Separation and Enrichment of Selected Polar and Non-Polar Organic Micro-Pollutants—The Dual Nature of Quaternary Ammonium Ionic Liquid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
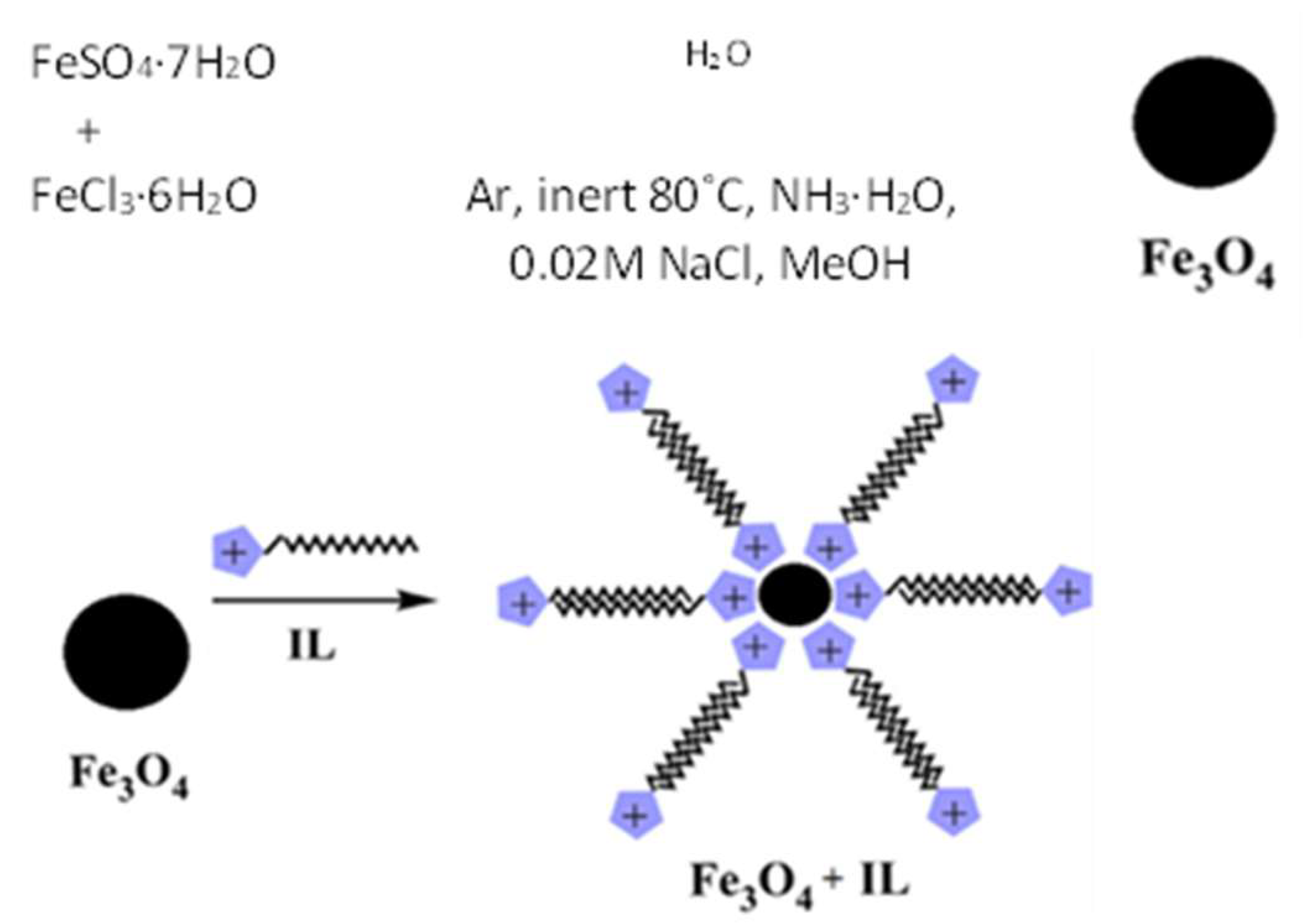
2.2. Preparation of Fe3O4 Magnetic Nanoparticles
2.3. Instrumentation
2.4. Procedure for In Situ IL-DLLME with Magnetic Retrieval of IL
3. Results and Discussion
3.1. Optimization of the IL Concentration
3.2. The Effect of DDAC to NaClO4 Molar Ratio
3.3. The Effect of the MNP Amount
3.4. Method Validation
3.5. Selectivity Parameters Determined by Inverse Gas Chromatography
3.6. Comparison with Similar Work
3.7. Analysis of Hoarfrost Samples
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rykowska, I.; Wasiak, W. Research trends on emerging environment pollutants—A review. Open Chem. 2015, 13, 1353–1370. [Google Scholar] [CrossRef]
- Shahriman, M.S.; Ramachandran, M.R.; Zain, N.N.M.; Mohamad, S.; Manan, N.S.A.; Yaman, S.M. Polyaniline-dicationic ionic liquid coated with magnetic nanoparticles composite for magnetic solid-phase extraction of polycyclic aromatic hydrocarbons in environmental samples. Talanta 2018, 178, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Hou, H.; Shangguan, Y.; Cheng, B.; Xu, Y.; Zhao, R.; Zhang, Y.; Hua, X.; Huo, X.; Zhao, X. Occurrence, sources, and potential human health risks of polycyclic aromatic hydrocarbons in agricultural soils of the coal production area surrounding Xinzhou, China. Ecotoxicol. Environ. Saf. 2014, 108, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Wise, S.A.; Sander, L.C.; Schantz, M.M. Analytical methods for determination of polycyclic aromatic hydrocarbons (PAHs)—A historical perspective on the 16 U.S. EPA priority pollutant PAHs. PAH 2015, 35, 187–247. [Google Scholar] [CrossRef]
- Zhou, H.; Wu, C.; Meng, A.; Zhang, Y.; Williams, P.T. Effect of interactions of biomass constituents on polycyclic aromatic hydrocarbons (PAH) formation during fast pyrolysis. J. Anal. Appl. Pyrolysis 2014, 110, 264–269. [Google Scholar] [CrossRef]
- Long, Y.; Chen, Y.; Yang, F.; Chen, C.; Pan, D.; Cai, Q.; Yao, S. Triphenylamine-functionalized magnetic microparticles as a new adsorbent coupled with high-performance liquid chromatography for the analysis of trace polycyclic aromatic hydrocarbons in aqueous samples. Analyst 2012, 137, 2716–2722. [Google Scholar] [CrossRef]
- Martinez, E.; Gros, M.; Lacorte, S.; Barceló, D. Simplified procedures for the analysis of polycyclic aromatic hydrocarbons in water, sediments and mussels. J. Chromatogr. A 2004, 1047, 181–188. [Google Scholar]
