Impact of Gum Arabic Coating Pretreatment on Quality Attributes of Oven-Dried Red Raspberry (Rubus idaeus L.) Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Chemicals
2.2. Gum Arabic Coating Preparation
2.3. Pretreatment and Drying
2.4. Determination of Physicochemical Properties
2.4.1. Moisture Content, Water Activity, and Colour
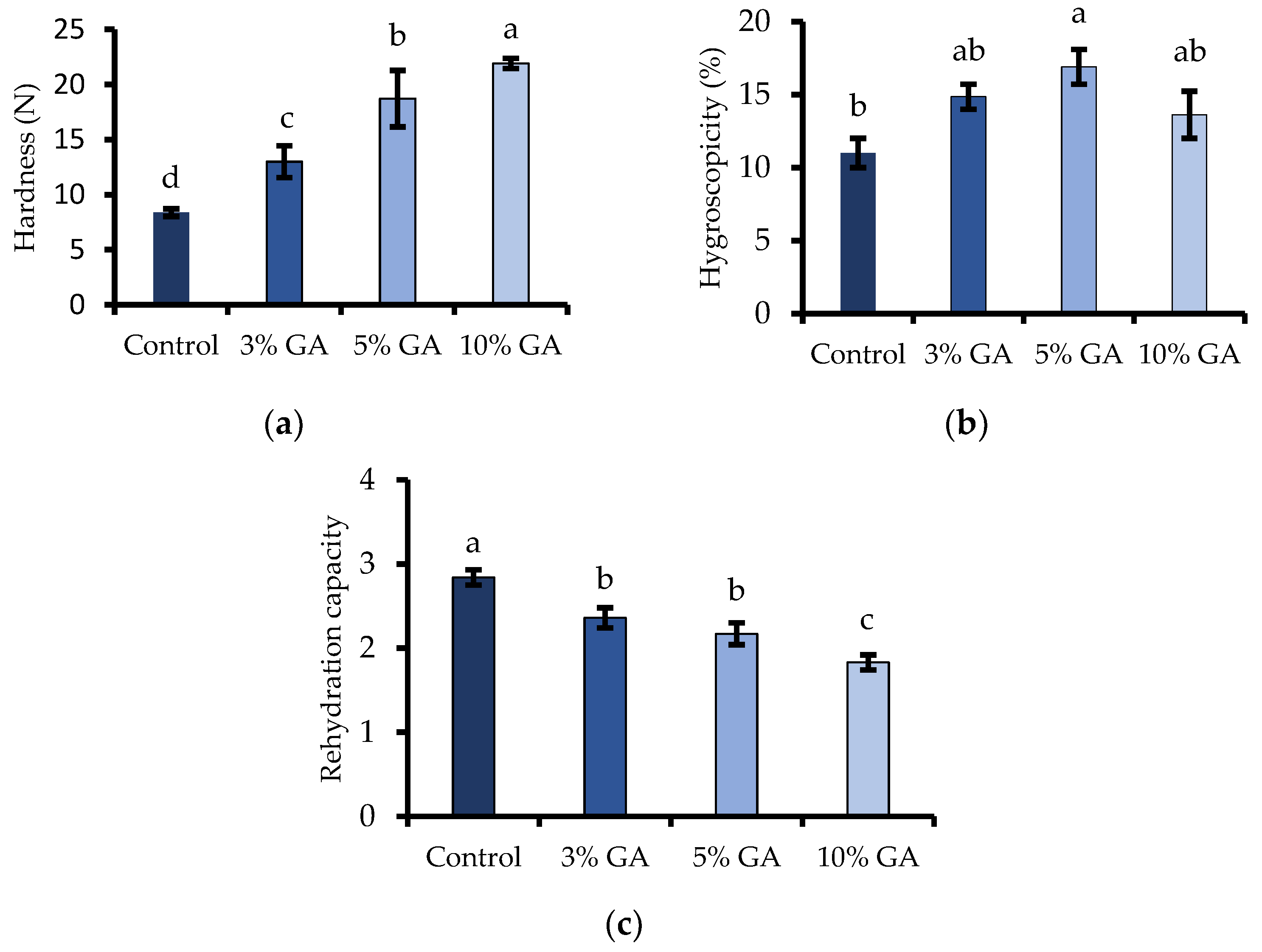
2.4.2. Hardness, Hygroscopicity, and Rehydration Capacity
2.4.3. Total Soluble Solids, pH, and Titratable Acidity
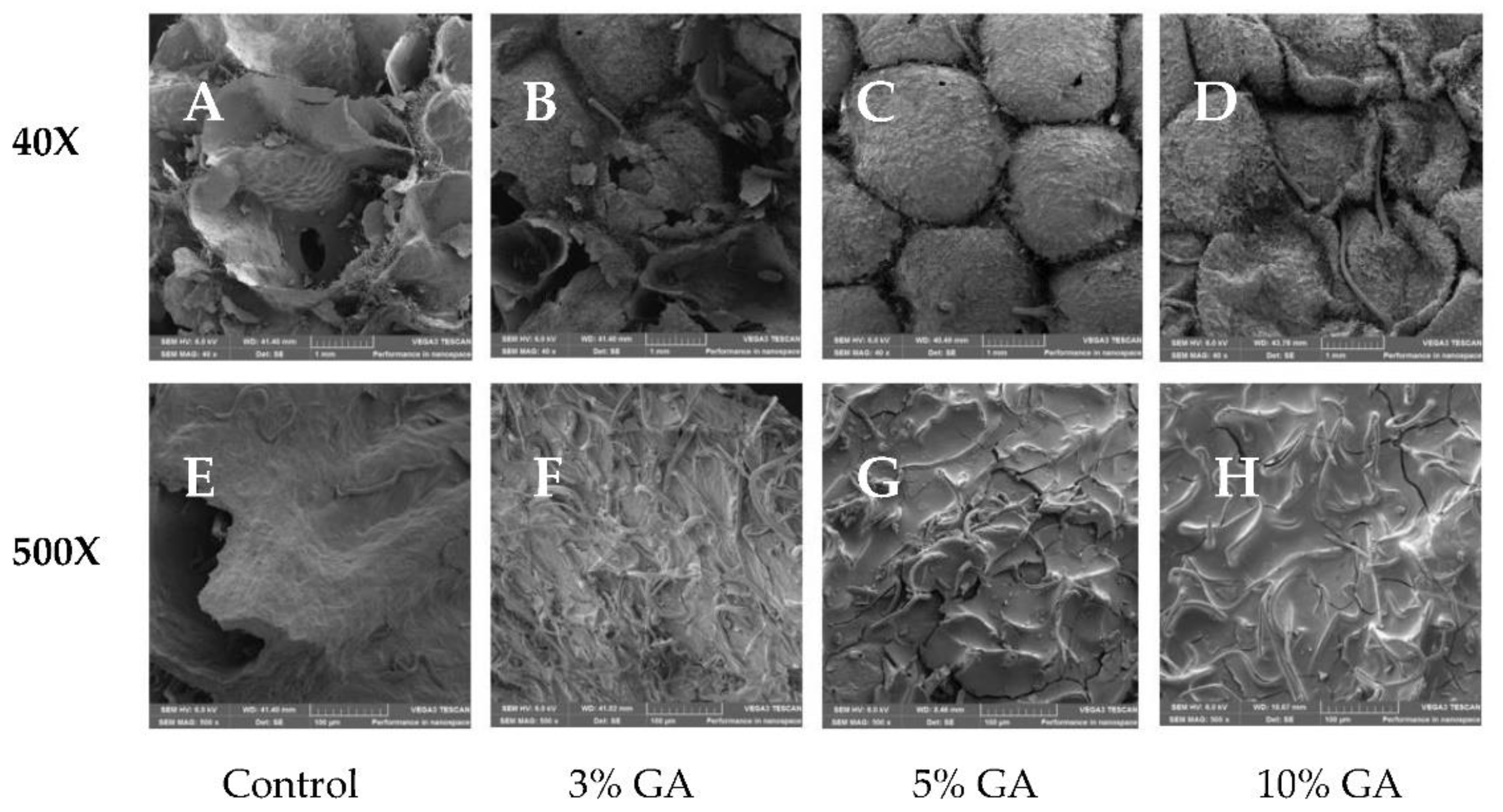
2.5. Microstructure Analysis
2.6. Bioactive Phytochemicals and Antioxidant Activity Determination
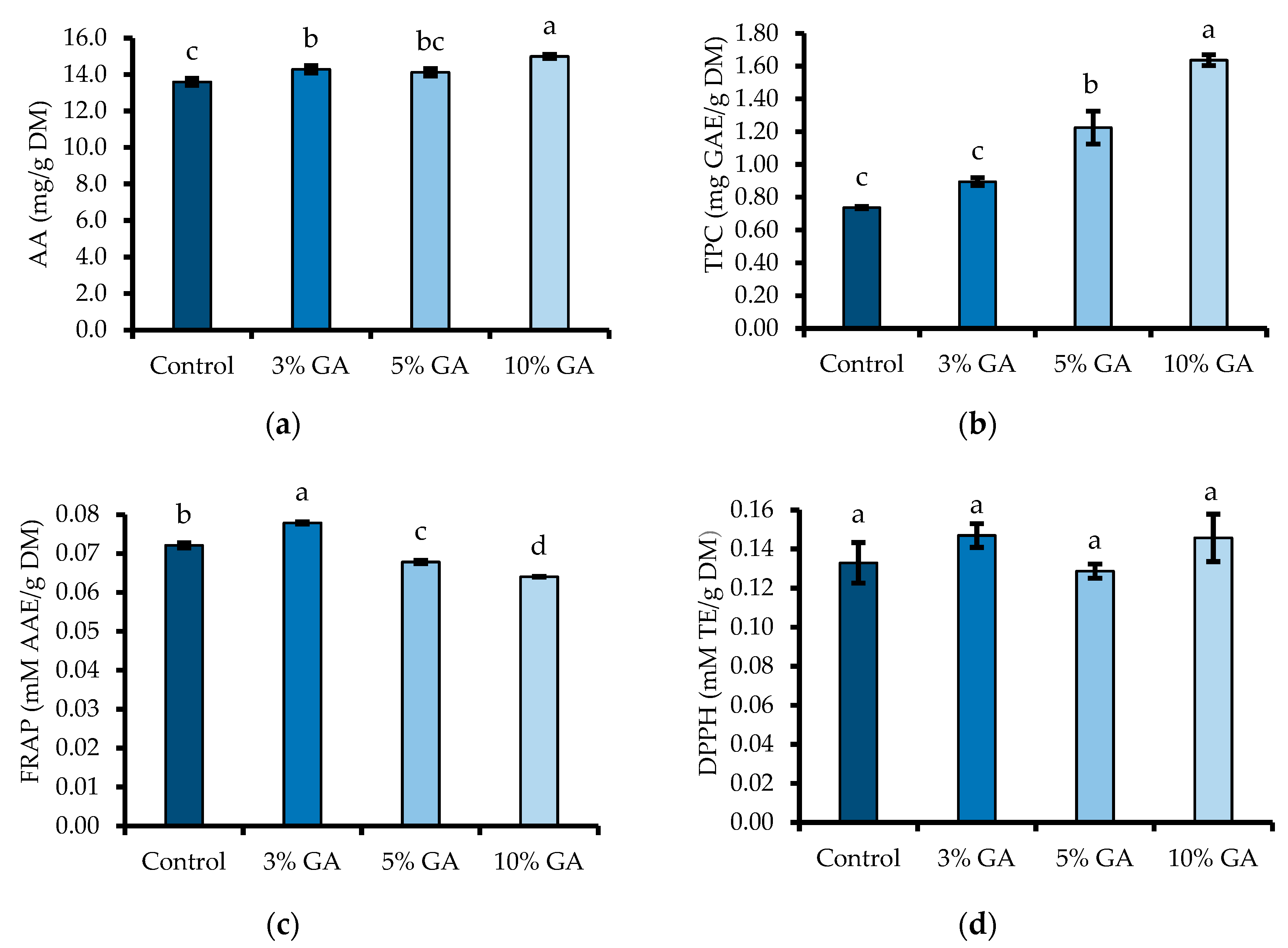
2.6.1. Ascorbic Acid, and Total Phenolic Content
2.6.2. Liquid Chromatography-Mass Spectrometry Analysis for Individual Anthocyanins
2.6.3. Quantification of the Individual Anthocyanins
2.6.4. Antioxidant Capacity
2.7. Determination of Activities of Enzymes Implicated in Browning Reaction
2.7.1. Enzyme Extraction
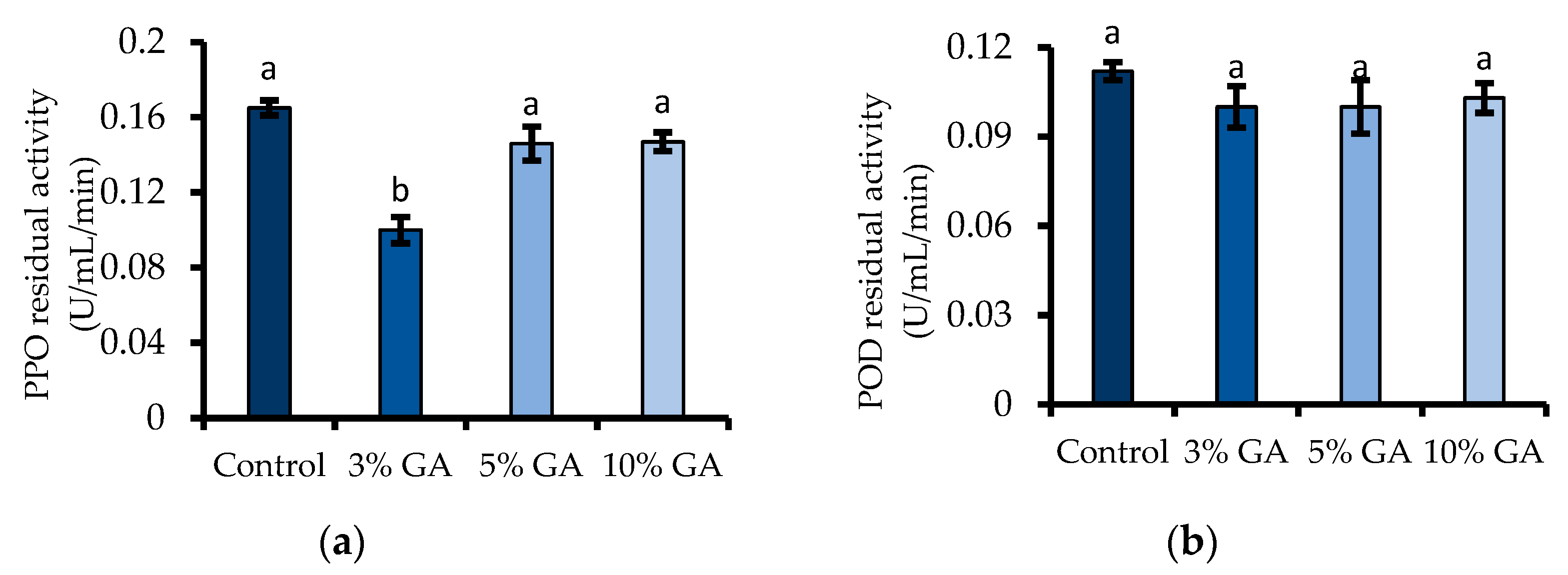
2.7.2. Polyphenol Oxidase
2.7.3. Peroxidase
2.8. Statistical Analysis
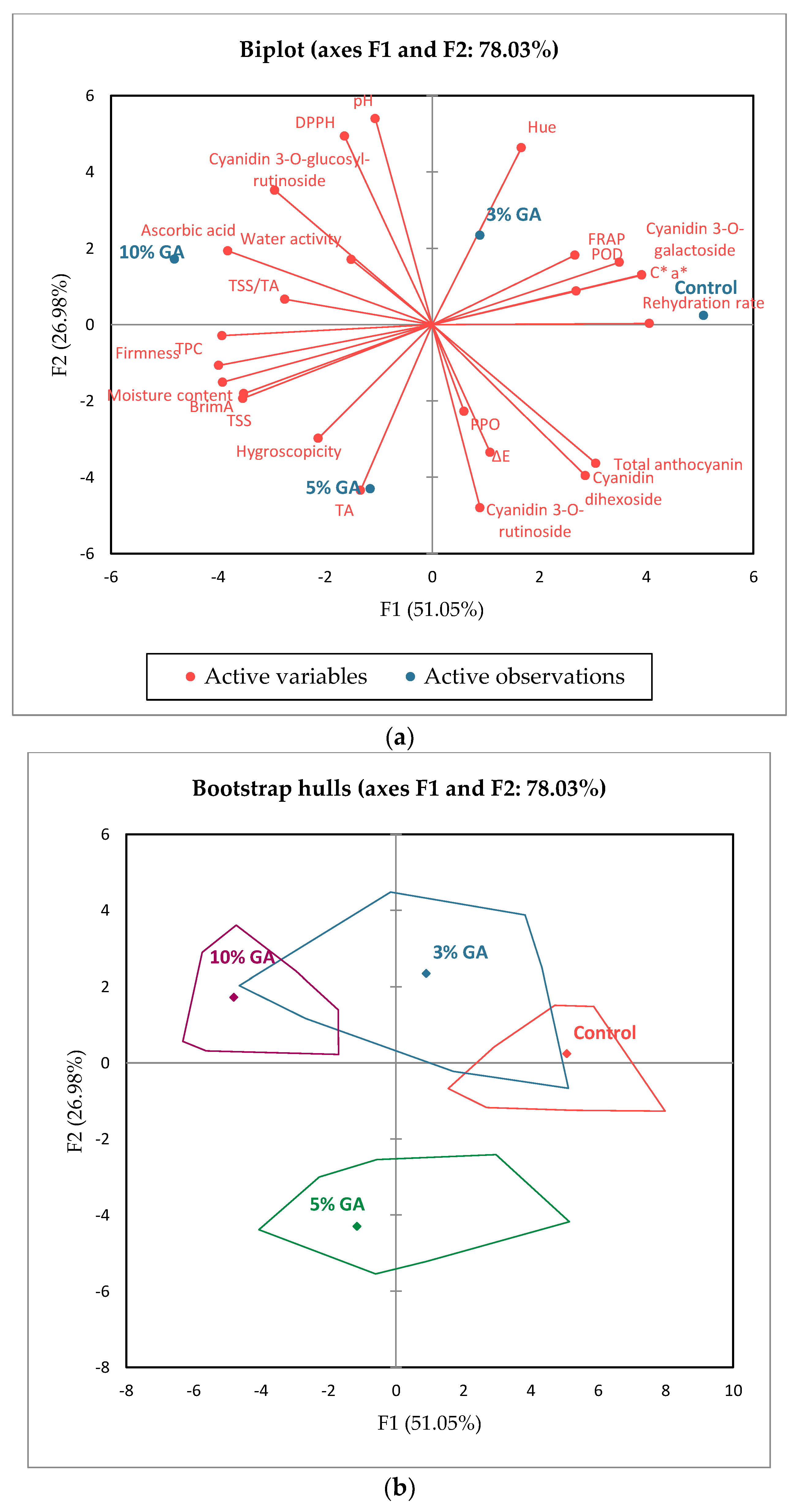
3. Results and Discussion
3.1. Colour Attributes, Moisture Content, and Water Activity
3.2. Hardness, Hygroscopicity, and Rehydration Capacity
3.3. Total Soluble Solids, pH, Titratable Acidity, and BrimA
3.4. Microstructure Analysis
3.5. Ascorbic Acid, Total Phenolic Content, and Antioxidant Activity (DPPH and FRAP)
3.6. Anthocyanin Composition
3.7. Enzyme Activity (PPO and POD)
3.8. Princial Component Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| DF | Dilution factor |
