An Overview of Emerging Cyanide Bioremediation Methods
Abstract
:1. Introduction
2. Emerging Cyanide Bioremediation Methods
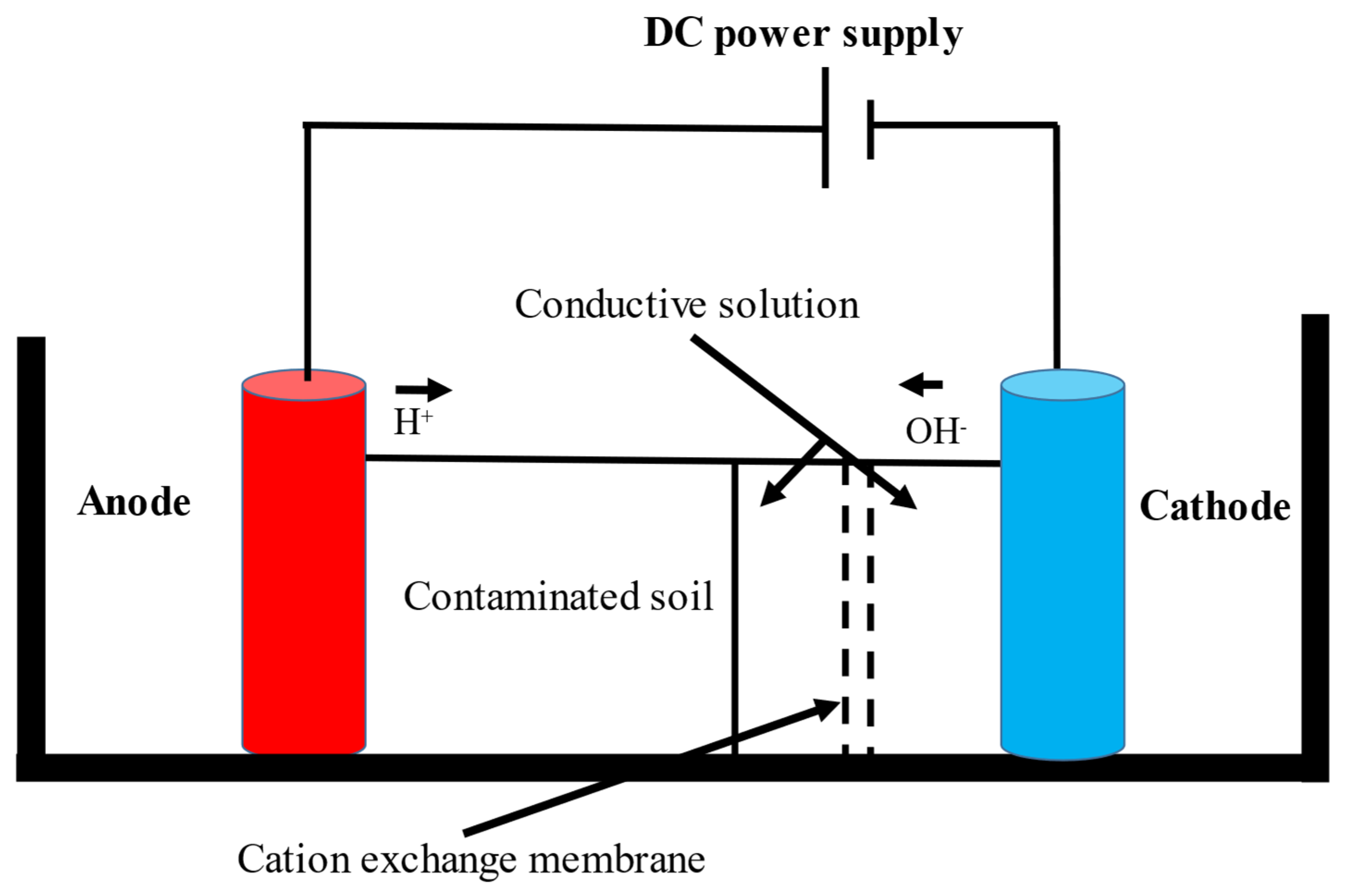
2.1. Electro-Biodegradation of Cyanide Compounds
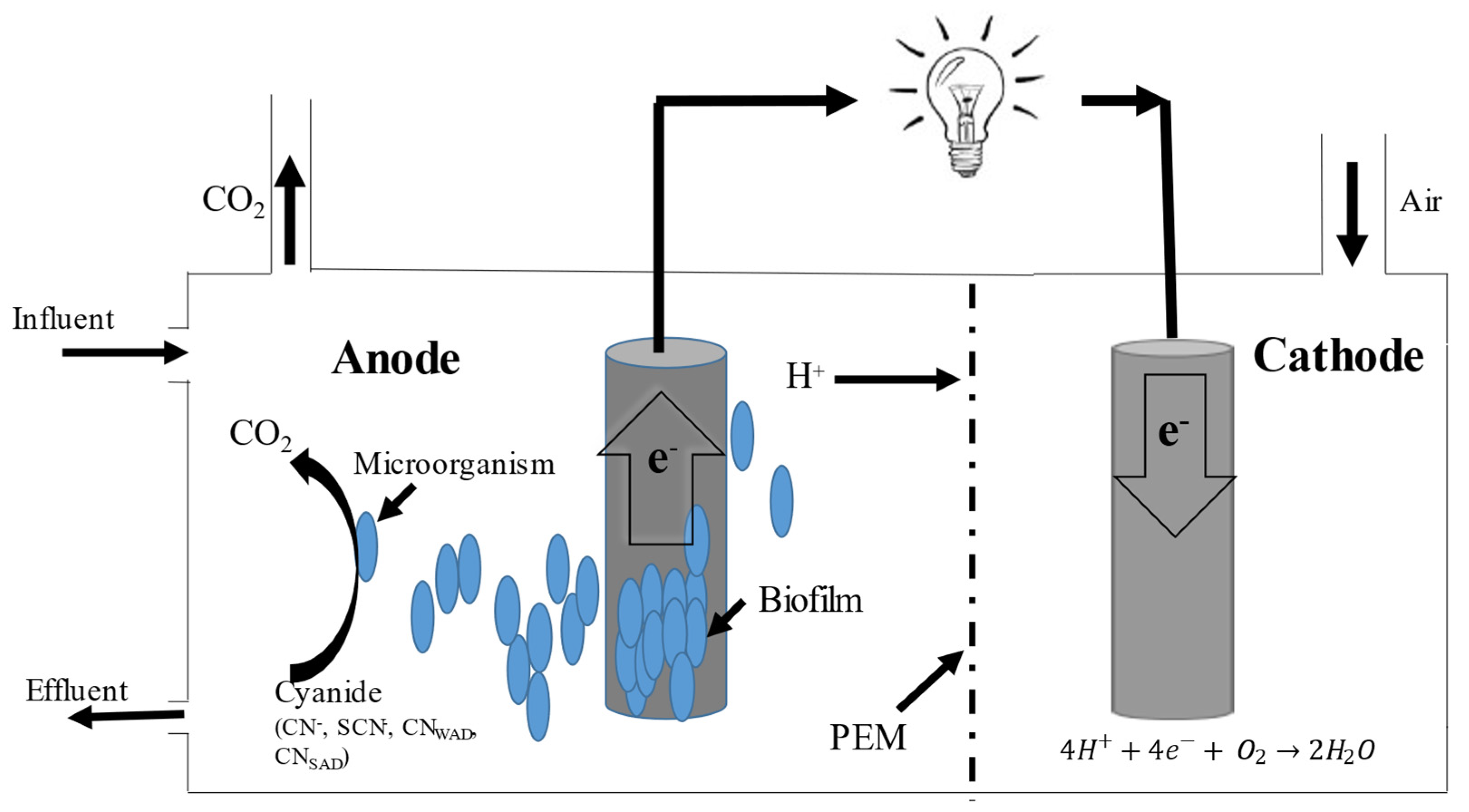
2.2. Microbial Fuel Cells in Cyanide Treatment
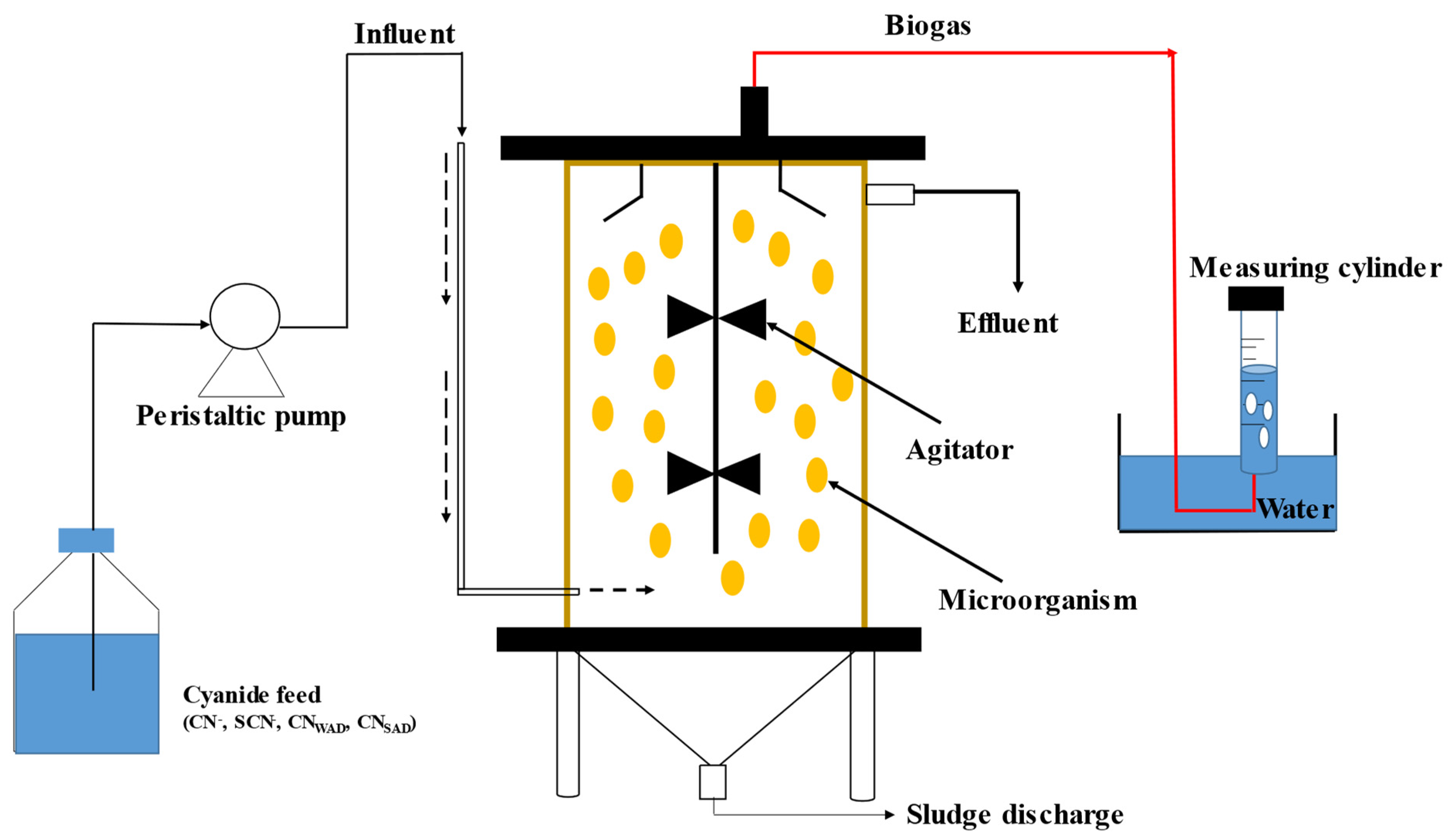
2.3. Anaerobic Cyanide Biodegradation
Pterin Production
2.4. Application of Omics in Cyanide Bioremediation
Genetic Engineering
3. Conclusions
- Accelerated cyanide biodegradation through electro-biodegradation;
- Electricity generation through the use of MFC technology;
- Methane production through anaerobic biodegradation systems;
- Production of bioactive compounds, such as pterins.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinedo-Rivilla, C.; Aleu, J.; Collado, I. Pollutants biodegradation by fungi. Curr. Org. Chem. 2009, 13, 1194–1214. [Google Scholar] [CrossRef]
- Chen, C.; Kao, C.; Chen, S. Application of Klebsiella oxytoca immobilized cells on the treatment of cyanide wastewater. Chemosphere 2008, 71, 133–139. [Google Scholar] [PubMed]
- Johnson, C.A. The fate of cyanide in leach wastes at gold mines: An environmental perspective. Appl. Geochem. 2015, 57, 194–205. [Google Scholar]
- Nsimba, E.B. Cyanide and cyanide complexes in the goldmine polluted land in the east and central rand goldfields, South Africa. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2009. [Google Scholar]
- Ryall, B.; Lee, X.; Zlosnik, J.E.A.; Hoshino, S.; Williams, H.D. Bacteria of the Burkholderia cepacia complex are cyanogenic under biofilm and colonial growth conditions. BMC Microbiol. 2008, 8, 108. [Google Scholar]
- Gracia, R.; Shepherd, G. Cyanide poisoning and its treatment. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 1358–1365. [Google Scholar]
- Ketterer, L.; Keusgen, M. Amperometric sensor for cyanide utilizing cyanidase and formate dehydrogenase. Anal. Chim. Acta 2010, 673, 54–59. [Google Scholar]
- Gupta, N.; Balomajumder, C.; Agarwal, V. Enzymatic mechanism and biochemistry for cyanide degradation: A review. J. Hazard. Mater. 2010, 176, 1–13. [Google Scholar]
- Devi, P. Hydrogen cyanide: Risk assessment, environmental, and health hazard. In Hazardous Gases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 183–195. [Google Scholar]
- Maniyam, M.N.; Sjahrir, F.; Ibrahim, A.L. Biodegradation of cyanide by rhodococcus strains isolated in Malaysia. Int. Conf. Food Eng. Biotechnol. 2011, 9, 21–25. [Google Scholar]
- Akcil, A. Destruction of cyanide in gold mill effluents: Biological versus chemical treatments. Biotechnol. Adv. 2003, 21, 501–511. [Google Scholar] [CrossRef]
- Akcil, A.; Mudder, T. Microbial destruction of cyanide wastes in gold mining: Process review. Biotechnol. Lett. 2003, 25, 445–450. [Google Scholar]
- Sirianuntapiboon, S.; Chuamkaew, C. Packed cage rotating biological contactor system for treatment of cyanide wastewater. Bioresour. Technol. 2007, 98, 266–272. [Google Scholar] [CrossRef]
- Mekuto, L.; Ntwampe, S.K.; Utomi, C.E.; Mobo, M.; Mudumbi, J.B.; Ngongang, M.M.; Akinpelu, E.A. Performance of a continuously stirred tank bioreactor system connected in series for the biodegradation of thiocyanate and free cyanide. J. Environ. Chem. Eng. 2017, 5, 1936–1945. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, V.; Bhalla, T.C. Packed bed reactor for degradation of simulated cyanide-containing wastewater. 3 Biotech 2015, 5, 641–646. [Google Scholar] [CrossRef]
- Wu, H.; Feng, Y.L.; Li, H.R.; Wang, H.J.; Wang, J.J. Co-metabolism kinetics and electrogenesis change during cyanide degradation in a microbial fuel cell. RSC Adv. 2018, 8, 40407–40416. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Agarwal, B.; Balomajumder, C. Simultaneous treatment of phenol and cyanide containing aqueous solution by adsorption, biotreatment and simultaneous adsorption and biotreatment (SAB) process. J. Environ. Chem. Eng. 2016, 4, 564–575. [Google Scholar] [CrossRef]
- Botz, M.; Mudder, T.; Akcil, A. Cyanide treatment: Physical, chemical and biological processes. Adv. Gold Ore Processing 2005, 4528, 672–700. [Google Scholar]
- Bazrafshan, E.; Mohammadi, L.; Ansari-Moghaddam, A.; Mahvi, A.H. Heavy metals removal from aqueous environments by electrocoagulation process—A systematic review. J. Environ. Health Sci. Eng. 2015, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, M.; Yang, J.K.; Lee, S.M.; Shirzad-Siboni, M. Effect of organic matter on cyanide removal by illuminated titanium dioxide or zinc oxide nanoparticles. J. Environ. Health Sci. Eng. 2013, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Mekuto, L.; Ntwampe, S.K.; Akcil, A. An integrated biological approach for treatment of cyanidation wastewater. Sci. Total Environ. 2016, 571, 711–720. [Google Scholar] [CrossRef]
- Barba, S.; López-Vizcaíno, R.; Saez, C.; Villaseñor, J.; Cañizares, P.; Navarro, V.; Rodrigo, M.A. Electro-bioremediation at the prototype scale: What it should be learned for the scale-u. Chem. Eng. J. 2018, 334, 2030–2038. [Google Scholar] [CrossRef]
- Hassan, I.; Mohamedelhassan, E.; Yanful, E.K.; Yuan, Z.C. A review article: Electrokinetic bioremediation current knowledge and new prospects. Adv. Microbiol. 2016, 6, 57. [Google Scholar] [CrossRef]
- Li, T.; Guo, S.; Zhang, L.; Li, F. Electro-biodegradation of the oil-contaminated soil through periodic electrode switching. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010. [Google Scholar]
