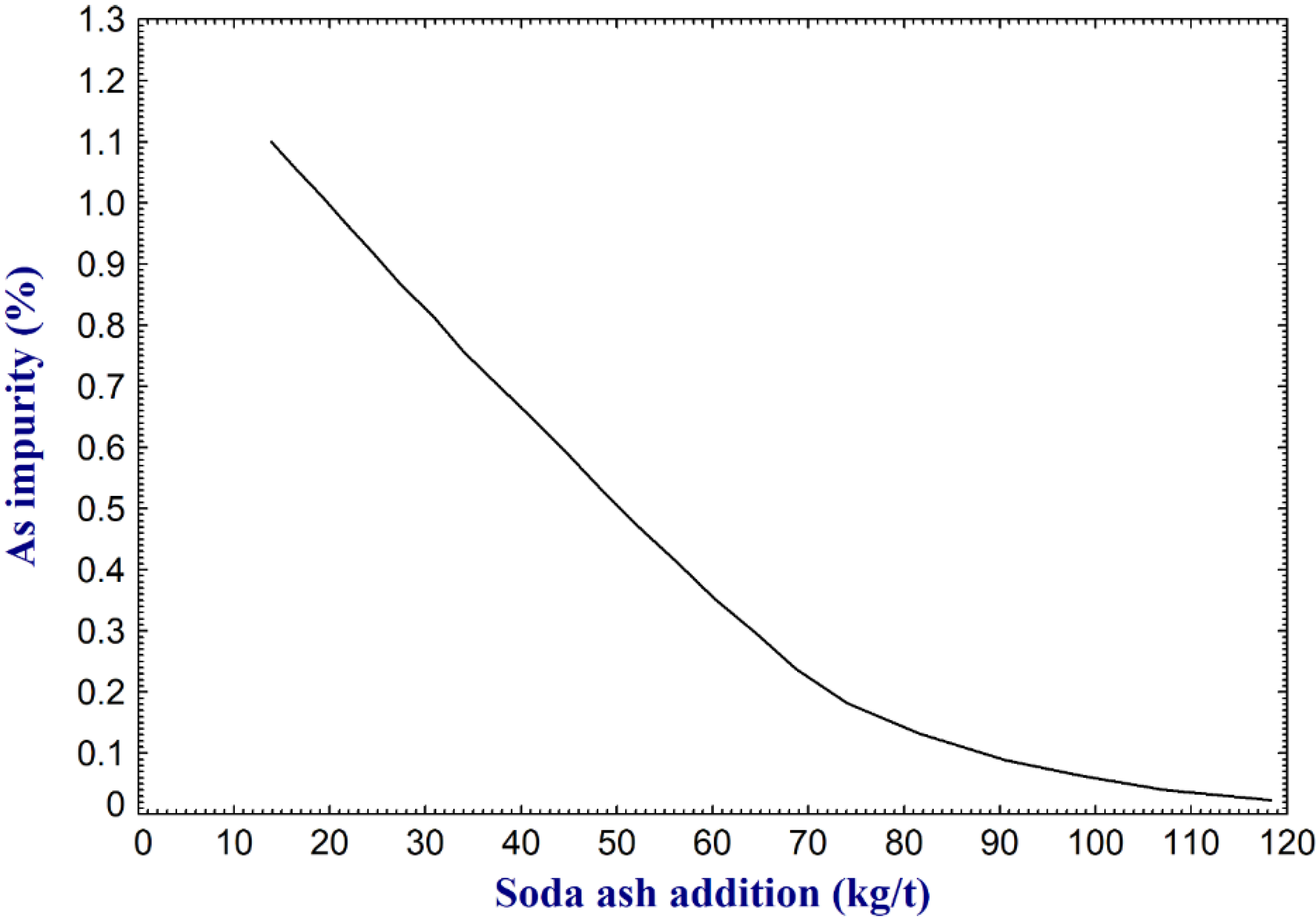
- Crude antimony contains various impurities (e.g., arsenic, sulfur, lead, iron, and copper), originating from the raw materials or production processes, and they should be removed to meet commercial and technical requirements. Often a multistage refining method should be selected based on the type of the impurity and applications of antimony product. For example, iron is removed via oxidation followed by sulfidation. Copper can be removed via selective crystallization and sulfidation. Lead can be removed via sulfidation followed by different methods such as phosphate addition, repeated oxidation volatilization-reduction, or vacuum distillation. For removal of arsenic, vacuum distillation or fire refining using soda flux were reported to be effective.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanley, G. Metals and metallurgy in the twentieth century. J. S. Afr. Inst. Min. Metall. 1946, 46, 235–267. [Google Scholar]
- Tian-Cong, Z. The Metallurgy of Antimony; Central South University of Technology Press: Changsha, China, 1988; p. 731. [Google Scholar]
- United States Geological Survey. Publications of the U.S. Geological Survey, 2000; United States Geological Survey: Washington, DC, USA, 2001.