| DM | Dry matter |
| DPPH | 2,2-diphenyl-1-picryl hydrazyl |
| FAO | Food and agriculture organisation |
| FRAP | Ferric reducing antioxidant power |
| GA | Gum Arabic |
| GRAS | Generally regarded as safe |
| LC-MS | Liquid Chromatography-Mass Spectrometry |
| MC | Moisture content |
| PCA | Principal component analysis |
| POD | Peroxidase |
| PPO | Polyphenol oxidase |
| SEM | Scanning electron microscope |
| TA | Titratable acidity |
| TE | Trolox equivalence |
| TPC | Total phenolic content |
| TPTZ | 2,4,6-tri(2-pyridyl)-s-triazine |
| TSS | Total soluble solids |
References
- Rodriguez, A.; Bruno, E.; Paola, C.; Campanone, L.; Mascheroni, R.H. Experimental study of dehydration process of raspberries (Rubus idaeus) with microwave and solar drying. Food Sci. Technol. Camp. 2020, 39, 336–343. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Crops. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 13 June 2022).
- Nicole, W. Market Intelligence Report: Raspberries; Agriculture Western Cape Government: Cape Town, South Africa, 2020; pp. 1–35. Available online: https:www.elsenburg.com (accessed on 14 June 2022).
- Kostecka-Gugala, A.; Ledwozyw-Smolen, I.; Augustynowicz, J.; Wyzgolik, G.; Kruczek, M.; Kaszycki, P. Antioxidant properties of fruits of raspberry and blackberry grown in central Europe. Open Chem. 2015, 13, 1313–1325. [Google Scholar] [CrossRef]
- Sette, P.; Franceschinis, L.; Schebor, C.; Salvatori, D. Fruit snacks from raspberries: Influence of drying parameters on colour degradation and bioactive potential. Int. J. Food Sci. 2017, 52, 313–328. [Google Scholar] [CrossRef]
