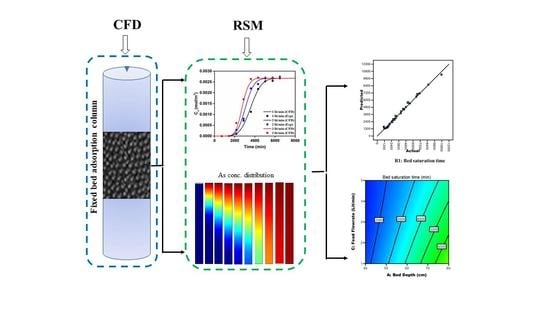
A Combined CFD-Response Surface Methodology Approach for Simulation and Optimization of Arsenic Removal in a Fixed Bed Adsorption Column
Abstract
:1. Introduction
2. Methodology
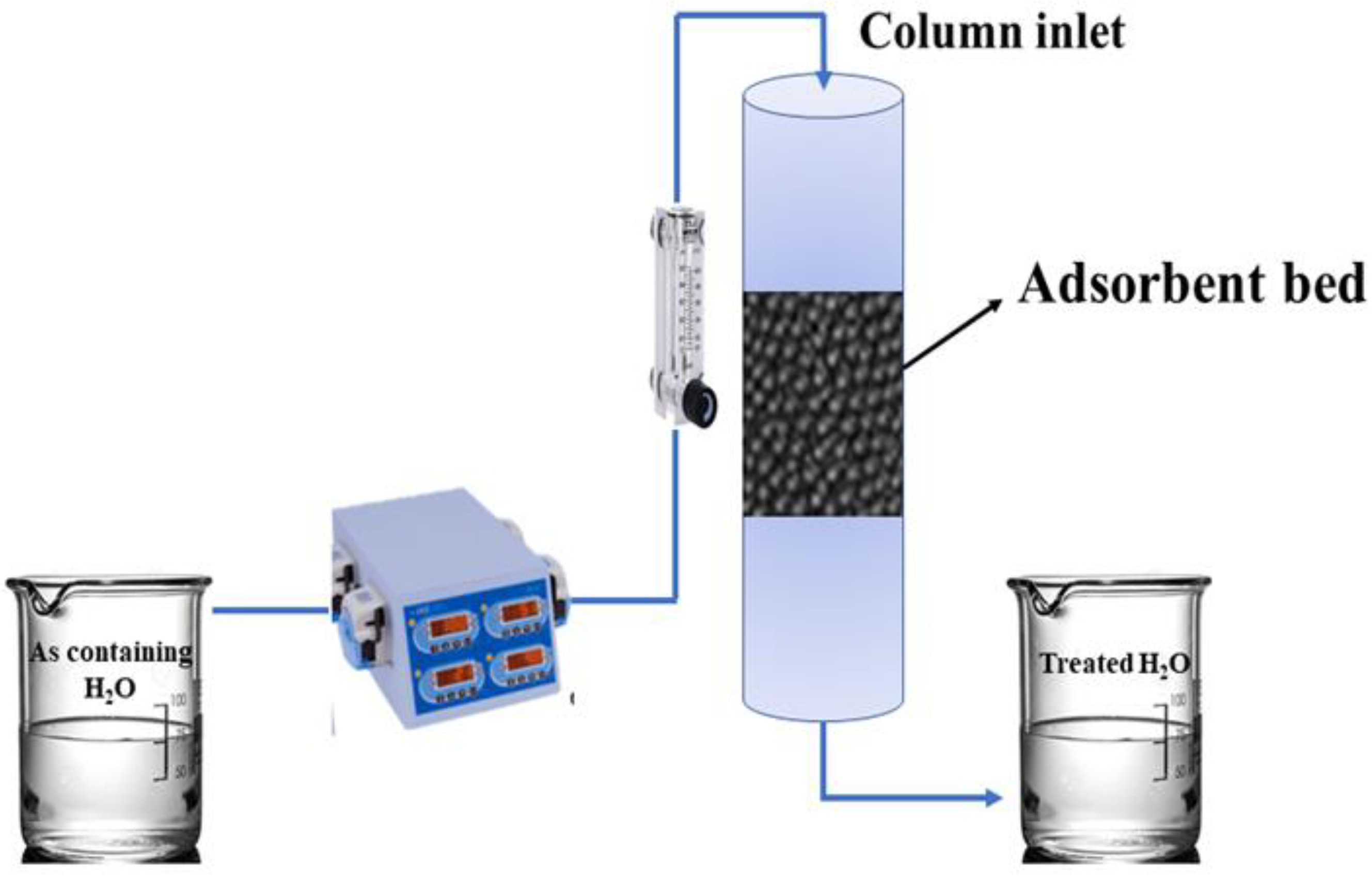
2.1. Experimental Setup and Material Selection
2.2. Model Development and Numerical Setting
2.2.1. General Assumptions
2.2.2. CFD Model Equations
Mass Balance Equation
Adsorption Mechanism
Convection in a Porous Medium
2.2.3. Simulation Parameters
2.3. Response Surface Methodology (RSM)
3. Results and Discussion
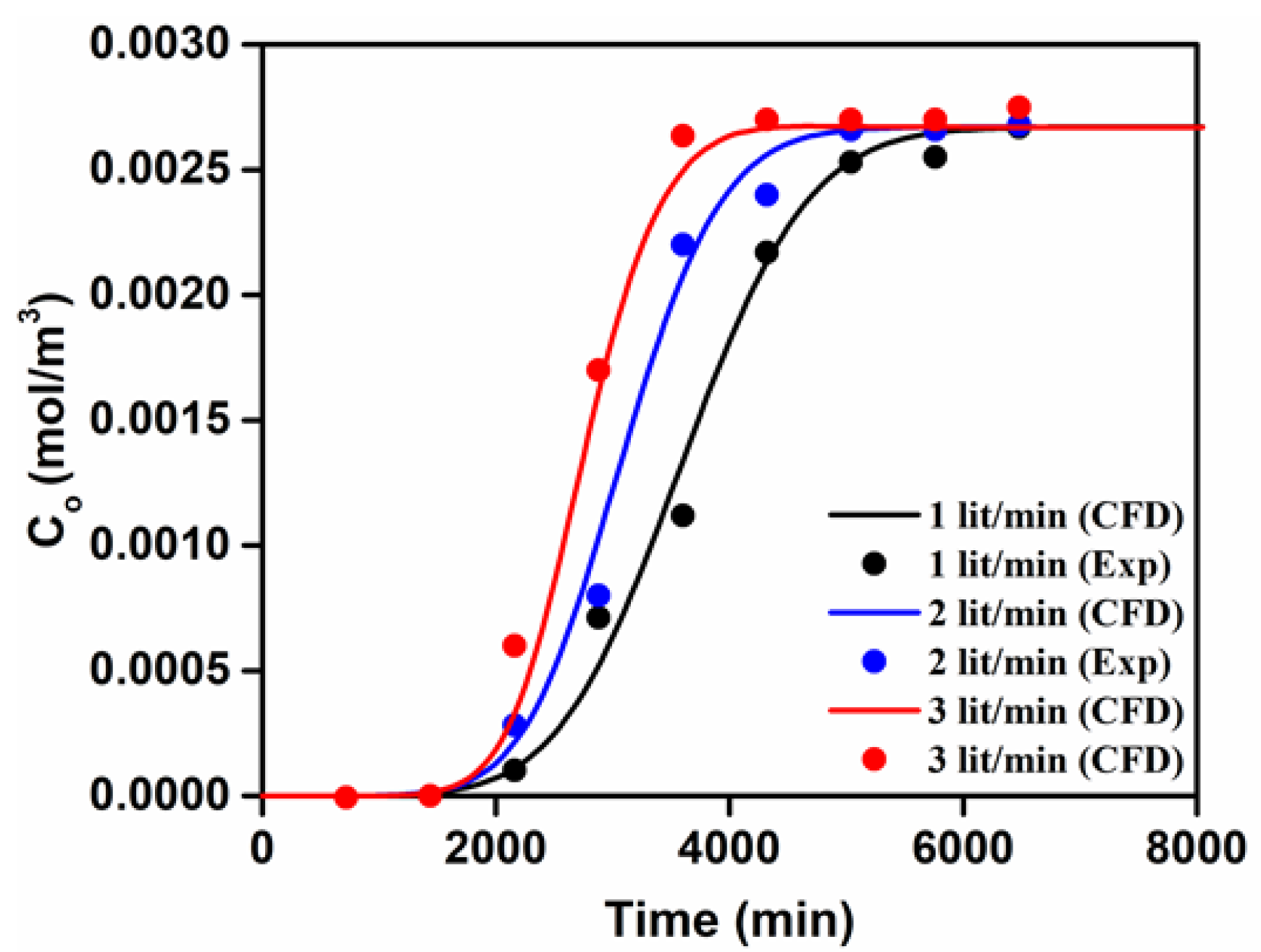
3.1. Model Validation
3.2. CFD Results—Effects of the Bed Depth, the Feed Flow Rate, and the Initial Arsenic Concentration
3.3. RSM Results
3.3.1. ANOVA Results and Models Regression
3.3.2. The Regression Model Equations
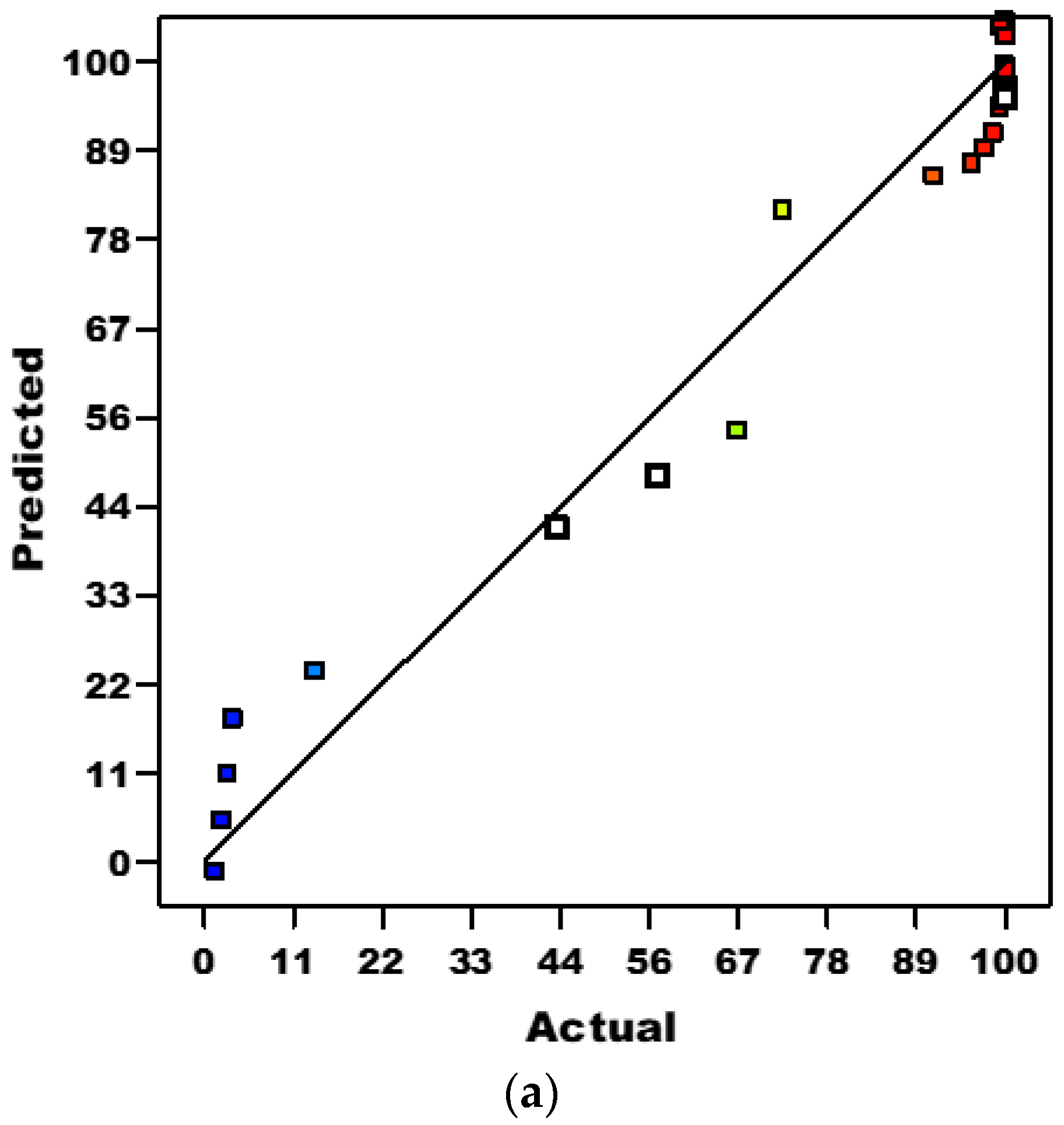
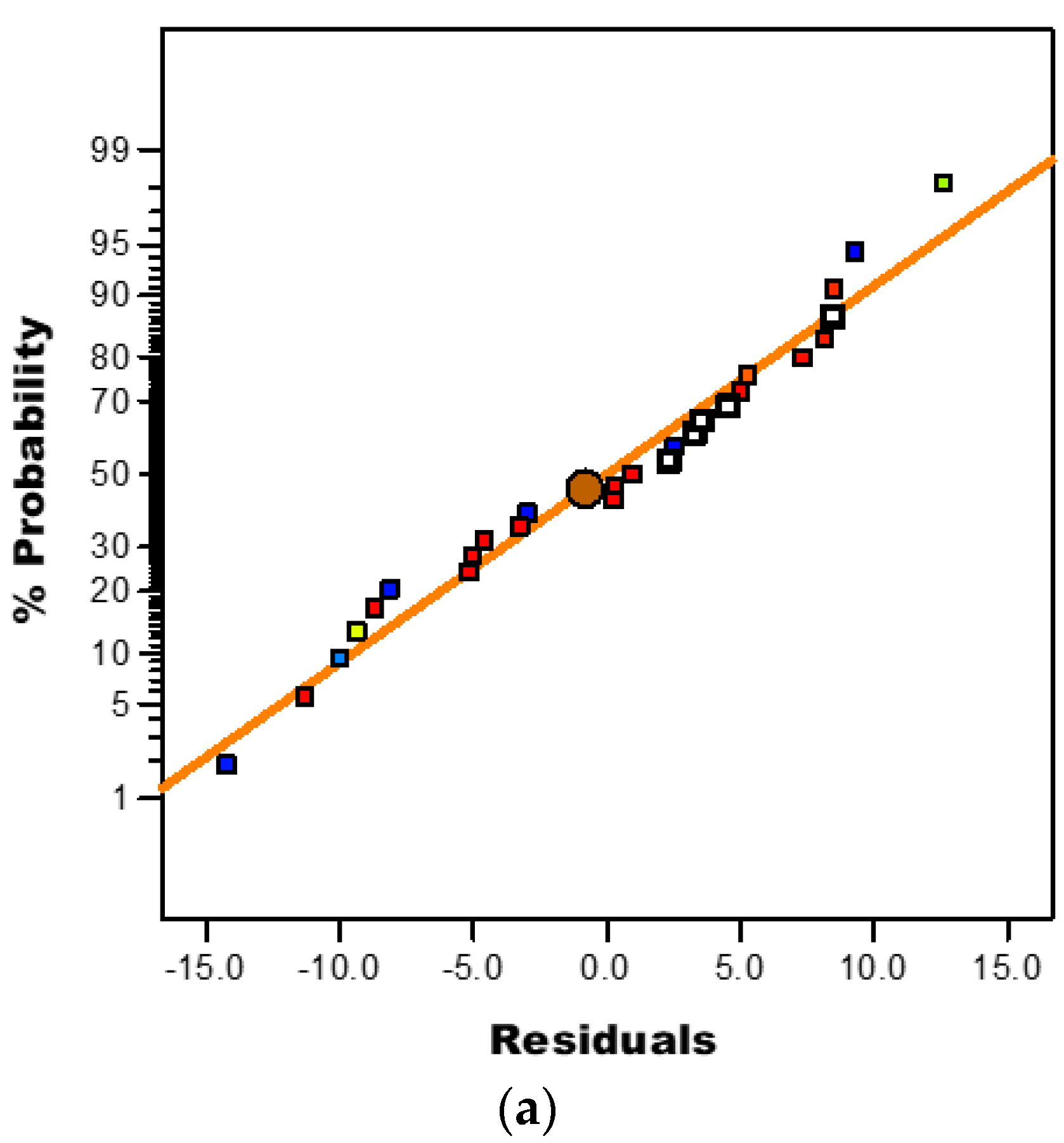
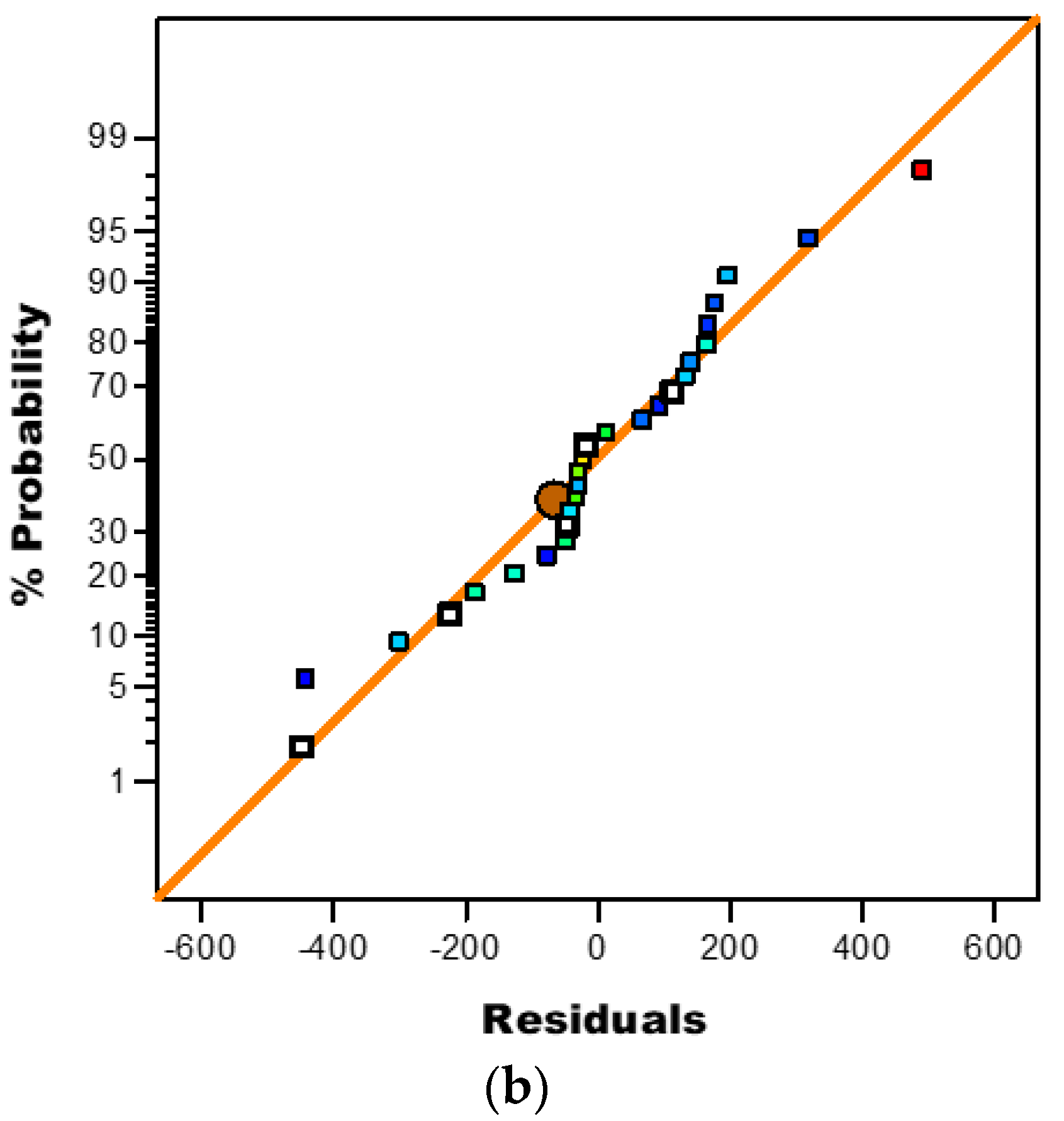
3.3.3. Diagnostic Plots
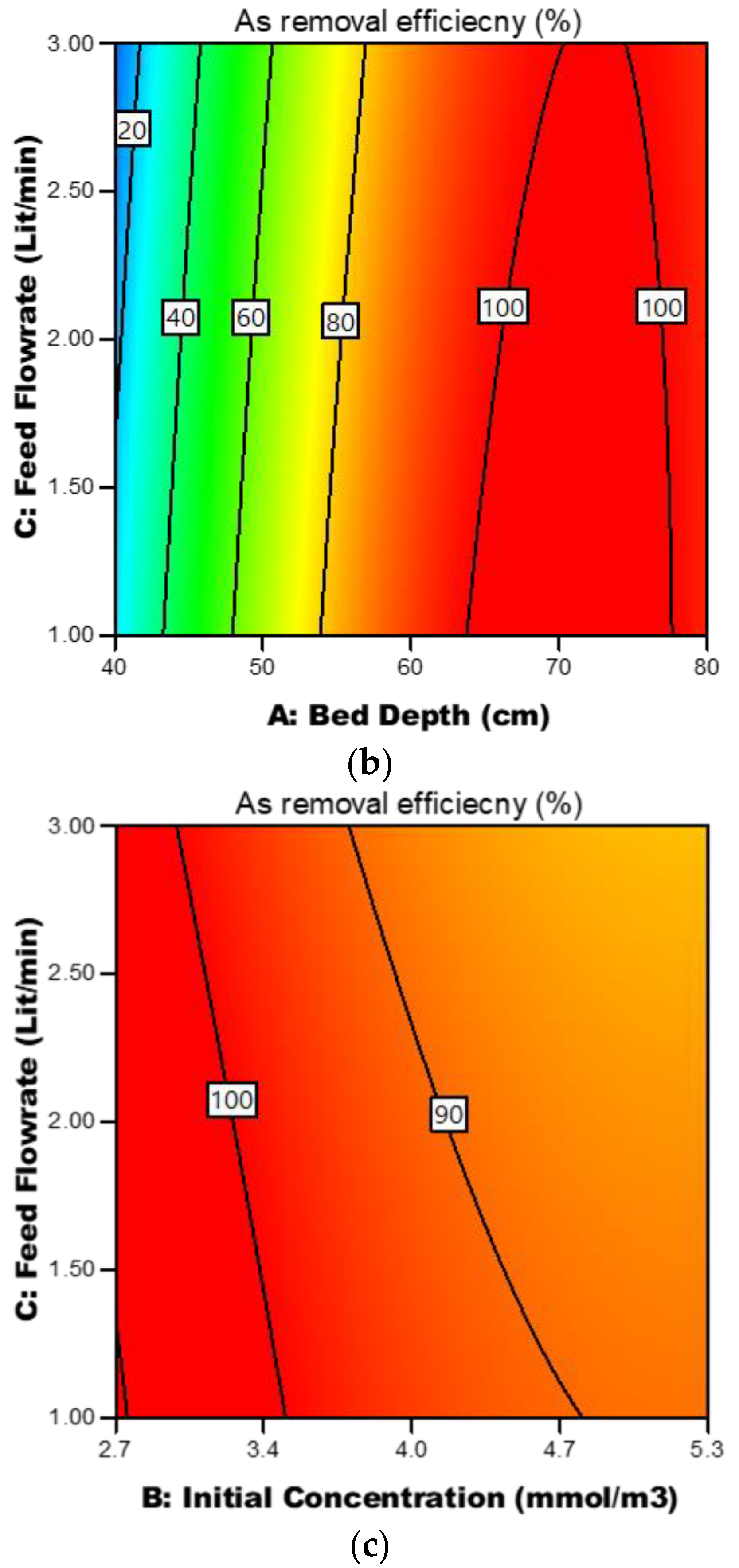
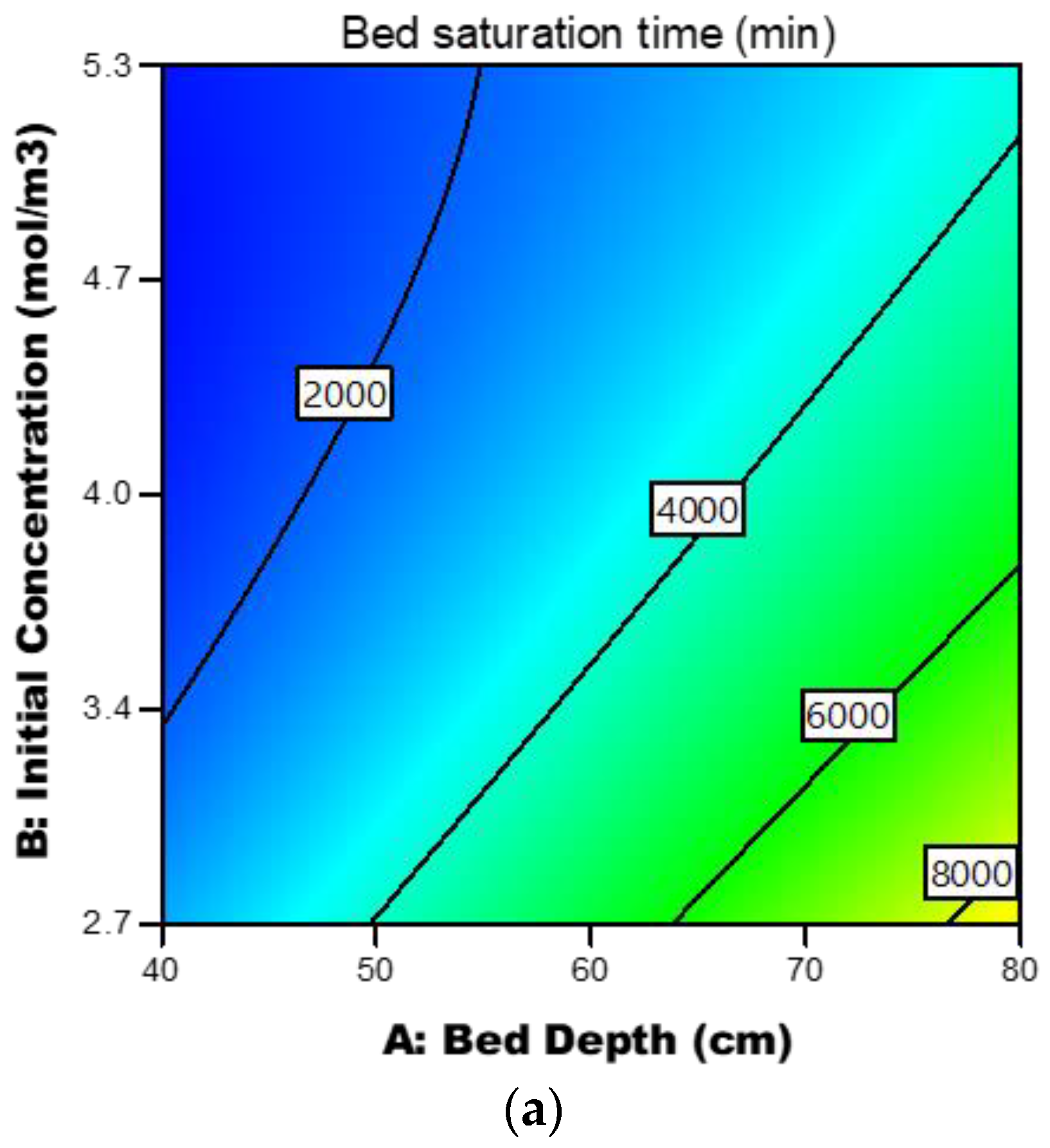
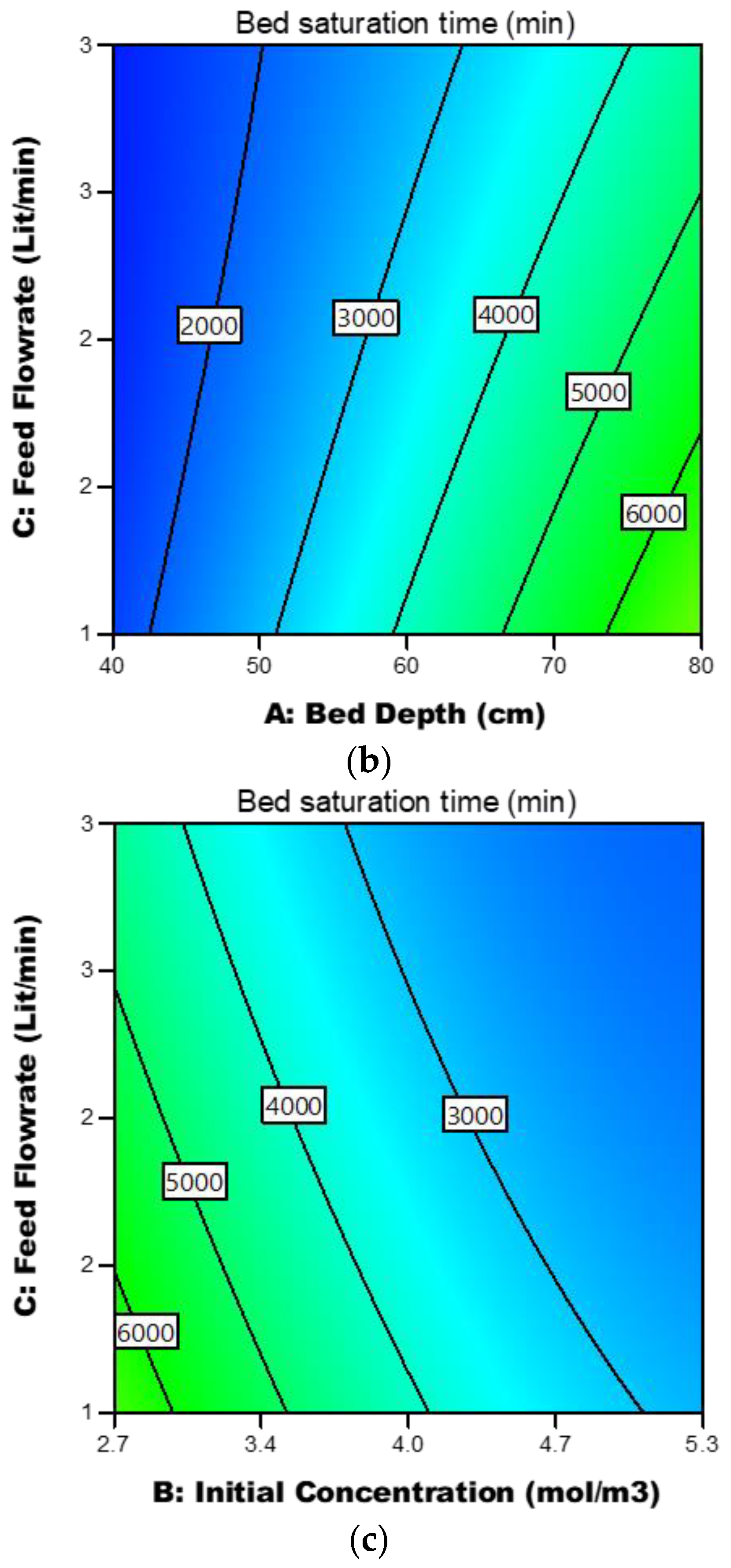
3.3.4. Response Contour Plots