- Rayu, S.; Karpouzas, D.G.; Singh, B.K. Emerging technologies in bioremediation: Constraints and opportunities. Biodegradation 2012, 23, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, E.M.; Camacho, J.V.; Rodrigo, M.R.; Cañizares, P.C. Feasibility of electrokinetic oxygen supply for soil bioremediation purposes. Chemosphere 2014, 117, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Müller, S.; Harms, H.; Wick, L.Y. Effect of electrokinetic transport on the vulnerability of PAH-degrading bacteria in a model aquifer. Environ. Geochem. Health 2008, 30, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Reible, D. Electro-bioremediation of contaminated sediment by electrode enhanced capping. J. Environ. Manag. 2015, 155, 154–161. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q. Electro-assisted groundwater bioremediation: Fundamentals, challenges and future perspectives. Bioresour. Technol. 2015, 196, 677–684. [Google Scholar] [CrossRef]
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Effect of a direct electric current on the activity of a hydrocarbon-degrading microorganism culture used as the flushing liquid in soil remediation processes. Sep. Purif. Technol. 2014, 124, 217–223. [Google Scholar] [CrossRef]
- Choi, J.-H.; Maruthamuthu, S.; Lee, H.G.; Ha, T.H.; Bae, J.H. Nitrate removal by electro-bioremediation technology in Korean soil. J. Hazard. Mater. 2009, 168, 1208–1216. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, S.; Li, F.; Wu, B. Distribution of ion contents and microorganisms during the electro-bioremediation of petroleum-contaminated saline soil. J. Environ. Sci. Health Part A 2017, 52, 1141–1149. [Google Scholar] [CrossRef]
- Barba, S.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Can electro-bioremediation of polluted soils perform as a self-sustainable process? J. Appl. Electrochem. 2018, 48, 579–588. [Google Scholar] [CrossRef]
- Mena, E.; Barba, S.; Sáez, C.; Navarro, V.; Villaseñor, J.; Rodrigo, M.A.; Cañizares, P. Prescale-up of electro-bioremediation processes. Geo-Chicago 2016, 264–273. Available online: https://ascelibrary.org/doi/10.1061/9780784480168.027 (accessed on 1 August 2022).
- Chaplin, B.P. Advantages, disadvantages, and future challenges of the use of electrochemical technologies for water and wastewater treatment. In Electrochemical Water and Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2018; pp. 451–494. [Google Scholar]
- Khodadadi, A.; Yousefi, D.; Ganjidoust, H.; Yari, M. Bioremediation of diesel-contaminated soil using Bacillus sp. (strain TMY-2) in soil by uniform and non-uniform electro kinetic technology field. J. Toxicol. Environ. Health Sci. 2011, 3, 376–384. [Google Scholar]
- Ramírez, E.M.; Camacho, J.V.; Rodrigo, M.A.; Cañizares, P. Combination of bioremediation and electrokinetics for the in-situ treatment of diesel polluted soil: A comparison of strategies. Sci. Total Environ. 2015, 533, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Mena, E.; Rubio, P.; Cañizares, P.; Villasenor, J.; Rodrigo, M.A. Electrokinetic transport of diesel-degrading microorganisms through soils of different textures using electric fields. J. Environ. Sci. Health Part A 2012, 47, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Azhar, A.; Nabila, A.T.A.; Nurshuhaila, M.S.; Shaylinda, M.Z.N.; Azim, M.A.M. Electromigration of contaminated soil by electro-bioremediation technique. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Balatonkenese, Hungary, 2016. [Google Scholar]
- Mena, E.; Villaseñor, J.; Cañizares, P.; Rodrigo, M.A. Effect of electric field on the performance of soil electro-bioremediation with a periodic polarity reversal strategy. Chemosphere 2016, 146, 300–307. [Google Scholar] [CrossRef]
- Alshawabkeh, A.N. Electrokinetic soil remediation: Challenges and opportunities. Sep. Sci. Technol. 2009, 44, 2171–2187. [Google Scholar] [CrossRef]
- Luo, Q.; Zhang, X.; Wang, H.; Qian, Y. The use of non-uniform electrokinetics to enhance in situ bioremediation of phenol-contaminated soil. J. Hazard. Mater. 2005, 121, 187–194. [Google Scholar] [CrossRef]
- Nyer, E.K.; Suarez, G. In situ biodegradation is better than monitored natural attenuation. Groundw. Monit. Remediat. 2002, 22, 30–39. [Google Scholar] [CrossRef]
- Inglis, T.J.; Sagripanti, J.-L. Environmental factors that affect the survival and persistence of Burkholderia pseudomallei. Appl. Environ. Microbiol. 2006, 72, 6865–6875. [Google Scholar] [CrossRef]
- Hansen, H.K.; Ottosen, L.M.; Kliem, B.K.; Villumsen, A. Electrodialytic remediation of soils polluted with Cu, Cr, Hg, Pb and Zn. J. Chem. Technol. Biotechnol. Int. Res. Process Environ.; Clean Technol. 1997, 70, 67–73. [Google Scholar] [CrossRef]
- Reed, B.E.; Berg, M.T.; Thompson, J.C.; Hatfield, J.H. Chemical conditioning of electrode reservoirs during electrokinetic soil flushing of Pb-contaminated silt loam. J. Environ. Eng. 1995, 121, 805–815. [Google Scholar] [CrossRef]
- Wong, J.S.; Hicks, R.E.; Probstein, R.F. EDTA-enhanced electroremediation of metal-contaminated soils. J. Hazard. Mater. 1997, 55, 61–79. [Google Scholar] [CrossRef]
- Acar, Y.B.; Alshawabkeh, A.N. Principles of electrokinetic remediation. Environ. Sci. Technol. 1993, 27, 2638–2647. [Google Scholar] [CrossRef]
- Acar, Y.B.; Hamidon, A.; Field, S.D.; Scott, L. The effect of organic fluids on hydraulic conductivity of compacted kaolinite. In Hydraulic Barriers in Soil and Rock; Johnson, A.I., Frobel, R.K., Cavalli, N.J., Patterson, C.B., Eds.; American Society for Testing Materials: West Conshohocken, PA, USA, 1985. [Google Scholar]