- Rao, A.V.; Snyder, D.M. Raspberries and Human Health: A review. J. Agric. Food Chem. 2010, 58, 3871–3883. [Google Scholar] [CrossRef]
- Lopez-Corona, A.V.; Valencia-Espinosa, I.; Gonzalez-Sanchez, F.; Sanchez-Lopez, A.L.; Garcia-Amezquita, L.; Garcia-Varela, R. Antioxidant, Anti-Inflammatory and Cytotoxic Activity of Phenolic Compound Family Extracted from Raspberries (Rubus idaeus): A General Review. Antioxidants 2022, 11, 1192. [Google Scholar] [CrossRef]
- Nthimole, C.T.; Kaseke, T.; Fawole, O.A. Microencapsulation and Characterization of Anthocyanin-Rich Raspberry Juice Powder for potential Applications in the Food industry. Processes 2022, 10, 1038. [Google Scholar] [CrossRef]
- Tezotto-Uliana, J.V.; Fargoni, G.P.; Geerdink, G.M.; Kluge, R.A. Chitosan applications pre- or postharvest prolong raspberry shelf-life quality. Postharvest Biol. Technol. 2014, 91, 72–77. [Google Scholar] [CrossRef]
- Adiamo, O.Q.; Eltoum, Y.A.I.; Babiker, E. Effects of Gum Arabic Edible coatings and Sun-Drying on the Storage Life and Quality of Raw and Blanched Tomato Slices. J. Culin. Sci. Technol. 2017, 17, 45–58. [Google Scholar] [CrossRef]
- Xu, B.; Tiliwa, E.S.; Yan, W.; Azam, R.; Wei, B.; Zhou, C.; Ma, H.; Bhandari, B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Int. Food Res. J. 2022, 152, 110744. [Google Scholar] [CrossRef]
- Oliveria, B.G.; Ventura, J.A.; Kondrayut, T.P.; Barroso, M.E.S.; Correia, R.M.; Pimentel, E.F.; Pinto, F.E.; Endringer, D.C.; Romao, W. Chemical profile of mango (Mangifera indica L.) using electrospray ionisation mass spectrometry (ESI-MS). Food Chem. 2016, 204, 37–45. [Google Scholar] [CrossRef]