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jamil, N.; Baqar, M.; Shaikh, I.A. Assessment of contamination in water and soil surrounding a chlor-alkali plant: A case study. J. Chem. Soc. Pak. 2015, 37, 173–178. [Google Scholar]
- Raza, A.; Farooq, A.; Ali, W.; Javed, A. Effect of human settlements on surface and groundwater quality: Statistical source identification of heavy and trace metals of siran river and its catchment area mansehra, pakistan. J. Chem. Soc. Pak. 2017, 39, 296–308. [Google Scholar]
- Yadav, A.; Dindorkar, S.S.; Ramisetti, S.B.; Sinha, N. Simultaneous adsorption of methylene blue and arsenic on graphene, boron nitride and boron carbon nitride nanosheets: Insights from molecular simulations. J. Water Process Eng. 2022, 46, 102653. [Google Scholar] [CrossRef]
- Ranjan, A. Spatial Analysis of Arsenic Contamination of Groundwater around the World and India. IJISSH 2019, 4, 1–10. [Google Scholar]
- Wang, Y.-Y.; Chai, L.-Y.; Yang, W.-C. Arsenic distribution and pollution characteristics. In Arsenic Pollution Control in Nonferrous Metallurgy; Springer: Cham, Switzerland, 2019; pp. 1–15. [Google Scholar]