- Anderson, C. Antimony production and commodities. SME Mineral Processing & Extractive Metallurgy Handbook; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2019. [Google Scholar]
- Umicore Antimony. Available online: https://www.umicore.com/en/about/our-metals/antimony/ (accessed on 30 April 2022).
- Umicore Antimony. Available online: https://www.usantimony.com/ (accessed on 3 May 2022).
- Keny, S.J.; Gokhale, B.K.; Kumbhar, A.G.; Bera, S.; Velmurugan, S.; Basu, S.; Suvarna, S.; Kiran, K. Antimony (Sb) Sorption at High Temperature and Pressure on Zircaloy, Carbon Steel (CS) and Magnetite Coated CS (MCS) Surfaces; Bhabha Atomic Research Centre Facilities: Kalpakkam, India, 2015. [Google Scholar]
- Antimony Substances. Available online: https://www.antimony.com/antimony/ (accessed on 30 April 2022).
- Binz, F.; Friedrich, B. Recovery of antimony trioxide flame retardants from lead refining residues by slag conditioning and fuming. Chem. Ing. Tech. 2015, 87, 1569–1579. [Google Scholar] [CrossRef]
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. How can we adapt to geological scarcity of antimony? Investigation of antimony’s substitutability and of other measures to achieve a sustainable use. Resour. Conserv. Recycl. 2016, 108, 54–62. [Google Scholar] [CrossRef]
- Claude, F. Non-ferrous metal: From Ag to Zn. Lannoo. Tielt 2002. [Google Scholar]
- Schmidt, M.L.M.; Domer, U. Rohstoffrisikobewertung—Antimon; Deutsche Rohstoffagentur (DERA) in der Bundesanstalt für Geowissenschaften und Rohstoffe (BGR): Berlin, Germany, 2015. [Google Scholar]
- Dupont, D.; Arnout, S.; Jones, P.T.; Binnemans, K. Antimony Recovery from End-of-Life Products and Industrial Process Residues: A Critical Review. J. Sustain. Metall. 2016, 2, 79–103. [Google Scholar] [CrossRef]
- Multani, R.S.; Feldmann, T.; Demopoulos, G.P. Antimony in the metallurgical industry: A review of its chemistry and environmental stabilization options. Hydrometallurgy 2016, 164, 141–153. [Google Scholar] [CrossRef]
- Schulz, K.J.; DeYoung, J.H., Jr.; Seal Ii, R.R.; Bradley, D.C. Critical Mineral Resources of the United States—An Introduction; 1802A; U.S. Geological Survey: Reston, VA, USA, 2017; p. 22.
- Habashi, F. Antimony. Handbook of Extractive Metallurgy; Habashi, F., Ed.; Wiley-VCH: Weinheim, Germany, 1997; Volume 2, pp. 822–844. [Google Scholar]
- Štrbac, N.; Mihajlović, I.; Minić, D.; Živković, Ž. Characterization of the natural mineral form from the PbS-Sb2S3 system. J. Min. Metall. B Metall. 2010, 46, 75–86. [Google Scholar] [CrossRef]
- Kovalev, K.R.; Kalinin, Y.A.; Naumov, E.A.; Myagkaya, M.K. Relationship of antimony with gold mineralization in the ore districts of Eastern Kazakhstan. Russ. Geol. Geophys. 2014, 55, 1170–1182. [Google Scholar] [CrossRef]
- Liu, W.-F.; Yang, T.-Z.; Chen, L.; Bin, S.; Bin, W.-D. Development of Antimony Smelting Technology in China. In 4th International Symposium on High-Temperature Metallurgical Processing; Springer: Berlin/Heidelberg, Germany, 2013; pp. 341–351. [Google Scholar]
- Other Metals. Available online: https://www.teck.com/products/other-metals/ (accessed on 30 April 2022).