- Rahimi, J.; Singh, A.; Adewale, P.O.; Adedeji, A.A.; Ngadi, M.O.; Raghavan, V. Effect of Carboxymethyl Cellulose Coating and Osmotic Dehydration on Freeze Drying Kinetics of Apple Slices. Foods 2013, 2, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Bassey, E.J.; Cheng, J.H.; Sun, D.W. Novel nonthermal and thermal pretreatments for enhancing drying performance and improving quality of dried fruits and vegetables. Trends Food Sci. Technol. 2021, 112, 137–148. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, M.; Mujumdar, A.S.; Chen, J. Effects of High Voltage Electrostatic Field and Gelatin-Gum Arabic Composite Film on Color Protection of Freeze-dried Grapefruit Slices. Food Bioprocess Technol. 2022, 15, 1881–1895. [Google Scholar] [CrossRef]
- Adetoro, A.O.; Opara, U.L.; Fawole, O.A. Effect of Blanching on Enzyme Inactivation, Physicochemical Attributes and Antioxidant Capacity of Hot-Air Dried Pomegranate (Punica granatum L.) Arils (cv. Wonderful). Processes 2019, 9, 25. [Google Scholar] [CrossRef]
- Magangana, T.P.; Makunga, N.P.; la Grange, C.; Stander, M.A.; Fawole, O.A.; Opara, U.L. Blanching Pre-Treatment Promotes High Yields, Bioactive Compounds, Antioxidants, Enzyme Inactivation and Antibacterial Activity of ‘Wonderful’ Pomegranate Peel Extracts at Three Different Harvest Maturities. Antioxidants 2021, 10, 1119. [Google Scholar] [CrossRef]
- Alberts, P.; Stander, M.A.; De Villiers, A. Advanced ultra-high pressure liquid chromatography-tandem mass spectrometric methods for the screening of red wine anthocyanins and derived pigments. J. Chromatogr. 2012, 1235, 92–102. [Google Scholar] [CrossRef]