- Chandrajith, R.; Diyabalanage, S.; Dissanayake, C. Geogenic fluoride and arsenic in groundwater of Sri Lanka and its implications to community health. Groundw. Sustain. Dev. 2020, 10, 100359. [Google Scholar] [CrossRef]
- Zanini, A.; Petrella, E.; Sanangelantoni, A.M.; Angelo, L.; Ventosi, B.; Viani, L.; Rizzo, P.; Remelli, S.; Bartoli, M.; Bolpagni, R.; et al. Groundwater characterization from an ecological and human perspective: An interdisciplinary approach in the Functional Urban Area of Parma, Italy. Rend. Lincei Sci. Fis. E Nat. 2019, 30, 93–108. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Rabia, M.; Elkader, Y.A.; Abd El-Halim, M.R. Investigation the adsorption properties of graphene oxide and polyaniline nano/micro structures for efficient removal of toxic Cr(VI) contaminants from aqueous solutions; kinetic and equilibrium studies. Rend. Lincei Sci. Fis. E Nat. 2018, 29, 141–154. [Google Scholar] [CrossRef]
- Mandal, B.K.; Suzuki, K.T. Arsenic round the world: A review. Talanta 2002, 58, 201–235. [Google Scholar] [CrossRef]
- Hocaoglu, S.M.; Wakui, Y.; Suzuki, T.M. Separation of arsenic(V) by composite adsorbents of metal oxide nanoparticles immobilized on silica flakes and use of adsorbent coated alumina tubes as an alternative method. J. Water Process Eng. 2019, 27, 134–142. [Google Scholar] [CrossRef]
- Hashim, M.A.; Kundu, A.; Mukherjee, S.; Ng, Y.-S.; Mukhopadhyay, S.; Redzwan, G.; Sen Gupta, B. Arsenic removal by adsorption on activated carbon in a rotating packed bed. J. Water Process Eng. 2019, 30, 100591. [Google Scholar] [CrossRef]
- Sujatha, S.; Rajasimman, M. Development of a green emulsion liquid membrane using waste cooking oil as diluent for the extraction of arsenic from aqueous solution—Screening, optimization, kinetics and thermodynamics studies. J. Water Process Eng. 2021, 41, 102055. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Ociński, D.; Mazur, P. Highly efficient arsenic sorbent based on residual from water deironing—Sorption mechanisms and column studies. J. Hazard. Mater. 2020, 382, 121062. [Google Scholar] [CrossRef]
- Syam Babu, D.; Nidheesh, P. A review on electrochemical treatment of arsenic from aqueous medium. Chem. Eng. Commun. 2020, 208, 389–410. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, G.; Siddhu, M.A.H.; Masroor, A.; Ali, M.F.; Abdeltawab, A.A.; Chen, X. Effect of impeller on sinking and floating behavior of suspending particle materials in stirred tank: A computational fluid dynamics and factorial design study. Adv. Powder Technol. 2017, 28, 1159–1169. [Google Scholar] [CrossRef]
- Cheng, S.-J.; Miao, J.-M.; Wu, S.-J. Investigating the effects of operational factors on PEMFC performance based on CFD simulations using a three-level full-factorial design. Renew. Energy 2012, 39, 250–260. [Google Scholar] [CrossRef]
- Lee, S.; Park, Y.; Kim, J. An evaluation of factors influencing drag coefficient in double-deck tunnels by CFD simulations using factorial design method. J. Wind Eng. Ind. Aerodyn. 2018, 180, 156–167. [Google Scholar] [CrossRef]
- Jabbari, B.; Jalilnejad, E.; Ghasemzadeh, K.; Iulianelli, A. Modeling and optimization of a membrane gas separation based bioreactor plant for biohydrogen production by CFD–RSM combined method. J. Water Process Eng. 2021, 43, 102288. [Google Scholar] [CrossRef]
- Awad, A.M.; Hussein, I.A.; Nasser, M.S.; Ghani, S.A.; Mahgoub, A.O. A CFD- RSM study of cuttings transport in non-Newtonian drilling fluids: Impact of operational parameters. J. Pet. Sci. Eng. 2022, 208, 109613. [Google Scholar] [CrossRef]