- Nikolaou, A.; Kostopoulou, M.; Petsas, A.; Vagi, M.; Lofrano, G.; Meric, S. Levels and toxicity of polycyclic aromatic hydrocarbons in marine sediments, TrAC-trend. Anal. Chem. 2009, 28, 653–664. [Google Scholar]
- Alidina, M.; Hoppe-Jones, C.; Yoon, M.; Hamadeh, A.F.; Li, D.; Drewes, J.E. The occurrence of emerging trace organic chemicals in wastewater effluents in Saudi Arabia. Sci. Total Environ. 2014, 478, 152–162. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Gao, Y.; Xu, B.; Wu, Q.; Zhang, H.; Song, D. Determination of five pyrethroids in tea drinks by dispersive solid-phase extraction with polyaniline coated magnetic particles. Talanta 2014, 119, 268–275. [Google Scholar] [CrossRef]
- Bachelot, M.; Li, Z.; Munaron, D.; Le Gall, P.; Casellas, C.; Fenet, H.; Gomez, E. Organic UV filter concentrations in marine mussels from French coastal regions. Sci. Total Environ. 2012, 420, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Giokal, D.L.; Salvador, A.; Cisvent, A. UV filters: From sunscreens to the human body and the environment. Trends Anal. Chem. 2007, 5, 360–374. [Google Scholar]
- Zenker, A.; Schutz, H.; Fent, K. Simultaneous trace determination of nine organic UV-absorbing compounds (UV filters) in environmental samples. J. Chromatogr. A 2008, 1202, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Maya, F.; Cabello, C.P.; Frizzarin, R.M.; Estela, J.M.; Palomino, G.T.; Cerd, V. Magnetic solid-phase extraction using metal-organic frameworks (MOFs) and their derived carbons. Trends Anal. Chem. 2017, 90, 142–152. [Google Scholar] [CrossRef]
- Rocío-Bautista, P.; Gonzalez-Hernandez, P.; Pino, V.; Pasan, J.; Afonso, A.M. Metal-organic frameworks as novel sorbents in dispersive-based microextraction approaches. Trends Anal. Chem. 2017, 90, 114–134. [Google Scholar] [CrossRef]
- Wen, Y.; Chen, L.; Li, J.; Liu, D.; Chen, L. Recent advances in solid-phase sorbents for sample preparation prior to chromatographic analysis. Trends Anal. Chem. 2014, 59, 26–41. [Google Scholar] [CrossRef]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Dimpe, K.M.; Nomngongo, P.N. Current sample preparation methodologies for the analysis of emerging pollutants in different environmental matrices. Trends Anal. Chem. 2016, 82, 199–207. [Google Scholar] [CrossRef]