- Fawole, O.A.; Opara, U.L. Seasonal variation in chemical composition, aroma volatiles and antioxidant capacity of pomegranate during fruit development. Afr. J. Biotechnol. 2013, 12, 4006–4019. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma: FRAP as a measure of “antioxidant power” the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Meighani, H.; Ghasemnezhad, M.; Bakshi, D. Evaluation of biochemical composition and enzyme activities in browned arils of pomegranate fruits. Int. J. Hortic. Sci. Technol. 2014, 1, 53–65. [Google Scholar]
- Shah, H.M.S.; Khan, A.S.; Ali, S. Pre-storage kojic acid application delays pericarp browning and maintains antioxidant activities of litchi fruit. Postharvest Biol. Technol. 2017, 132, 154–161. [Google Scholar] [CrossRef]
- Islam, M.Z.; Saha, T.; Monalisa, K.; Hoque, M.M. Effect of starch edible coating on drying characteristics and antioxidant properties of papaya. J. Food. Meas. Charact. 2019, 13, 2951–2960. [Google Scholar] [CrossRef]
- Yu, F.; Li, Y.; Wu, Z.; Wang, X.; Wan, N.; Yang, M. Dehydration of wolfberry fruit using pulsed vacuum drying combined with carboxymethyl cellulose coating pretreatment. LWT Food Sci. Technol. 2020, 134, 110159. [Google Scholar] [CrossRef]
- Ozcelik, M.; Ambros, S.; Morais, S.I.; Kulozik, U. Storage stability of dried raspberry foam as a snack product: Effect of foam structure and microwave-assisted freeze drying on the stability of plant bioactives and ascorbic acid. J. Food Eng. 2020, 270, 109779. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Li, D.; Liu, C.; Yang, Q.; Zhang, M. Effect of starch osmo-coating on carotenoids, colour and microstructure of dehydrated pumpkin slices. J. Food Sci. Technol. 2018, 55, 3249–3256. [Google Scholar] [CrossRef] [PubMed]