- Denisov, G.; Hicks, R.E.; Probstein, R.F. On the kinetics of charged contaminant removal from soils using electric fields. J. Colloid Interface Sci. 1996, 178, 309–323. [Google Scholar] [CrossRef]
- Niqui-Arroyo, J.-L.; Bueno-Montes, M.; Posada-Baquero, R.; Ortega-Calvo, J.J. Electrokinetic enhancement of phenanthrene biodegradation in creosote-polluted clay soil. Environ. Pollut. 2006, 142, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Shen, Z.M.; Yuan, T.; Zheng, S.S.; Ju, B.X.; Wang, W.H. Enhancing electrokinetic remediation of cadmium-contaminated soils with stepwise moving anode method. J. Environ. Sci. Health Part A 2006, 41, 2517–2530. [Google Scholar] [CrossRef] [PubMed]
- Rajić, L.; Dalmacija, B.; Dalmacija, M.; Rončević, S.; Perović, S.U. Enhancing electrokinetic lead removal from sediment: Utilizing the moving anode technique and increasing the cathode compartment length. Electrochim. Acta 2012, 86, 36–40. [Google Scholar] [CrossRef]
- Pazos, M.; Sanroman, M.; Cameselle, C. Improvement in electrokinetic remediation of heavy metal spiked kaolin with the polarity exchange technique. Chemosphere 2006, 62, 817–822. [Google Scholar] [CrossRef]
- Kim, S.; Han, S. Application of an enhanced electrokinetic ion injection system to bioremediation. Water Air Soil Pollut. 2003, 146, 365–377. [Google Scholar] [CrossRef]
- Mao, X.; Wang, J.; Ciblak, A.; Cox, E.E.; Riis, C.; Terkelsen, M.; Gent, D.B.; Alshawabkeh, A.N. Electrokinetic-enhanced bioaugmentation for remediation of chlorinated solvents contaminated clay. J. Hazard. Mater. 2012, 213, 311–317. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Gent, D.B.; Davis, J.L.; Alshawabkeh, A.N. Lactate injection by electric currents for bioremediation of tetrachloroethylene in clay. Electrochim. Acta 2012, 86, 157–163. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, J.Y.; Lee, Y.J.; Lee, J.Y.; Yang, J.W. Application of a new electrolyte circulation method for the ex situ electrokinetic bioremediation of a laboratory-prepared pentadecane contaminated kaolinite. J. Hazard. Mater. 2005, 118, 171–176. [Google Scholar] [CrossRef]
- Mohamedelhassan, E.; Shang, J.Q. Electrokinetic cementation of calcareous sand for offshore foundations. Int. J. Offshore Polar Eng. 2008, 18, 13–19. [Google Scholar]
- Ho, S.V.; Athmer, C.; Sheridan, P.W.; Hughes, B.M.; Orth, R.; McKenzie, D.; Brodsky, P.H.; Shapiro, A.M.; Sivavec, T.M.; Salvo, J.; et al. The Lasagna technology for in situ soil remediation. 2. Large field test. Environ. Sci. Technol. 1999, 33, 1092–1099. [Google Scholar] [CrossRef]
- Kim, S.-H.; Han, H.Y.; Lee, Y.J.; Kim, C.W.; Yang, J.W. Effect of electrokinetic remediation on indigenous microbial activity and community within diesel contaminated soil. Sci. Total Environ. 2010, 408, 3162–3168. [Google Scholar] [CrossRef]
- Wan, C.; Du, M.; Lee, D.J.; Yang, X.; Ma, W.; Zheng, L. Electrokinetic remediation and microbial community shift of β-cyclodextrin-dissolved petroleum hydrocarbon-contaminated soil. Appl. Microbiol. Biotechnol. 2011, 89, 2019–2025. [Google Scholar] [CrossRef]
- Tiehm, A.; Lohner, S.T.; Augenstein, T. Effects of direct electric current and electrode reactions on vinyl chloride degrading microorganisms. Electrochim. Acta 2009, 54, 3453–3459. [Google Scholar] [CrossRef]
- Mohamedelhassan, E.; Shang, J. Effects of electrode materials and current intermittence in electro-osmosis. Proc. Inst. Civ. Eng. -Ground Improv. 2001, 5, 3–11. [Google Scholar] [CrossRef]
- Malik, A.; Grohmann, E. Environmental Protection Strategies for Sustainable Development; Springer: Berlin, Germany, 2011. [Google Scholar]
- Hassan, I.; Mohamedelhassan, E. Electrokinetic remediation with solar power for a homogeneous soft clay contaminated with copper. Int. J. Environ. Pollut. Remediat. (IJEPR) 2012, 1, 67–74. [Google Scholar] [CrossRef]
- Yuan, S.; Zheng, Z.; Chen, J.; Lu, X. Use of solar cell in electrokinetic remediation of cadmium-contaminated soil. J. Hazard. Mater. 2009, 162, 1583–1587. [Google Scholar] [CrossRef]
- Hansen, H.K.; Rojo, A. Testing pulsed electric fields in electroremediation of copper mine tailings. Electrochim. Acta 2007, 52, 3399–3405. [Google Scholar] [CrossRef]
- Hassan, I.; Mohamedelhassan, E.; Yanful, E.K. Solar powered electrokinetic remediation of Cu polluted soil using a novel anode configuration. Electrochim. Acta 2015, 181, 58–67. [Google Scholar] [CrossRef]
- Xu, H.; Li, A.; Feng, L.; Cheng, X.; Ding, S. Destruction of cyanide in aqueous solution by electrochemical oxidation method. Int. J. Electrochem. Sci. 2012, 7, 7516–7525. [Google Scholar]
- Ojaghi, A.; Shafaie Tonkaboni, S.Z.; Shariati, P.; Doulati Ardejani, F. Novel cyanide electro-biodegradation using Bacillus pumilus ATCC 7061 in aqueous solution. J. Environ. Health Sci. Eng. 2018, 16, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Bond, D.R.; Holmes, D.E.; Tender, L.M.; Lovley, D.R. Electrode-reducing microorganisms that harvest energy from marine sediments. Science 2002, 295, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, H.S.; Hyun, M.S.; Chang, I.S.; Kim, M.; Kim, B.H. A mediator-less microbial fuel cell using a metal reducing bacterium, Shewanella putrefaciens. Enzym. Microb. Technol. 2002, 30, 145–152. [Google Scholar] [CrossRef]
- Kim, H.J.; Moon, S.H.; Byung, H.K. A microbial fuel cell type lactate biosensor using a metal-reducing bacterium, Shewanella putrefaciens. J. Microbiol. Biotechnol. 1999, 9, 365–367. [Google Scholar]