- Martín-Esteban, A. Molecularly-imprinted polymers as a versatile, highly selective tool in sample preparation. Trends Anal. Chem. 2013, 45, 169–181. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Beiraghi, A.; Shokri, M. A novel task-specific magnetic polymeric ionic liquid for selective pre-concentration of potassium in oil samples using centrifuge-less dispersive liquid-liquid microextraction technique and its determination by flame atomic emission spectroscopy. Talanta 2018, 178, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Liao, Y.; Huang, X. Fabrication of polymeric ionic liquid-modified magnetic adsorbent for extraction of apolar and polar pollutants in complicated samples. Talanta 2017, 172, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, Y.; Chen, J.; Xu, P.; Zhou, Y. Preparation of ionic liquid modified magnetic metal-organic frameworks composites for the solid-phase extraction of α–chymotrypsin. Talanta 2018, 182, 484–491. [Google Scholar] [CrossRef]
- Wasserscheid, P.; Keim, W. Ionic Liquids-New “Solutions” for Transition Metal Catalysis. Angew. Chem. Int. Ed. 2000, 39, 3772–3789. [Google Scholar] [CrossRef]
- Plechkova, N.V.; Seddon, K.R. Applications of ionic liquids in the chemical industry. Chem. Soc. Rev. 2008, 37, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Shahriman, M.S.; Mohamad, S.; Zain, N.N.M.; Alias, Y. Paper-based polymeric ionic liquid for thin film micro extraction of sulfonamides in environmental water samples prior to HPLC-DAD analysis. Microchem. J. 2021, 171, 106798. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Berthod, A.; Ruiz-Ángel, M.J.; Carda-Broch, S. Review Ionic liquids in separation techniques. J. Chromatogr. A 2008, 1184, 6–18. [Google Scholar] [CrossRef]
- Nerín, C.; Salafranca, J.; Aznar, M.; Batlle, R. Critical review on recent developments in solventless techniques for extraction of analytes Review. Anal. Bional. Chem. 2009, 393, 809–833. [Google Scholar] [CrossRef]
- Ahmad, W.; Al-Sibaai, A.A.; Bashammakh, A.S.; Alwael, H.; El-Shahawi, M.S. Ionic liquids in analytical chemistry. Anal. Chim. Acta 2010, 661, 1–16. [Google Scholar]
- Liu, R.; Liu, J.-F.; Yin, Y.-G.; Hu, X.; Jiang, G.-B. Ionic liquids in sample preparation. Anal. Bioanal. Chem. 2009, 393, 871–883. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, Z.; Xing, J.; Liu, H. Preparation and characterization of amino–silane-modified superparamagnetic silica nanospheres. J. Magn. Magn. Mater. 2004, 270, 1–6. [Google Scholar] [CrossRef]
- Mutelet, F.; Jaubert, J.-N. Accurate measurements of thermodynamic properties of solutes in ionic liquids using inverse gas chromatography. J. Chromatogr. A 2006, 1102, 256–267. [Google Scholar] [CrossRef]
- Zhao, O.; Anderson, J.L. Task-specific microextractions using ionic liquids. Anal. Bioanal. Chem. 2011, 400, 1613–1618. [Google Scholar] [CrossRef]