- Lago-Vanzela, E.S.; Nascimento, P.D.; Fontes, E.A.F.; Mauro, M.A.; Kimura, M. Edible coatings from native and modified starches retain carotenoids in pumpkin during drying. LWT Food Sci. Technol. 2013, 50, 420–425. [Google Scholar] [CrossRef]
- Udomkun, P.; Mahayothee, B.; Nagle, M.; Muller, J. Effects of calcium chloride and calcium lactate applications with osmotic pretreatment on physicochemical aspects and consumer acceptances of dried papaya. J. Food Sci. Technol. 2014, 49, 1122–1131. [Google Scholar] [CrossRef]
- Bchir, B.; Besbes, S.; Karoui, R.; Atria, H.; Paquot, M.; Blecker, C. Effect of Air-Drying Conditions on Physico-Chemical Properties of Osmotically Pre-Treated Pomegranate Seeds. Food Bioprocess Technol. 2012, 5, 1840. [Google Scholar] [CrossRef]
- Xu, B.; Chen, J.; Tiliwa, E.S.; Yan, W.; Azam, S.M.R.; Wei, B.; Zhou, C.; Ma, H. Effect of multi-mode dual-frequency ultrasound pretreatment on the vacuum freeze-drying process and quality attributes of the strawberry slices. Ultrason. Sonochem. 2021, 78, 105714. [Google Scholar] [CrossRef]
- GEA Niro. Hygroscopicity—Method A14a. In GEA Niro Analytical methods of dry milk products; GEA Niro: Soeborg, Denmark, 2005; pp. 1–3. Available online: https://efps.gr/products/dryers-particle-processing-plants/spray-dryers/food-dairy-products/analytical-methods-dry-milk-products (accessed on 22 April 2022).