- Zhou, M.; Wang, H.; Hassett, D.J.; Gu, T. Recent advances in microbial fuel cells (MFCs) and microbial electrolysis cells (MECs) for wastewater treatment, bioenergy and bioproducts. J. Chem. Technol. Biotechnol. 2013, 88, 508–518. [Google Scholar] [CrossRef]
- Franks, A.E.; Nevin, K.P. Microbial fuel cells, a current review. Energies 2010, 3, 899–919. [Google Scholar] [CrossRef]
- Pandey, P.; Shinde, V.N.; Deopurkar, R.L.; Kale, S.P.; Patil, S.A.; Pant, D. Recent advances in the use of different substrates in microbial fuel cells toward wastewater treatment and simultaneous energy recovery. Appl. Energy 2016, 168, 706–723. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Zularisam, A.W.; Hai, F.I. Microbial fuel cell is emerging as a versatile technology: A review on its possible applications, challenges and strategies to improve the performances. Int. J. Energy Res. 2018, 42, 369–394. [Google Scholar] [CrossRef]
- Chung, K.; Okabe, S. Continuous power generation and microbial community structure of the anode biofilms in a three-stage microbial fuel cell system. Appl. Microbiol. Biotechnol. 2009, 83, 965–977. [Google Scholar] [CrossRef]
- Davis, F.; Higson, S.P. Biofuel cells—recent advances and applications. Biosens. Bioelectron. 2007, 22, 1224–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ieropoulos, I.; Melhuish, C.; Greenman, J.; Horsfield, I.; Hart, J. Energy autonomy in robots through Microbial Fuel Cells. In CiteSeerX-Scientific Literature Digital Library and Search Engine; CiteSeerX: PA, USA, 2004. [Google Scholar]
- Kumar, R.; Singh, L.; Zularisam, A. Microbial fuel cells: Types and applications. In Waste Biomass Management—A Holistic Approach; Springer: Berlin/Heidelberg, Germany, 2017; pp. 367–384. [Google Scholar]
- Ya-li, F.; Wei-da, W.; Xin-hua, T.; Hao-ran, L.; Zhuwei, D.; Zhi-chao, Y.; Yun-long, D. Isolation and characterization of an electrochemically active and cyanide-degrading bacterium isolated from a microbial fuel cell. RSC Adv. 2014, 4, 36458–36463. [Google Scholar] [CrossRef]
- Rabaey, K.; Verstraete, W. Microbial fuel cells: Novel biotechnology for energy generation. TRENDS Biotechnol. 2005, 23, 291–298. [Google Scholar] [CrossRef]
- Rodrigo, M.; Canizares, P.; Lobato, J.; Paz, R.; Sáez, C.; Linares, J.J. Production of electricity from the treatment of urban waste water using a microbial fuel cell. J. Power Sources 2007, 169, 198–204. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, L.; Wahid, Z.A.; Din, M.F.M. Exoelectrogens in microbial fuel cells toward bioelectricity generation: A review. Int. J. Energy Res. 2015, 39, 1048–1067. [Google Scholar] [CrossRef]
- Borole, A.P.; Reguera, G.; Ringeisen, B.; Wang, Z.W.; Feng, Y.; Kim, B.H. Electroactive biofilms: Current status and future research needs. Energy Environ. Sci. 2011, 4, 4813–4834. [Google Scholar] [CrossRef]
- Chaudhuri, S.K.; Lovley, D.R. Electricity generation by direct oxidation of glucose in mediatorless microbial fuel cells. Nat. Biotechnol. 2003, 21, 1229. [Google Scholar] [CrossRef]
- Inoue, K.; Leang, C.; Franks, A.E.; Woodard, T.L.; Nevin, K.P.; Lovley, D.R. Specific localization of the c-type cytochrome OmcZ at the anode surface in current-producing biofilms of Geobacter sulfurreducens. Environ. Microbiol. Rep. 2011, 3, 211–217. [Google Scholar] [CrossRef]
- Jiang, X.; Hu, J.; Lieber, A.M.; Jackan, C.S.; Biffinger, J.C.; Fitzgerald, L.A.; Ringeisen, B.R.; Lieber, C.M. Nanoparticle facilitated extracellular electron transfer in microbial fuel cells. NanoLett. 2014, 14, 6737–6742. [Google Scholar] [CrossRef] [PubMed]
- Gude, V.G. Wastewater treatment in microbial fuel cells–an overview. J. Clean. Prod. 2016, 122, 287–307. [Google Scholar] [CrossRef]
- Huang, L.; Yang, X.; Quan, X.; Chen, J.; Yang, F. A microbial fuel cell–electro-oxidation system for coking wastewater treatment and bioelectricity generation. J. Chem. Technol. Biotechnol. 2010, 85, 621–627. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q.; He, Z. Towards sustainable wastewater treatment by using microbial fuel cells-centered technologies. Energy Environ. Sci. 2014, 7, 911–924. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Y.; Puranik, S.; Lei, Y.; Vadas, T.; Li, B. Metals as electron acceptors in single-chamber microbial fuel cells. J. Power Sources 2014, 269, 430–439. [Google Scholar] [CrossRef]
- Li, W.-W.; Yu, H.-Q. From wastewater to bioenergy and biochemicals via two-stage bioconversion processes: A future paradigm. Biotechnol. Adv. 2011, 29, 972–982. [Google Scholar] [CrossRef]
- Kaewkannetra, P.; Chiwes, W.; Chiu, T. Treatment of cassava mill wastewater and production of electricity through microbial fuel cell technology. Fuel 2011, 90, 2746–2750. [Google Scholar] [CrossRef]
- Logan, B.E.; Hamelers, B.; Rozendal, R.; Schröder, U.; Keller, J.; Freguia, S.; Aelterman, P.; Verstraete, W.; Rabaey, K. Microbial fuel cells: Methodology and technology. Environ. Sci. Technol. 2006, 40, 5181–5192. [Google Scholar] [CrossRef]
- Logan, B.E. Scaling up microbial fuel cells and other bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1665–1671. [Google Scholar] [CrossRef]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Wang, X.; Cheng, S.; Feng, Y.; Merrill, M.D.; Saito, T.; Logan, B.E. Use of carbon mesh anodes and the effect of different pretreatment methods on power production in microbial fuel cells. Environ. Sci. Technol. 2009, 43, 6870–6874. [Google Scholar] [CrossRef] [PubMed]