- Mineral Commodity Summaries 2022; U.S. Geological Survey: Reston, VA, USA, 2022; p. 202.
- Englman, T.; Terkieltaub, E.; Etgar, L. High Open Circuit Voltage in Sb2S3/Metal Oxide-Based Solar Cells. J. Phys. Chem. C 2015, 119, 12904–12909. [Google Scholar] [CrossRef]
- Itzhaik, Y.; Bendikov, T.; Hines, D.; Kamat, P.V.; Cohen, H.; Hodes, G. Band Diagram and Effects of the KSCN Treatment in TiO2/Sb2S3/CuSCN ETA Cells. J. Phys. Chem. C 2016, 120, 31–41. [Google Scholar] [CrossRef]
- Perpetua Resources Could Help Secure U.S. Production of Critical Mineral Antimony. Available online: https://perpetuaresources.com/news/american-antimony-production/ (accessed on 30 April 2022).
- Guo, X.; Wang, K.; He, M.; Liu, Z.; Yang, H.; Li, S. Antimony smelting process generating solid wastes and dust: Characterization and leaching behaviors. J. Environ. Sci. 2014, 26, 1549–1556. [Google Scholar] [CrossRef]
- Antimony Trioxide. Available online: https://www.sica-chauny.com/en/products/antimony (accessed on 3 May 2022).
- SPMP. Available online: https://www.spmp.co.om/who-we-are (accessed on 3 May 2022).
- Henckens, M.L.C.M.; Driessen, P.P.J.; Worrell, E. Metal scarcity and sustainability, analyzing the necessity to reduce the extraction of scarce metals. Resour. Conserv. Recycl. 2014, 93, 1–8. [Google Scholar] [CrossRef]
- Juan Cancio, J.-L.; Ricardo Gerardo, S.-A.; Alejandro, C.-R.; José Antonio, R.-S.; Aurelio, H.-R.; Jorge Enrique, R.-S. Antimony recovery from recycled terminals of lead-acid batteries with Na2CO3 and SiC after the formation of Sb2O3. J. Min. Metall. Sect. B Metall. 2022, 58, 97–108. [Google Scholar]
- Ubaldini, S.; Veglio, F.; Toro, L.; Abbruzzese, C. Combined bio-hydrometallurgical process for gold recovery from refractory stibnite. Miner. Eng. 2000, 13, 1641–1646. [Google Scholar] [CrossRef]
- de Carvalho, L.C.; da Silva, S.R.; Giardini, R.M.N.; de Souza, L.F.C.; Leão, V.A. Bio-oxidation of refractory gold ores containing stibnite and gudmundite. Environ. Technol. Innov. 2019, 15, 100390. [Google Scholar] [CrossRef]
- Dembele, S.; Akcil, A.; Panda, S. Technological trends, emerging applications and metallurgical strategies in antimony recovery from stibnite. Miner. Eng. 2022, 175, 107304. [Google Scholar] [CrossRef]
- Panda, S.; Sarangi, C.K.; Pradhan, N.; Subbaiah, T.; Sukla, L.B.; Mishra, B.K.; Bhatoa, G.L.; Prasad, M.; Ray, S.K. Bio-hydrometallurgical processing of low grade chalcopyrite for the recovery of copper metal. Korean J. Chem. Eng. 2012, 29, 781–785. [Google Scholar] [CrossRef]
- Akcil, A.; Vegliò, F.; Ferella, F.; Okudan, M.D.; Tuncuk, A. A review of metal recovery from spent petroleum catalysts and ash. Waste Manag. 2015, 45, 420–433. [Google Scholar] [CrossRef]
- Anderson, C. Hydrometallurgically treating antimony-bearing industrial wastes. JoM 2001, 53, 18–20. [Google Scholar] [CrossRef]
- Anderson, C.; Twidwell, L. The alkaline sulfide hydrometallurgical separation, recovery and fixation of tin, arsenic, antimony, mercury and gold. Lead Zinc 2008, 2008, 121–132. [Google Scholar]
- Awe, S.A.; Sundkvist, J.-E.; Bolin, N.-J.; Sandström, Å. Process flowsheet development for recovering antimony from Sb-bearing copper concentrates. Miner. Eng. 2013, 49, 45–53. [Google Scholar] [CrossRef]