- Jafari, S.M.; Azizi, D.; Mirzaei, H.; Dehnad, D. Comparing quality characteristics of oven-dried and refractance window-dried kiwifruit. J. Food Processing Preserv. 2016, 40, 362–372. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on the functional properties and colour of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Lemus-Mondace, R.; Bilbao-Sainz, C.; Andres, A. Effect of air drying temperature on the quality of rehydrated dried red bell pepper (var. Lamuyo). J. Food Eng. 2008, 85, 42–50. [Google Scholar] [CrossRef]
- Garcia, C.C.; Caetano, L.C.; de Souza Silva, K.; Mauro, M.A. Influence of Edible Coating on the Drying and Quality of Papaya. Food Bioprocess Technol. 2014, 7, 2828–2839. [Google Scholar] [CrossRef]
- Carr, A.C.; Lykkesfeldt, J. Discrepancies in global vitamin C recommendations: A review of RFA criteria and underlying health perspectives. Crit. Rev. Food Sci. Nutr. 2021, 61, 742–755. [Google Scholar] [CrossRef] [PubMed]
- Noguer, M.; Cerezo, A.B.; Moya, M.L.; Troncoso, A.M.; Carcia-Parilla, C. Synergism Effect between Phenolic Metabolites and Endogenous Antioxidants in Terms of Antioxidant Activity. Adv. Chem. Eng. 2014, 4, 258–265. [Google Scholar] [CrossRef]
- Chottanom, P.; Amornsin, A.; Yodthava, N.; Wunnapong, S. Effect of Edible Coating on Antioxidants and Certain Properties of Dried Jerusalem Artichoke. Pak. J. Biol. Sci. 2020, 23, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Vara, A.L.; Pinela, J.; Dias, M.I.; Petrovic, J.; Nogueira, A.; Sokovic, M.; Ferreira, I.C.F.R.; Barros, L. Compositional Features of the “Kweli” Red Raspberry and Its Antioxidant and Antimicrobial Activities. Foods 2020, 9, 1522. [Google Scholar] [CrossRef]
- Symanowska, U.; Baraniak, B. Antioxidant and Potentially Anti-Inflammatory Activity of Anthocyanin Fractions from Pomace Obtained from Enzymatically Treated Raspberries. Antioxidants 2019, 8, 299. [Google Scholar] [CrossRef]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and their changes during processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef]
- Stamenkovic, Z.; Pavkov, I.; Radojcin, M.; Horecki, A.T.; Keseli, K.; Kovacevic, D.B.; Putnik, P. Convective Drying of Fresh and Frozen Raspberries and Change of Their Physical and Nutritive Properties. Foods 2019, 8, 251. [Google Scholar] [CrossRef]
- Lee, J. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit juices, Beverages, Natural Colorants, and Wines by the pH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Sandoval-Ramirez, B.A.; Catalan, U.; Fernandez-Castillejo, S.; Pedret, A.; Llaurado, E.; Sola, R. Cyanidin-3-glucoside as a possible biomarker of anthocyanin-rich berry intake in body fluids of healthy humans: A systematic review of clinical trials. Nutr. Rev. 2019, 78, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Adetoro, A.O.; Tsige, A.A.; Opara, U.L.; Fawole, O.A. Mathematical Modelling of Blanch-Assisted Drying of Pomegranate (Punica granatum) Arils in a Hot-Air Drier. Processes 2020, 8, 611. [Google Scholar] [CrossRef]
- Wan, C.; Kahramano, L.; Chen, J.; Gan, Z.; Chen, C. Effects of Hot Air Treatments on Postharvest Storage of Newhall Navel Orange. Plants 2020, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Piochi, M.; Chiacaro, E.; Cichelli, A.; Torri, L.; Cerretani, L. Sensory properties of iodine-biofortified potatoes. Ital. J. Food Sci. 2021, 33, 52–60. [Google Scholar] [CrossRef]