- Park, D.H.; Kim, S.K.; Shin, I.H.; Jeong, Y.J. Electricity production in biofuel cell using modified graphite electrode with neutral red. Biotechnol. Lett. 2000, 22, 1301–1304. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Najafpour, G.D.; Ghoreyshi, A.A.; Shakeri, M.; Zare, H. Methylene blue as electron promoters in microbial fuel cell. Int. J. Hydrog. Energy 2011, 36, 13335–13341. [Google Scholar] [CrossRef]
- Park, D.; Zeikus, J. Utilization of electrically reduced neutral Red byActinobacillus succinogenes: Physiological function of neutral Red in membrane-driven fumarate reduction and energy conservation. J. Bacteriol. 1999, 181, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Ghoreyshi, A.A.; Najafpour, G.; Jafary, T. Power generation from organic substrate in batch and continuous flow microbial fuel cell operations. Appl. Energy 2011, 88, 3999–4004. [Google Scholar] [CrossRef]
- Shukla, A.; Suresh, P.; Sheela, B.; Rajendran, A.J.C.S. Biological fuel cells and their applications. Curr. Sci. 2004, 87, 455–468. [Google Scholar]
- Aghababaie, M.; Farhadian, M.; Jeihanipour, A.; Biria, D. Effective factors on the performance of microbial fuel cells in wastewater treatment—A review. Environ. Technol. Rev. 2015, 4, 71–89. [Google Scholar] [CrossRef]
- Song, R.B.; Zhao, C.E.; Gai, P.P.; Guo, D.; Jiang, L.P.; Zhang, Q.; Zhang, J.R.; Zhu, J.J. Graphene/Fe3O4 nanocomposites as efficient anodes to boost the lifetime and current output of microbial fuel cells. Chem.–Asian J. 2017, 12, 308–313. [Google Scholar] [CrossRef]
- Ieropoulos, I.A.; Greenman, J.; Melhuish, C.; Hart, J. Comparative study of three types of microbial fuel cell. Enzym. Microb. Technol. 2005, 37, 238–245. [Google Scholar] [CrossRef]
- Chen, Y.-M.; Wang, C.T.; Yang, Y.C.; Chen, W.J. Application of aluminum-alloy mesh composite carbon cloth for the design of anode/cathode electrodes in Escherichia coli microbial fuel cell. Int. J. Hydrog. Energy 2013, 38, 11131–11137. [Google Scholar] [CrossRef]
- Bond, D.R.; Lovley, D.R. Electricity production by Geobacter sulfurreducens attached to electrodes. Appl. Environ. Microbiol. 2003, 69, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Mathuriya, A.S. Inoculum selection to enhance performance of a microbial fuel cell for electricity generation during wastewater treatment. Environ. Technol. 2013, 34, 1957–1964. [Google Scholar] [CrossRef] [PubMed]
- Bergel, A.; Féron, D.; Mollica, A. Catalysis of oxygen reduction in PEM fuel cell by seawater biofilm. Electrochem. Commun. 2005, 7, 900–904. [Google Scholar] [CrossRef]
- Li, Y.; Williams, I.; Xu, Z.; Li, B.; Li, B. Energy-positive nitrogen removal using the integrated short-cut nitrification and autotrophic denitrification microbial fuel cells (MFCs). Appl. Energy 2016, 163, 352–360. [Google Scholar] [CrossRef]
- Li, W.; Zhang, S.; Chen, G.; Hua, Y. Simultaneous electricity generation and pollutant removal in microbial fuel cell with denitrifying biocathode over nitrite. Appl. Energy 2014, 126, 136–141. [Google Scholar] [CrossRef]
- Yang, Y. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. Enhancing bidirectional electron transfer of Shewanella oneidensis by a synthetic flavin pathway. ACS Synth. Biol. 2015, 4, 815–823. [Google Scholar] [CrossRef]
- Tang, J.; Chen, S.; Yuan, Y.; Cai, X.; Zhou, S. In situ formation of graphene layers on graphite surfaces for efficient anodes of microbial fuel cells. Biosens. Bioelectron. 2015, 71, 387–395. [Google Scholar] [CrossRef]
- Hai, F.I.; Yamamoto, K.; Fukushi, K. Hybrid treatment systems for dye wastewater. Crit. Rev. Environ. Sci. Technol. 2007, 37, 315–377. [Google Scholar] [CrossRef]
- Fornero, J.J.; Rosenbaum, M.; Angenent, L.T. Electric power generation from municipal, food, and animal wastewaters using microbial fuel cells. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2010, 22, 832–843. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Yang, Q.; Chen, Y.; Zhu, S.; Shen, S. Power generation using polyaniline/multi-walled carbon nanotubes as an alternative cathode catalyst in microbial fuel cells. Int. J. Energy Res. 2014, 38, 1416–1423. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Jafary, T.; Haghparast, F. Nafion as a nanoproton conductor in microbial fuel cells. Turk. J. Eng. Environ. Sci. 2011, 34, 289–292. [Google Scholar]
- Rahimnejad, M.; Ghasemi, M.; Najafpour, G.D.; Ismail, M.; Mohammad, A.W.; Ghoreyshi, A.A.; Hassan, S.H. Synthesis, characterization and application studies of self-made Fe3O4/PES nanocomposite membranes in microbial fuel cell. Electrochim. Acta 2012, 85, 700–706. [Google Scholar] [CrossRef]
- Pant, D.; Van Bogaert, G.; Diels, L.; Vanbroekhoven, K. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour. Technol. 2010, 101, 1533–1543. [Google Scholar] [CrossRef] [PubMed]
- Pant, D.; Arslan, D.; Van Bogaert, G.; Gallego, Y.A.; De Wever, H.; Diels, L.; Vanbroekhoven, K. Integrated conversion of food waste diluted with sewage into volatile fatty acids through fermentation and electricity through a fuel cell. Environ. Technol. 2013, 34, 1935–1945. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Z.; Wu, J.; Zhang, Y.; Li, M.; Lin, Z.Q.; Bañuelos, G. Simultaneous removal of selenite and electricity production from Seladen wastewater by constructed wetland coupled with microbial fuel cells. Selenium Environ. Hum. Health 2013, 212, 180–191. [Google Scholar]