- Baláž, P.; Briančin, J.; Šepelák, V.; Havlik, T.; Škrobian, M. Non-oxidative leaching of mechanically activated stibnite. Hydrometallurgy 1992, 31, 201–212. [Google Scholar] [CrossRef]
- Garboś, S.; Bulska, E.; Hulanicki, A.; Fijałek, Z.; Sołtyk, K. Determination of total antimony and antimony(V) by inductively coupled plasma mass spectrometry after selective separation of antimony(III) by solvent extraction with N-benzoyl-N-phenylhydroxylamine. Spectrochim. Acta Part B At. Spectrosc. 2000, 55, 795–802. [Google Scholar] [CrossRef]
- Kyle, J.H.; Breuer, P.L.; Bunney, K.G.; Pleysier, R.; May, P.M. Review of trace toxic elements (Pb, Cd, Hg, As, Sb, Bi, Se, Te) and their deportment in gold processing. Part 1: Mineralogy, aqueous chemistry and toxicity. Hydrometallurgy 2011, 107, 91–100. [Google Scholar] [CrossRef]
- Lin, H.K. Extraction of antimony by a copper chloride extractant. Hydrometallurgy 2004, 73, 283–291. [Google Scholar] [CrossRef]
- Nordwick, S.; Anderson, C. Advances in antimony electrowinning at the Sunshine mine. In Proceedings of the Fourth International Symposium on Hydrometallurgy Fundamentals, Technology and Innovations, Salt Lake City, UT, USA, 13 August 1993; pp. 1107–1128. [Google Scholar]
- Solozhenkin, P.M.; Alekseev, A.N. Innovative processing and hydrometallurgical treatment methods for complex antimony ores and concentrates. Part I. J. Min. Sci. 2010, 46, 203–209. [Google Scholar] [CrossRef]
- Solozhenkin, P.M.; Alekseev, A.N. Innovative Processing and Hydrometallurgical Treatment Methods for Complex Antimony Ores and Concentrates. Part II: Hydrometallurgy of Complex Antimony Ores. J. Min. Sci. 2010, 46, 446–452. [Google Scholar] [CrossRef]
- Thibault, J.D.; MacDonald, M.D.; Stevens, D.A. Process for producing antimony trioxide. US Patent Patent no. 5,783,166, 1998. [Google Scholar]
- Vigdorovitch, V.N.; Ivleva, V.S.; Krol, L.I. Purification of antimony by zone recrystallization. Izvestia Akademii Nauk SSSR Metally Topi. 1960, 44, 29–35. [Google Scholar]
- Xu, Y.; Feng, P.; Yu, X.; Li, Y.; Yu, S.; Xie, G. Study on the Separation of Antimony by Hydrometallurgy with Typical Material Containing Sb. Multipurp. Util. Miner. Resour. 2017, 6, 31–35. [Google Scholar]
- Yang, J.-G.; Wu, Y.-T. A hydrometallurgical process for the separation and recovery of antimony. Hydrometallurgy 2014, 143, 68–74. [Google Scholar] [CrossRef]
- Anderson, C.; Nordwick, S.; Krys, L. Processing of antimony at the Sunshine mine. Residues Effl. Process. Environ. Consid. 1992, 349–366. [Google Scholar]
- Wang, Z.; Ru, J.; Bu, J.; Hua, Y.; Zhang, Y.; Xu, C. Direct Electrochemical Desulfurization of Solid Sb2S3 to Antimony Powders in Deep Eutectic Solvent. J. Electrochem. Soc. 2019, 166, D747–D754. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, J.; Tang, C.; Nan, T.; Ding, R.; Liu, J. Electroreduction of Antimony Sulfide Enhanced by Nitrogen Bottom Blowing in Molten NaCl-KCl-Na2S. JOM 2022, 74, 1889–1899. [Google Scholar] [CrossRef]
- Yin, H.; Chung, B.; Sadoway, D.R. Electrolysis of a molten semiconductor. Nat. Commun. 2016, 7, 12584. [Google Scholar] [CrossRef]
- Wang, C.Y. Antimony, Its History, Chemistry, Etc., 2nd ed.; Charles Griffin & Company Ltd.: London, UK, 1919. [Google Scholar]
- Bray, J.L. Non-Ferrous Production Metallurgy; J. Wiley: New York, NY, USA, 1947. [Google Scholar]