 |  |  |  | |
|---|---|---|---|---|
| Pretreatment | Control | 3% GA | 5% GA | 10% GA |
| a* | 35.84 ± 0.99 a | 26.65 ± 2.42 b | 19.40 ± 0.75 c | 17.38 ± 0.58 c |
| h° | 11.88 ± 0.53 a | 11.42 ± 0.76 a | 10.21 ± 1.76 a | 11.44 ± 1.61 a |
| C* | 36.63 ±0.96 a | 27.20 ±2.47 b | 19.76 ±0.82 c | 17.76 ±0.56 c |
| ∆E | 15.20 ± 2.45 a | 8.11 ± 0.95 b | 13.96 ± 1.09 a | 12.06 ± 1.08 ab |
| MC | 4.60 ± 0.13 b | 5.02 ± 0.44 ab | 5.64 ± 0.36 ab | 5.83 ± 0.37 a |
| aw | 0.31 ± 0.006 b | 0.27 ±0.003 c | 0.28 ±0.006 c | 0.34 ±0.002 a |
| Pretreatment | Control | 3% GA | 5% GA | 10% GA |
|---|---|---|---|---|
| TSS | 5.60 ± 0.06 b | 5.57 ± 0.09 b | 6.00 ± 0.15 a | 6.12 ± 0.13 a |
| TA | 0.76 ± 0.02 a | 0.78 ± 0.02 a | 0.81 ± 0.01 a | 0.77 ± 0.01 a |
| TSS/TA ratio | 6.80 ± 0.30 b | 6.87 ± 0.22 b | 7.70 ± 0.22 a | 7.69 ± 0.13 a |
| BrimA | 3.91 ± 0.03 b | 4.00 ± 0.13 b | 4.11 ± 0.07 b | 4.54 ± 0.08 a |
| pH | 4.23 ± 0.009 b | 4.27 ± 0.001 a | 4.18 ± 0.004 c | 4.28 ± 0.014 a |
| No. | Experimental m/z [M-H]− | Mass Accuracy (ppm) | Retention Time (min) | Elemental Formula | Sensitivity (ppm) | MSE Fragments | UV (nm) | Tentative Identity | Chemical Structure | Control | 3% GA | 5% GA | 10% GA | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 611.1610 | 0.3 | 11.36 | C27H31O16 | - | 611.1608, 287.0554 | 550 | Cyanidin dihexoside |  | ✓ | ✓ | ✓ | ✓ | [17,18] |
| 2 | 449.1082 | 3.2 | 12.31 | C21H21O11 | 1.09 | 449.1070, 287.0558 | 550 | Cyanidin 3-O-galactoside |  | ✓ | ✓ | ✓ | ✓ | Std * |
| 3 | 757.2170 | 3.6 | 12.53 | C33H41O20 | - | 757.2189, 287.0552 | 550 | Cyanidin 3-O-glucosyl-rutinoside | 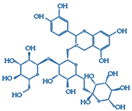 | ✓ | ✓ | ✓ | ✓ | [17,18] |
| 4 | 595.1665 | 2.4 | 13.42 | C27H31O15 | - | 595.1680, 287.0554 | 550 | Cyanidin 3-O-rutinoside | 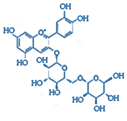 | ✓ | ✓ | ✓ | ✓ | [17,18] |
| Anthocyanin/Pretreatment | Control | 3% GA | 5% GA | 10% GA |
|---|---|---|---|---|
| Cyanidin dihexoside | 1049.07 ± 22.37 a | 998.30 ± 3.39 b | 1053.70 ± 15.12 a | 951.18 ± 22.74 b |
| Cyanidin 3-O-galactoside | 350.55 ± 3.08 a | 339.09 ± 2.31 b | 334.02 ± 3.58 b | 336.09 ± 2.75 b |
| Cyanidin 3-O-glucosyl-rutinoside | 211.78 ± 11.57 c | 219.01 ± 1.65 b | 209.22 ± 1.65 c | 237.37 ± 2.56 a |
| Cyanidin 3-O-rutinoside | 41.98 ± 0.05 a | 34.09 ± 0.18 b | 43.76 ± 0.53 a | 37.62 ± 0.34 b |
| Total Anthocyanins | 1650 ± 37 a | 1590 ± 8 b | 1640 ± 21 a | 1560 ± 28 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morodi, V.; Kaseke, T.; Fawole, O.A. Impact of Gum Arabic Coating Pretreatment on Quality Attributes of Oven-Dried Red Raspberry (Rubus idaeus L.) Fruit. Processes 2022, 10, 1629. https://doi.org/10.3390/pr10081629
Morodi V, Kaseke T, Fawole OA. Impact of Gum Arabic Coating Pretreatment on Quality Attributes of Oven-Dried Red Raspberry (Rubus idaeus L.) Fruit. Processes. 2022; 10(8):1629. https://doi.org/10.3390/pr10081629
Chicago/Turabian StyleMorodi, Veronica, Tafadzwa Kaseke, and Olaniyi Amos Fawole. 2022. "Impact of Gum Arabic Coating Pretreatment on Quality Attributes of Oven-Dried Red Raspberry (Rubus idaeus L.) Fruit" Processes 10, no. 8: 1629. https://doi.org/10.3390/pr10081629