- Villasenor, J.; Capilla, P.; Rodrigo, M.A.; Canizares, P.; Fernandez, F.J. Operation of a horizontal subsurface flow constructed wetland–microbial fuel cell treating wastewater under different organic loading rates. Water Res. 2013, 47, 6731–6738. [Google Scholar] [CrossRef]
- Huang, L.; Chai, X.; Chen, G.; Logan, B.E. Effect of set potential on hexavalent chromium reduction and electricity generation from biocathode microbial fuel cells. Environ. Sci. Technol. 2011, 45, 5025–5031. [Google Scholar] [CrossRef]
- Liu, S.-H.; Wu, C.-H.; Lin, C.-W. Enhancement of bioelectricity generation for an air-cathode microbial fuel cell using polyvinyl alcohol-membrane electrode assemblies. Biochem. Eng. J. 2017, 128, 210–217. [Google Scholar] [CrossRef]
- Xie, S.; Liang, P.; Chen, Y.; Xia, X.; Huang, X. Simultaneous carbon and nitrogen removal using an oxic/anoxic-biocathode microbial fuel cells coupled system. Bioresour. Technol. 2011, 102, 348–354. [Google Scholar] [CrossRef]
- Eom, H.; Chung, K.; Kim, I.; Han, J.I. Development of a hybrid microbial fuel cell (MFC) and fuel cell (FC) system for improved cathodic efficiency and sustainability: The M2FC reactor. Chemosphere 2011, 85, 672–676. [Google Scholar] [CrossRef]
- Chen, Z.; Huang, Y.C.; Liang, J.H.; Zhao, F.; Zhu, Y.G. A novel sediment microbial fuel cell with a biocathode in the rice rhizosphere. Bioresour. Technol. 2012, 108, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Behera, M.; Jana, P.S.; More, T.T.; Ghangrekar, M.M. Rice mill wastewater treatment in microbial fuel cells fabricated using proton exchange membrane and earthen pot at different pH. Bioelectrochemistry 2010, 79, 228–233. [Google Scholar] [CrossRef]
- Tota-Maharaj, K.; Paul, P. Performance of pilot-scale microbial fuel cells treating wastewater with associated bioenergy production in the Caribbean context. Int. J. Energy Environ. Eng. 2015, 6, 213–220. [Google Scholar] [CrossRef]
- Ge, Z.; Wu, L.; Zhang, F.; He, Z. Energy extraction from a large-scale microbial fuel cell system treating municipal wastewater. J. Power Sources 2015, 297, 260–264. [Google Scholar] [CrossRef]
- Walter, X.A.; Merino-Jiménez, I.; Greenman, J.; Ieropoulos, I. PEE POWER® urinal II–Urinal scale-up with microbial fuel cell scale-down for improved lighting. J. Power Sources 2018, 392, 150–158. [Google Scholar] [CrossRef]
- Pirc, E.T.; Novosel, B.; Bukovec, P. Comparison of GC and OxiTop Analysis of Biogas Composition Produced by Anaerobic Digestion of Glucose in Cyanide Inhibited Systems. Acta Chim. Slov. 2012, 59. [Google Scholar]
- Fallon, R.D. Evidence of hydrolytic route for anaerobic cyanide degradation. Appl. Environ. Microbiol. 1992, 58, 3163–3164. [Google Scholar] [CrossRef] [Green Version]
- Luque-Almagro, V.M.; Cabello, P.; Sáez, L.P.; Olaya-Abril, A.; Moreno-Vivián, C.; Roldán, M.D. Exploring anaerobic environments for cyanide and cyano-derivatives microbial degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1067–1074. [Google Scholar] [CrossRef]
- Pirc, E.T.; Levstek, M.; Bukovec, P. Influence of cyanide on the anaerobic degradation of glucose. Water Sci. Technol. 2010, 62, 1799–1806. [Google Scholar] [CrossRef]
- Ebbs, S. Biological degradation of cyanide compounds. Curr. Opin. Biotechnol. 2004, 15, 231–236. [Google Scholar] [CrossRef]
- Baxter, J.; Cummings, S.P. The current and future applications of microorganism in the bioremediation of cyanide contamination. Antonie Van Leeuwenhoek 2006, 90, 1–17. [Google Scholar] [CrossRef]
- Dash, R.R.; Gaur, A.; Balomajumder, C. Cyanide in industrial wastewaters and its removal: A review on biotreatment. J. Hazard. Mater. 2009, 163, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Saha, S.; Dhaka, S.; Kurade, M.B.; Kang, C.U.; Baek, S.H.; Jeon, B.H. Remediation of cyanide-contaminated environments through microbes and plants: A review of current knowledge and future perspectives. Geosystem Eng. 2017, 20, 28–40. [Google Scholar] [CrossRef]
- Park, J.M.; Sewell, B.T.; Benedik, M.J. Cyanide bioremediation: The potential of engineered nitrilases. Appl. Microbiol. Biotechnol. 2017, 101, 3029–3042. [Google Scholar] [CrossRef] [PubMed]
- Luque-Almagro, V.M.; Moreno-Vivián, C.; Roldán, M.D. Biodegradation of cyanide wastes from mining and jewellery industries. Curr. Opin. Biotechnol. 2016, 38, 9–13. [Google Scholar] [CrossRef]
- Haarstrick, A.; Hempel, D.C.; Ostermann, L.; Ahrens, H.; Dinkler, D. Modelling of the biodegradation of organic matter in municipal landfills. Waste Manag. Res. 2001, 19, 320–331. [Google Scholar] [CrossRef]
- Gavala, H.N.; Angelidaki, I.; Ahring, B.K. Kinetics and modeling of anaerobic digestion process. In Biomethanation I; Springer: Berlin/Heidelberg, Germany, 2003; pp. 57–93. [Google Scholar]
- Nishio, N.; Nakashimada, Y. High rate production of hydrogen/methane from various substrates and wastes. In Recent Progress of Biochemical and Biomedical Engineering in Japan I; Springer: Berlin/Heidelberg, Germany, 2004; pp. 63–87. [Google Scholar]
- Ozturk, I.; Anderson, G.; Saw, C. Anaerobic fluidized-bed treatment of brewery wastes and bioenergy recovery. Water Sci. Technol. 1989, 21, 1681–1684. [Google Scholar] [CrossRef]
- Switzenbaum, M.S.; Danskin, S.C. Anaerobic expanded bed treatment of whey. Agric. Wastes 1982, 4, 411–426. [Google Scholar] [CrossRef]
- Gijzen, H.J.; Bernal, E.; Ferrer, H. Cyanide toxicity and cyanide degradation in anaerobic wastewater treatment. Water Res. 2000, 34, 2447–2454. [Google Scholar] [CrossRef]