- Vičkačkaite, V.; Padarauskas, A. Ionic liquids in microextraction techniques. Review article. Cent. Eur. J. Chem. 2012, 10, 652–674. [Google Scholar]
- Fontanals, N.; Borrull, F.; Marcé, R.M. Ionic liquids in solid-phase extraction. Trends Anal. Chem. 2012, 41, 15–26. [Google Scholar] [CrossRef]
- Potami, F.; Castiglione, S.; Zuccato, E.; Fanelli, A.; Vigetti, D.; Rossetti, C.; Calamari, D. Effects of a Complex Mixture of Therapeutic Drugs at Environmental Levels on Human Embryonic Cells. Environ. Sci. Technol. 2006, 40, 2442–2447. [Google Scholar]
- Rykowska, I.; Ziemblińska, J.; Nowak, I. Modern approaches in dispersive liquid-liquid microextraction (DLLME) based on ionic liquids: A review. J. Mol. Liq. 2018, 259, 319–339. [Google Scholar] [CrossRef]
- Nie, L.; Cai, C.; Guo, R.; Yao, S.; Zhu, Z.; Hong, Y.; Guo, D. Ionic Liquid-Assisted DLLME and SPME for the Determination of Contaminants in Food Samples. Separations 2022, 9, 170. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Yang, M.; Peng, B.; Li, Y.; Zhou, W.; Gao, H.; Lu, R. Magnetic retrieval of ionic liquids: Fast dispersive liquid-liquid microextraction for the determination of benzoylurea insecticides in environmental water samples. J. Chromatogr. A 2012, 1254, 23–29. [Google Scholar] [CrossRef]
- Bagheri, H.; Zandi, O.; Aghakhani, A. Magnetic nanoparticle-based micro-solid phase extraction and GC–MS determination of oxadiargyl in aqueous samples. Chromatographia 2011, 74, 483–488. [Google Scholar] [CrossRef]
- Song, Y.R.; Zhao, S.L.; Tchounwou, P.; Liu, Y.M. A nanoparticle-based solid-phase extraction method for liquid chromatography-electrospray ionization-tandem mass spectrometric analysis. J. Chromatogr. A 2007, 1166, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Song, S.; Wang, C.; Wu, Q.; Wang, Z. Determination of triazine herbicides in environmental water samples by high-performance liquid chromatography using graphene-coated magnetic nanoparticles as adsorbent. Anal. Chim. Acta 2011, 708, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Roman, I.P.; Chisvert, A.; Canals, A. Dispersive solid-phase extraction based on oleic acid-coated magnetic nanoparticles followed by gas chromatography-mass spectrometry for UV-filter determination in water samples. J. Chromatogr. A 2011, 1218, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Musarurwa, H.; Tavengwa, N.T. Homogenous liquid-liquid micro-extraction of pollutants in complex matrices. Microchem. J. 2021, 170, 106750. [Google Scholar] [CrossRef]