- Anderson, C.G. The metallurgy of antimony. Geochemistry 2012, 72, 3–8. [Google Scholar] [CrossRef]
- Padilla, R.; Chambi, L.C.; Ruiz, M.C. Antimony production by carbothermic reduction of stibnite in the presence of lime. J. Min. Metall. B Metall. 2014, 50, 5–13. [Google Scholar] [CrossRef]
- Guo-Hui, S. Chinese blast-furnace for volatilization smelting of antimony. Inst. Min. Metall. Trans. 1984, 93, 186–192. [Google Scholar]
- Lager, T.; Forssberg, K.S.E. Current processing technology for antimony-bearing ores a review, part 2. Miner. Eng. 1989, 2, 543–556. [Google Scholar] [CrossRef]
- Ziao, Y.F.; Zhou, W.T. Improved Smelting and Refining Processes for Production of Antimony and its Oxides in Xikuangshan, China; The Institute of Scientific and Technical Information of China: Beijing, China, 1987. [Google Scholar]
- Padilla, R.; Ramírez, G.; Ruiz, M.C. High-Temperature Volatilization Mechanism of Stibnite in Nitrogen-Oxygen Atmospheres. Metall. Mater. Trans. B 2010, 41, 1284–1292. [Google Scholar] [CrossRef]
- Ren, B.; Zhou, Y.; Ma, H.; Deng, R.; Zhang, P.; Hou, B. Sb release characteristics of the solid waste produced in antimony mining smelting process. J. Mater. Cycles Waste Manag. 2018, 20, 193–200. [Google Scholar] [CrossRef]
- Liu, W.; Luo, H.; Qing, W.; Zheng, Y.; Yang, K.; Han, J. Investigation into Oxygen-Enriched Bottom-Blown Stibnite and Direct Reduction. Metall. Mater. Trans. B 2014, 45, 1281–1290. [Google Scholar] [CrossRef]
- Yang, J.-G.; Tang, C.-B.; Chen, Y.-M.; Tang, M.-T. Separation of Antimony from a Stibnite Concentrate Through a Low-Temperature Smelting Process to Eliminate SO2 Emission. Metall. Mater. Trans. B 2011, 42, 30–36. [Google Scholar] [CrossRef]
- Qin, W.-Q.; Luo, H.-L.; Liu, W.; Zheng, Y.-X.; Yang, K.; Han, J.-W. Mechanism of stibnite volatilization at high temperature. J. Cent. South Univ. 2015, 22, 868–873. [Google Scholar] [CrossRef]
- Wang, H.; You, C. Photocatalytic removal of low concentration SO2 by titanium dioxide. Chem. Eng. J. 2016, 292, 199–206. [Google Scholar] [CrossRef]
- Liu, T.; Qiu, K. Removing antimony from waste lead storage batteries alloy by vacuum displacement reaction technology. J. Hazard. Mater. 2018, 347, 334–340. [Google Scholar] [CrossRef]
- Bo, C. Method and Special Equipment for Direct Production of Ultrafine Antimony Trioxide by Volatilization and Smelting in Blast Furnace. CN Patent 1339612A, 13 March 2000. [Google Scholar]
- Mo-Tang, T.; Gui-Zhong, J. Industrial experiment on the volatilization-matt making smelting of antimony concentration. China Nonferrous Metall. 2007, 3, 34–36. [Google Scholar]
- Padilla, R.; Moscoso, I.; Ruiz, M.C. Mechanism and Kinetics of Stibnite Chloridizing with Calcium Chloride-Oxygen at 500 °C to 650 °C. Metall. Mater. Trans. B 2019, 50, 219–228. [Google Scholar] [CrossRef]
- Wu, X. Application of CSC Technology in Nonferrous Metallurgy. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: Cham, Switzerland, 2020; pp. 201–217. [Google Scholar]
- Zhang, X.; Friedrich, S.; Friedrich, B. Separation behavior of arsenic and lead from antimony during vacuum distillation and zone refining. J. Mater. Res. Technol. 2020, 9, 4386–4398. [Google Scholar] [CrossRef]
- Worrell, A.C.; Taft, M.A. Overview of Foreign Nonferrous Smelter Technology; Industrial Environmental Research Laboratory: Cincinnati, OH, USA, 1980. [Google Scholar]