- Chakraborty, S.; Veeramani, H. Effect of HRT and recycle ratio on removal of cyanide, phenol, thiocyanate and ammonia in an anaerobic–anoxic–aerobic continuous system. Process Biochem. 2006, 41, 96–105. [Google Scholar] [CrossRef]
- Novak, D.; Franke-Whittle, I.H.; Pirc, E.T.; Jerman, V.; Insam, H.; Logar, R.M.; Stres, B. Biotic and abiotic processes contribute to successful anaerobic degradation of cyanide by UASB reactor biomass treating brewery waste water. Water Res. 2013, 47, 3644–3653. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.R.; Zhang, Y.; Tian, Z.; Gao, Y.; Yang, M. Performance and microbial community composition in a long-term sequential anaerobic-aerobic bioreactor operation treating coking wastewater. Appl. Microbiol. Biotechnol. 2016, 100, 8191–8202. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, M.; Kumar, V.; Mahajan, R.; Bhalla, T.C.; Chatterjee, S.; Akhter, Y. Molecular insights into the activity and mechanism of cyanide hydratase enzyme associated with cyanide biodegradation by Serratia marcescens. Arch. Microbiol. 2018, 200, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Ahammad, S.; Sreekrishnan, T. Improving the cyanide toxicity tolerance of anaerobic reactor: Microbial interactions and toxin reduction. J. Hazard. Mater. 2016, 315, 52–60. [Google Scholar] [CrossRef]
- Kuyucak, N.; Akcil, A. Cyanide and removal options from effluents in gold mining and metallurgical processes. Miner. Eng. 2013, 50, 13–29. [Google Scholar] [CrossRef]
- Smith, M.R.; Lequerica, J.; Hart, M. Inhibition of methanogenesis and carbon metabolism in Methanosarcina sby cyanide. J. Bacteriol. 1985, 162, 67–71. [Google Scholar] [CrossRef]
- Ibrahim, K.K.; Syed, M.A.; Shukor, M.Y.; Ahmad, S.A. Biological remediation of cyanide: A review. Biotropia-Southeast Asian J. Trop. Biol. 2016, 22, 151–163. [Google Scholar]
- Gupta, P.; Sreekrishnan, T.; Shaikh, Z. Application of hybrid anaerobic reactor: Treatment of increasing cyanide containing effluents and microbial composition identification. J. Environ. Manag. 2018, 226, 448–456. [Google Scholar] [CrossRef]
- Glanpracha, N.; Annachhatre, A.P. Anaerobic co-digestion of cyanide containing cassava pulp with pig manure. Bioresour. Technol. 2016, 214, 112–121. [Google Scholar] [CrossRef]
- Fisher, K.; Dilworth, M.J.; Newton, W.E. Azotobacter vinelandii vanadium nitrogenase: Formaldehyde is a product of catalyzed HCN reduction, and excess ammonia arises directly from catalyzed azide reduction. Biochemistry 2006, 45, 4190–4198. [Google Scholar] [CrossRef]
- Seefeldt, L.C.; Yang, Z.Y.; Duval, S.; Dean, D.R. Nitrogenase reduction of carbon-containing compounds. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2013, 1827, 1102–1111. [Google Scholar] [CrossRef] [PubMed]
- Ni, G.; Canizales, S.; Broman, E.; Simone, D.; Palwai, V.R.; Lundin, D.; Lopez-Fernandez, M.; Sleutels, T.; Dopson, M. Microbial community and metabolic activity in thiocyanate degrading low temperature microbial fuel cells. Front. Microbiol. 2018, 9, 2308. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-S.; Wu, X.-Y.; Zhou, C.C. Effect of different acclimation methods on the performance of microbial fuel cells using phenol as substrate. Bioprocess Biosyst. Eng. 2014, 37, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Saito, T.; Regan, J.M. Nitrogen removal in a single-chamber microbial fuel cell with nitrifying biofilm enriched at the air cathode. Water Res. 2012, 46, 2215–2224. [Google Scholar] [CrossRef]
- Yang, N.; Liu, H.; Zhan, G.Q.; Li, D.P. Sustainable ammonia-contaminated wastewater treatment in heterotrophic nitrifying/denitrifying microbial fuel cell. J. Clean. Prod. 2020, 245, 118923. [Google Scholar] [CrossRef]
- Chen, C.; Kao, C.M.; Chen, S.C.; Chen, T.Y. Biodegradation of tetracyanonickelate by Klebsiella oxytoca under anaerobic conditions. Desalination 2009, 249, 1212–1216. [Google Scholar] [CrossRef]
- Landkamer, L.L.; Bucknam, C.H.; Figueroa, L.A. Anaerobic nitrogen transformations in a gold-cyanide leach residue. Environ. Sci. Technol. Lett. 2015, 2, 357–361. [Google Scholar] [CrossRef]
- Kao, C.; Liu, J.K.; Lou, H.R.; Lin, C.S.; Chen, S.C. Biotransformation of cyanide to methane and ammonia by Klebsiella oxytoca. Chemosphere 2003, 50, 1055–1061. [Google Scholar] [CrossRef]
- Marín-Yaseli, M.R.; Mompeán, C.; Ruiz-Bermejo, M. A prebiotic synthesis of pterins. Chem.–A Eur. J. 2015, 21, 13531–13534. [Google Scholar] [CrossRef]
- Nisshanthini, S.D.; Teresa Infanta S., A.K.; Raja, D.S.; Natarajan, K.; Palaniswamy, M.; Angayarkanni, J. Spectral characterization of a pteridine derivative from cyanide-utilizing bacterium Bacillus subtilis-JN989651. J. Microbiol. 2015, 53, 262–271. [Google Scholar] [CrossRef]
- Jayaraman, A.; Thandeeswaran, M.; Priyadarsini, U.; Sabarathinam, S.; Nawaz, K.A.; Palaniswamy, M. Characterization of unexplored amidohydrolase enzyme—pterin deaminase. Appl. Microbiol. Biotechnol. 2016, 100, 4779–4789. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, R.; Thandeeswaran, M.; Kiran, G.; Arulkumar, M.; Ayub Nawaz, K.A.; Jabastin, J.; Janani, B.; Anto Thomas, T.; Angayarkanni, J. Evaluation of Pterin, a Promising Drug Candidate from Cyanide Degrading Bacteria. Curr. Microbiol. 2018, 75, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, T.; Durairaj, N.; Ramasamy, M.; Jayaraman, K.; Palaniswamy, M.; Jayaraman, A. Analeptic agent from microbes upon cyanide degradation. Appl. Microbiol. Biotechnol. 2018, 102, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Thandeeswaran, M.; Bijukumar, S.; Arulkumar, M.; Mahendran, R.; Palaniswamy, M.; Angayarkanni, J. Production and optimization of pterin deaminase from cyanide utilizing bacterium Bacillus cereus AM12. Biotechnol. Res. Innov. 2019, 3, 159–167. [Google Scholar] [CrossRef]