- Kola, P.V.K.V.; Pisipaty, S.K.; Mendu, S.S.; Ghosh, R. Optimization of performance parameters of a double pipe heat exchanger with cut twisted tapes using CFD and RSM. Chem. Eng. Process. Process Intensif. 2021, 163, 108362. [Google Scholar] [CrossRef]
- Singh, S.; Chakraborty, J.P.; Mondal, M.K. Pyrolysis of torrefied biomass: Optimization of process parameters using response surface methodology, characterization, and comparison of properties of pyrolysis oil from raw biomass. J. Clean. Prod. 2020, 272, 122517. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, H.; Le, P.H.A.; Nguyen, D.T.C.; Nguyen, T.T.; Nguyen, C.V.; Vo, D.-V.N.; Nguyen, T.D. Microwave-assisted solvothermal fabrication of hybrid zeolitic–imidazolate framework (ZIF-8) for optimizing dyes adsorption efficiency using response surface methodology. J. Environ. Chem. Eng. 2020, 8, 104189. [Google Scholar] [CrossRef]
- Lysova, N.; Solari, F.; Vignali, G. Optimization of an indirect heating process for food fluids through the combined use of CFD and Response Surface Methodology. Food Bioprod. Process. 2022, 131, 60–76. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H.; Lee, W. Investigating micellar-enhanced ultrafiltration (MEUF) of mercury and arsenic from aqueous solution using response surface methodology and gene expression programming. Sep. Purif. Technol. 2022, 281, 119880. [Google Scholar] [CrossRef]
- Gokcek, O.B.; Uzal, N. Arsenic removal by the micellar-enhanced ultrafiltration using response surface methodology. Water Supply 2020, 20, 574–585. [Google Scholar] [CrossRef]
- Yaqub, M.; Lee, S.H. Experimental and neural network modeling of micellar enhanced ultrafiltration for arsenic removal from aqueous solution. Environ. Eng. Res. 2021, 26, 190261. [Google Scholar] [CrossRef]
- Baskan, M.B.; Pala, A. Determination of arsenic removal efficiency by ferric ions using response surface methodology. J. Hazard. Mater. 2009, 166, 796–801. [Google Scholar] [CrossRef]
- Noguchi, H.; Yin, Q.; Lee, S.C.; Xia, T.; Niwa, T.; Lay, W.; Chua, S.C.; Yu, L.; Tay, Y.J.; Nassir, M.J. Performance of Newly Developed Intermittent Aerator for Flat-Sheet Ceramic Membrane in Industrial MBR System. Water 2022, 14, 2286. [Google Scholar] [CrossRef]