- Zhou, Z.; Liu, D.; Xiong, H.; Zhang, B.; Yang, B.; Deng, Y.; Zhao, J. Separation and purification Sb2S3 from stibnite by vacuum distillation. Vacuum 2018, 157, 487–491. [Google Scholar] [CrossRef]
- Ye, L.; Ouyang, Z.; Chen, Y.; Chen, Y.; Xiao, L. Sulfur fixation and reduction roasting of stibnite for clean extraction of antimony by a combined metallurgy and beneficiation process. Miner. Eng. 2019, 144, 106049. [Google Scholar] [CrossRef]
- Wei, W.X. Practice of producing high lead-antimony alloy by fire process of brittle sulfur antimony lead ore. China Nonferrous Metall. 1992, 2, 42–44. [Google Scholar]
- Dunne, R. SME Mineral Processing and Extractive Metallurgy Handbook; Society of Mining, Metallurgy & Exploration: Englewood, CO, USA, 2019. [Google Scholar]
- Li, Y.; Chen, Y.; Xue, H.; Tang, C.; Yang, S.; Tang, M. One-Step Extraction of Antimony in Low Temperature from Stibnite Concentrate Using Iron Oxide as Sulfur-Fixing Agent. Metals 2016, 6, 153. [Google Scholar] [CrossRef]
- Ye, L.; Tang, C.; Yang, S.; Chen, Y.; Zhang, W. Removal of lead from crude antimony by using NaPO3 as lead elimination reagent. J. Min. Metall. B Metall. 2015, 51, 97–103. [Google Scholar] [CrossRef]
- Ouyang, Z.; Chen, Y.F.; Tian, S.Y.; Xiao, L.; Tang, C.B.; Hu, Y.J.; Xia, Z.M.; Chen, Y.M.; Ye, L.G. Thermodynamic and kinetics analysis of the sulfur-fixed roasting of antimony sulfide using ZnO as sulfur-fixing agent. J. Min. Metall. Sect. B Metall. 2018, 54, 411–418. [Google Scholar] [CrossRef]
- Igiehon, U.; Terry, B.; Grieveson, P. Carbothermic reduction of antimony sulphide. Trans. Inst. Min. Metall. Sect. C 1992, 101, 144–154. [Google Scholar]
- Wu, X. The Recovery of Pb and Zn in Antimony Smelting Slag. In PbZn 2020: 9th International Symposium on Lead and Zinc Processing; Springer: Cham, Switzerland, 2020; pp. 653–660. [Google Scholar]
- Yong, D.; Bin, Y.; Baoqiang, X.; Dachun, L.; Youth, Y.; Heng, X.; Yongnian, D.; Yang, T.; Hualong, Y.; Wei, W.; et al. Method for Removal of Lead and Arsenic in Crude Antimony Vacuum Refining Process. CN Patent Patent no. 104328289 A, 2014. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Min-Chin, L. A simple fractional distillation method for refining high purity antimony. Acta Metallurgica Sinica 1964, 7, 442–445. [Google Scholar]
- Bonnier, E.; Charveriat, M. Purification de l′antimoine par sublimation. Metallurgie 1965–1966, 5, 319. [Google Scholar]
- Jevtic, D.; Vitorovic, D. A New Procedure for the Preparation of Antimony of High Purity Combined with the Production of Antimony Trioxide of High Purity. Prod. RD 1974, 13, 275–279. [Google Scholar] [CrossRef]
- Ivanov, V.Y.; Papirov, I.I. Pure and ultrapure metals. Metallurgia 1965. [Google Scholar]
- Terlikbayeva, A.Z.; Sydykov, A.O.; Berdikulova, F.A.; Mazulevsky, E.A. Producing Metallic Antimony with Low Arsenic Content from Antimony Concentrate. Russ. J. Non-Ferr. Met. 2018, 59, 256–260. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Li, Q. Arsenic pre-removal from antimony oxide powder by roasting with pyrite (FeS2) for decreasing arsenic transfer and pollution in the followed antimony smelting process. Sep. Sci. Technol. 2021, 57, 1978–1991. [Google Scholar] [CrossRef]