- Dong, X.; Gao, X.; Song, J.; Zhao, L. A novel dispersive magnetic solid phase microextraction using ionic liquid-coated amino silanized magnetic graphene oxide nanocomposite for high efficient separation/preconcentration of toxic ions from shellfish samples. Food Chem. 2021, 360, 130023. [Google Scholar] [CrossRef]
- Boon, Y.H.; Zain, N.N.M.; Mohamad, S.; Osman, H.; Raoov, M. Magnetic poly(β-cyclodextrin-ionic liquid) nanocomposites for micro solid phase extraction of selected polycyclic aromatic hydrocarbons in rice samples prior to GC-FID analysis. Food Chem. 2019, 278, 322–332. [Google Scholar] [CrossRef]
- Erdem, P.; Tağaç, A.A.; Bozkurt, S.S.; Merdivan, M. Chitosan and dicationic ionic liquid intercalated clay-coated solid-phase microextraction fiber for determination of sixteen polycyclic aromatic hydrocarbons in coffee and tea samples. Talanta 2021, 235, 122764. [Google Scholar] [CrossRef]
- Hui, B.Y.; Zain, N.N.M. Sharifah Mohamad, Puttaruksa Varanusupakul, Hasnah Osman, Muggundha Raoov, Poly(cyclodextrin-ionic liquid) based ferrofluid: A new class of magnetic colloid for dispersive liquid phase microextraction of polycyclic aromatic hydrocarbons from food samples prior to GC-FID analysis. Food Chem. 2020, 314, 126214. [Google Scholar]
- Zhang, W.; Zhoua, P.; Liu, W.; Wang, H.; Wang, X. Enhanced adsorption/extraction of five typical polycyclic aromatic hydrocarbons from meat samples using magnetic effervescent tablets composed of dicationic ionic liquids and NiFe2O4 nanoparticles. J. Mol. Liq. 2020, 315, 113682. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, R.; Fan, R.; Yang, X.; Mei, H.; Chen, H.; Wang, H.; Wang, Z.; Wang, X. Magnetic amino-functionalized metal-organic frameworks as a novel solid support in ionic liquids-based effervescent tablets for efficient extraction of polycyclic aromatic hydrocarbons in milks. Ecotoxicol. Environ. Saf. 2021, 222, 112482. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Feng, J.; Loussala, H.M.; Han, S.; Ji, X.; Li, C.; Sun, M. Recent advances of ionic liquids in sample preparation. Trends Anal. Chem. 2020, 125, 115833. [Google Scholar] [CrossRef]






| PAHs | ||
|---|---|---|
| Fluorene (F) |  |  (a) fluorene, (b) anthracene, (c) pyrene, (d) fluorantene, (e) benzo(a)anthracene, (f) benzo(a)pyrene |
| Anthracene (Ant) |  | |
| Fluoranthene (FL) |  | |
| Pyrene (Pyr) |  | |
| Benzo(a)pyrene (BaP) |  | |
| Benzo(a)anthracene (BaA) |  | |
| Benzophenones | ||
| 2,4-dihydroxybenzo-phenone (BP1) |  |  |
| 2,2′,4,4′-tetrahydroxybenzo-phenone (BP2) |  | |
| 2-hydroxy-4-metoxybenzo-phenone (BP3) |  | |
| PARAMETER | PAHs | BPs | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F | Ant | FL | Pyr | BaA | BaP | BP1 | BP2 | BP3 | |