- Feirer, N.; Fuqua, C. Pterin function in bacteria. Pteridines 2017, 28, 23–36. [Google Scholar] [CrossRef]
- Kunz, D.A.; Wang, C.-S.; Chen, J.-L. Alternative routes of enzymic cyanide metabolism in Pseudomonas fluorescens NCIMB 11764. Microbiology 1994, 140, 1705–1712. [Google Scholar] [CrossRef]
- Fernandez, R.F.; Dolghih, E.; Kunz, D.A. Enzymatic assimilation of cyanide via pterin-dependent oxygenolytic cleavage to ammonia and formate in Pseudomonas fluorescens NCIMB 11764. Appl. Environ. Microbiol. 2004, 70, 121–128. [Google Scholar] [CrossRef]
- Luque-Almagro, V.M.; Escribano, M.P.; Manso, I.; Sáez, L.P.; Cabello, P.; Moreno-Vivián, C.; Roldán, M.D. DNA microarray analysis of the cyanotroph Pseudomonas pseudoalcaligenes CECT5344 in response to nitrogen starvation, cyanide and a jewelry wastewater. J. Biotechnol. 2015, 214, 171–181. [Google Scholar] [CrossRef]
- Park, S.; Ely, R.L. Whole-genome transcriptional and physiological responses of Nitrosomonas europaea to cyanide: Identification of cyanide stress response genes. Biotechnol. Bioeng. 2009, 102, 1645–1653. [Google Scholar] [CrossRef]
- Tang, P.; Hseu, Y.C.; Chou, H.H.; Huang, K.Y.; Chen, S.C. Proteomic analysis of the effect of cyanide on Klebsiella oxytoca. Curr. Microbiol. 2010, 60, 224–228. [Google Scholar] [CrossRef]
- Chen, W.J.; Tang, P.; Hseu, Y.C.; Chen, C.C.; Huang, K.Y.; Chen, S.C. A proteome analysis of the tetracyanonickelate (II) responses in Klebsiella oxytoca. Environ. Microbiol. Rep. 2011, 3, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Martínková, L.; Křen, V. Biotransformations with nitrilases. Curr. Opin. Chem. Biol. 2010, 14, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.; Birkemeyer, C.; Ballschmiter, M. A nitrilase from a metagenomic library acts regioselectively on aliphatic dinitriles. Appl. Microbiol. Biotechnol. 2011, 89, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.K.; Shukla, P. Advanced technologies for improved expression of recombinant proteins in bacteria: Perspectives and applications. Crit. Rev. Biotechnol. 2016, 36, 1089–1098. [Google Scholar] [CrossRef]
- Alcalde, M.; Ferrer, M.; Plou, F.J.; Ballesteros, A. Environmental biocatalysis: From remediation with enzymes to novel green processes. Trends Biotechnol. 2006, 24, 281–287. [Google Scholar] [CrossRef]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manag. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Zhou, X.; Xu, S.; Liu, L.; Chen, J. Degradation of cyanide by Trichoderma mutants constructed by restriction enzyme mediated integration (REMI). Bioresour. Technol. 2007, 98, 2958–2962. [Google Scholar] [CrossRef]
- Sexton, A.; Howlett, B. Characterisation of a cyanide hydratase gene in the phytopathogenic fungus Leptosphaeria maculans. Mol. Gen. Genet. MGG 2000, 263, 463–470. [Google Scholar] [CrossRef]
| Method | Performance Parameters | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Reactor Type | Contaminant | Removal Efficiency (%) | Microbial Source | Anode | Cathode | pH | Temperature (°C) | Reference | |
| EK-BIO | |||||||||
| EBC | Free cyanide | 99.7 | Bacillus pumilus ATCC 7061 | Aluminum | Aluminum | NM | 30 | [163] | |
| MFC | |||||||||
| sMFC | Free cyanide | 100 | Klebsiella sp. (MC-1) | Disc graphite felt | Disc graphite felt with Pt | - | 25 | [16] | |
| dMFC | COD Cyanide | 88.34 99.51 | Klebsiella sp. | - | - | - | 25 | [82] | |
| dMFC | Thiocyanate | 100 | Thiobacillus sp. | Graphite felt | Graphite felt | 7 | 8 | [164] | |
| sMFC | Phenol | 88.9% | NM | Carbon felt | Carbon cloth with Pt | 7 | 25 | [165] | |
| sMFC | Ammonium | 96.8% | WWTP sludge | Carbon cloth | Carbon Cloth | - | 30 | [166] | |
| ACMFC | COD Ammonium | 91% 99% | Aerobic denitrifying sludge | Carbon fiber felts | Carbon fiber felts | 8.0 | - | [167] | |
| AB | |||||||||
| Serum bottles | Tetracyano nickelate | 100 | Klebsiella oxytoca NSYSU-011 | - | - | 7.0 | 30 | [168] | |
| UASB | Free cyanide | 100 | UASB biomass | - | - | - | - | [151] | |
| Stirred conical reactor | Free cyanide | 90-93% | UASB biomass | - | - | 7.2–7.8 | 31 | [160] | |
| Conical flasks | Free cyanide Nitrate | 100 ≤ 40 | Heap leach residue and water | - | - | 8.5–9.5 | 22 | [169] | |
| ABR | Potassium tetrahydroxy zinc(II) | 100 | Cow dung and wastewater sludge | - | - | 6.8–8 | 37 | [155] | |
| Bottle | Potassium cyanide | 100 | Klebsiella oxytoca | - | - | 7.0 | 30 | [170] | |
| SGR | Phenol Cyanide Thiocyanate | 100 96 100 | Sewage | - | - | 8.0 | 27 | [150] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malmir, N.; Fard, N.A.; Aminzadeh, S.; Moghaddassi-Jahromi, Z.; Mekuto, L. An Overview of Emerging Cyanide Bioremediation Methods. Processes 2022, 10, 1724. https://doi.org/10.3390/pr10091724
Malmir N, Fard NA, Aminzadeh S, Moghaddassi-Jahromi Z, Mekuto L. An Overview of Emerging Cyanide Bioremediation Methods. Processes. 2022; 10(9):1724. https://doi.org/10.3390/pr10091724
Chicago/Turabian StyleMalmir, Narges, Najaf Allahyari Fard, Saeed Aminzadeh, Zahra Moghaddassi-Jahromi, and Lukhanyo Mekuto. 2022. "An Overview of Emerging Cyanide Bioremediation Methods" Processes 10, no. 9: 1724. https://doi.org/10.3390/pr10091724