- Cui, Y.; Matsuura, H.; Hamano, T.; Tsukihashi, F. Removal and recycling of antimony from liquid copper by using CuCl-CaO fluxes at 1423 K. Metall. Mater. Trans. B 2007, 38, 485–489. [Google Scholar] [CrossRef]
- Cui, Y.; Matsuura, H.; Hamano, T.; Tsukihashi, F. Removal of antimony from liquid copper by using CuCl–Na2CO3 fluxes at 1423 K. ISIJ Int. 2008, 48, 23–27. [Google Scholar] [CrossRef]
- Tao, Z.; Xiaolan, Z.; Bogun, W.; Jiangen, W., Jr. Antimony Fire Refining Lead Removal Agent and Its Application. CN Patent 102041399 A, 2011. [Google Scholar]
- Xiaolan, Z.; Tao, Z.; Chi, Z. A Lead Removal Agent for Melt Extraction Refining of Antimony. CN Patent 1455010A, 2002. [Google Scholar]






| Properties/Characteristics | Value | Unit |
|---|---|---|
| Atomic number | 51 | N/A |
| Atomic weight | 121.76 | u |
| Melting point | 630.5 | °C |
| Boiling point (at 101.3 kPa) | 1325 | °C |
| Density (at 20 °C) | 6.688 | g/cm3 |
| Tensile strength | 10.8 | N/mm2 |
| Mohs hardness | 3.0-3.5 | N/A |
| Modulus of elasticity | 566 | N/mm2 |
| Surface tension of solid (at 432 °C) | 317.2 | mN/m |
| Surface tension of liquid (at 1200 °C) | 255 | mN/m |
| Crystal structure | Rhombohedral | N/A |
| Lattice constant | a = 0.437, c = 1.1273 | nm |
| Latent heat of fusion | 10.49 | kJ/mol |
| Latent heat of evaporation | 195.10 | kJ/mol |
| Coefficient of linear expansion (at 20 °C) | 8-11 | µm/m-°C |
| Electrical resistivity (at 0 °C) | 37 | µΩ.cm |
| Molar heat capacity of solid (at 630.5 °C) | 30.446 | J/mol-K |
| Molar heat capacity of liquid (at 630.5 °C) | 31.401 | J/mol-K |
| Thermal conductivity (at 0 °C) | 25.9 | W/m-K |
| Physical Form | Powder | ||
| Particle size (µm) | 0.2–44 | ||
| Constituents | Low PbO | Medium PbO | High PbO |
| Sb2O3 (wt%) | >98 | >97.1 and ≤99.6 | >97 and ≤99.6 |
| PbO (wt%) | <0.25 | >0.25 and <0.3 | ≥0.3 and <2.5 |
| As2O3 (wt%) | <0.1 | <0.1 | <0.1 |
| Other impurities (wt%) | <1.75 | ≤2.6 | ≤0.4 |
| Mineral | Chemical Formula | Sb (wt%) | |
|---|---|---|---|
| Sulfides | Stibnite | Sb2S3 | 71.7 |
| Tetrahedrite | Cu6Sb2S6 | 29.8 | |
| Jamesonite | Pb4FeSb6S14 | 35.4 | |
| Zinckenite | PbSb2S4 | 42.1 | |
| Oxides | Senarmontite (cubic) | Sb2O3 | 83.5 |
| Valentinite (rhombohedral) | Sb2O3 | 83.5 | |
| Cervantite (orthorhombic) | Sb2O4/Sb2O3⋅Sb2O5 | 79.2 | |
| Stibiconite (antimony hydroxides) | Sb2O4⋅H2O | 74.8 | |
| Mixed | Kermesite | 2Sb2S3⋅Sb2O3 | 83.5 |
| Element/ Compound | Sb | Sb2S3 | Sb2O3 | Sb2S5 | Sb2O5 | Sb2O4 |
|---|---|---|---|---|---|---|
| Name(s) |
|
|
|
|
|
|
| Melting point | 630 °C | 546–548 °C | 656 °C | 120–170 °C (decomposes to Sb2S3 and S) | 380 °C (decomposes) | 930 °C (decomposes to ATO and oxygen) |
| Boiling point | 1635 °C | 1000–1150 °C | 1425 °C (sublimes) | N/A | N/A | N/A |
| Parameter (Unit) | Value |
|---|---|
| Furnace area (m2) | 2.2 |
| Bed capacity (t/m2-d) | 35–60 |
| Concentrate grade (%) | 30–50 |
| Antimony in ATO (%) | 79–81 |
| Antimony in slag (%) | 0.28–0.9 |
| Antimony recovery (%) | >96 |
| Coal rate (%) | 13–16 |
| Oxygen enrichment (%) | 60–70 |
| SO2 content of off-gas (%) | 10–20 |
| Sulfur capture rate (%) | >99 |
| Parameter | Value |
|---|---|
| Sodium sulfate | 40–55% of concentrate |
| Reducing coal | 3–6% of concentrate |
| Operating temperature | 1150–1200 °C |
| Antimony-recovery in matte | 78–86% of input |
| Antimony-loss to slag | 0.6–2% of input |
| Antimony to off-gas | Balance of antimony |