| Calibration curve range [µg L−1] | 1–100 | 1–100 | 1–100 | 1–100 | 1–100 | 1–100 | 1–1000 | 1–1000 | 1–1000 |
| Correlation coefficient (r2) | 0.9998 | 0.9999 | 0.9995 | 0.9998 | 0.9999 | 0.9999 | 0.9999 | 0.9995 | 0.9998 |
| Limit of detection (LOD) [µg L−1] | 0.035 | 0.011 | 0.061 | 0.079 | 0.025 | 0.024 | 0.020 | 0.012 | 0.016 |
| Limit of quantitation (LOQ) [µg L−1] | 0.115 | 0.036 | 0.203 | 0.263 | 0.083 | 0.081 | 0.067 | 0.041 | 0.054 |
| Recovery (%) (RSD (%)) | 77.1–88.5 (0.9–4.8) | 26.6–42.0 (1.3–9.9) | 34.6–60.4 (2.7–11.0) | 52.2–102.7 (1.0–8.8) | 28.2–44.0 (2.3–8.3) | 26.2–45.3 (6.2–9.5) | 70.5–90.3 (3.4–2.9) | 68.0–92.5 (7.8–5.4) | 79.5–91.3 (3.5–1.3) |
| Enrichment factor (EF) | 164.3 | 48.0 | 94.2 | 117.8 | 76.7 | 69.6 | 135.5 | 138.8 | 137.0 |
| Binary Mixture | [DDA][ClO4] | ||
|---|---|---|---|
| Sij∞ | |||
| Hexane(i)/benzene(j) | 2.477 | 0.499 | 4.96 |
| Hexane(i)/diethyl ether(j) | 1.029 | 2.41 | |
| Hexane(i)/tetrahydrofuran(j) | 0.478 | 5.18 | |
| Hexane(i)/acetonitryle(j) | 0.543 | 4.56 | |
| Hexane(i)/ethanol(j) | 0.497 | 4.98 | |
| Hexane(i)/methanol(j) | 0.351 | 7.06 | |
| Sampling Place | Concentration [µg·L−1] | |||||
|---|---|---|---|---|---|---|
| F | Ant | Fl | Pyr | BaA | BaP | |
| 1 | 0.079 ± 0.012 | 0.0504 ± 0.010 | 0.066 ± 0.011 | 0.013 ± 0.005 | 0.062 ± 0.012 | <LOD |
| 2 | 0.071 ± 0.014 | 0.2479 ± 0.034 | 0.126 ± 0.022 | <LOD | 0.113 ± 0.019 | 0.026 ± 0.002 |
| 3 | 0.059 ± 0.010 | <LOD | 0.060 ± 0.011 | <LOD | <LOD | <LOD |
| 4 | 0.044 ± 0.007 | <LOD | <LOD | <LOD | <LOD | <LOD |
| 5 | 0.037 ± 0.004 | <LOD | <LOD | <LOD | 0.025 ± 0.005 | <LOD |
| 6 | 0.054 ± 0.009 | <LOD | <LOD | <LOD | 0.032 ± 0.004 | <LOD |
| 7 | 0.040 ± 0.008 | <LOD | <LOD | <LOD | <LOD | <LOD |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziemblińska-Bernart, J.; Rykowska, I.; Nowak, I. Separation and Enrichment of Selected Polar and Non-Polar Organic Micro-Pollutants—The Dual Nature of Quaternary Ammonium Ionic Liquid. Processes 2022, 10, 1636. https://doi.org/10.3390/pr10081636
Ziemblińska-Bernart J, Rykowska I, Nowak I. Separation and Enrichment of Selected Polar and Non-Polar Organic Micro-Pollutants—The Dual Nature of Quaternary Ammonium Ionic Liquid. Processes. 2022; 10(8):1636. https://doi.org/10.3390/pr10081636
Chicago/Turabian StyleZiemblińska-Bernart, Justyna, Iwona Rykowska, and Iwona Nowak. 2022. "Separation and Enrichment of Selected Polar and Non-Polar Organic Micro-Pollutants—The Dual Nature of Quaternary Ammonium Ionic Liquid" Processes 10, no. 8: 1636. https://doi.org/10.3390/pr10081636