| Antimony-grade of concentrate | 40–50% |
| Dissolution of antimony in leachant | 98–99% |
| Process Routes | Process Technology Options | Major Disadvantage (-)/Advantage (+) | |
|---|---|---|---|
| Volatilization roasting/smelting-Reduction smelting (sulfides) | Blast furnace; Conventional volatilization in rotary kiln; Flash volatilization in rotary kiln; Cyclone smelting; Side-blown vessels; Vacuum distillation | (−) Mainly used for low-grade and high-grade concentrates, but not intermediate grades | (+) Efficient recovery of PMs |
| Non-volatilization roasting—Reduction smelting (sulfides) | Dead roasting (different units); O2-rich bottom-blown smelting and reduction | (−) Stronger reducing condition is required in the reduction step (i.e., more CO2 generation) | (+) Relatively easier process control |
| Direct reduction smelting to metal (sulfides/oxides) | Blast furnace; Reaction smelting (mutual reduction); Hydrogen reduction; Electric furnace reduction smelting (only for primary oxidic ores/intermediate oxidic products) | (+)/(−) Most suitable for intermediate grades but not for high- or low-grades | |
| Precipitation processes (sulfides) | Iron (English) precipitation; Liquation—reduction smelting; Alkaline smelting—reduction smelting; S-fixing smelting methods (using CaO, ZnO, or FeO) | (−) Yet to be proven at demonstration scale | (+) Potentially more environmentally friendly processes |
| Matte smelting (sulfides) | Molten matte—alkaline leaching—electrowinning; molten matte—electrolysis | (−) Not a common process, low recovery, and only applicable for a narrow window of feed composition (40–50% Sb) | (+) None can be highlighted |
| Ore type | Sb | Process |
|---|---|---|
| Sulfide (extremely high grade) | >60% | Iron precipitation |
| Cyclone smelting | ||
| Sulfide (high grade) | 40–60% | Flash volatilization in rotary kiln—Reduction smelting |
| Volatilization smelting in blast furnace—Reduction smelting | ||
| Liquation—iron precipitation | ||
| Sulfide (intermediate grade) | 25–40% | Direct reduction in blast furnace |
| Sulfide (low grade) | 15–25% | Volatilization roasting in rotary kiln—Reduction smelting |
| Oxide (all grades) | - | Reduction smelting in reverberatory, electric or blast furnace |
| Mixed sulfide and oxide | - | Volatilization smelting blast furnace—Reduction smelting |
| Direct reduction in blast furnace |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moosavi-Khoonsari, E.; Mostaghel, S.; Siegmund, A.; Cloutier, J.-P. A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes. Processes 2022, 10, 1590. https://doi.org/10.3390/pr10081590
Moosavi-Khoonsari E, Mostaghel S, Siegmund A, Cloutier J-P. A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes. Processes. 2022; 10(8):1590. https://doi.org/10.3390/pr10081590
Chicago/Turabian StyleMoosavi-Khoonsari, Elmira, Sina Mostaghel, Andreas Siegmund, and Jean-Pierre Cloutier. 2022. "A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes" Processes 10, no. 8: 1590. https://doi.org/10.3390/pr10081590
APA StyleMoosavi-Khoonsari, E., Mostaghel, S., Siegmund, A., & Cloutier, J.-P. (2022). A Review on Pyrometallurgical Extraction of Antimony from Primary Resources: Current Practices and Evolving Processes. Processes, 10(8), 1590. https://doi.org/10.3390/pr10081590