- Mackay, D.M.; Freyberg, D.L.; Roberts, P.V.; Cherry, J.A. A natural gradient experiment on solute transport in a sand aquifer: 1. Approach and overview of plume movement. Water Resour. Res. 1986, 22, 2017–2029. [Google Scholar] [CrossRef]
- Solangi, Z.A.; Bhatti, I.; Qureshi, K. Modeling and Simulation of Fixed Bed Column for Arsenic Removal using Iron Ore and PAN Fiber Adsorbents. Int. J. Emerg. Technol. 2021, 12, 183–191. [Google Scholar]
- Nguyen, D.T.C.; Vo, D.-V.N.; Nguyen, T.T.; Nguyen, T.T.T.; Nguyen, L.T.T.; Tran, T.V. Optimization of tetracycline adsorption onto zeolitic–imidazolate framework-based carbon using response surface methodology. Surf. Interfaces 2022, 28, 101549. [Google Scholar] [CrossRef]
- Hosseinpour, M.; Soltani, M.; Noofeli, A.; Nathwani, J. An optimization study on heavy oil upgrading in supercritical water through the response surface methodology (RSM). Fuel 2020, 271, 117618. [Google Scholar] [CrossRef]





| Sr. No. | Parameter | Value |
|---|---|---|
| 1 | Sequence selected in COMSOL | Physics-controlled mesh |
| 2 | Element size feature | Extremely fine |
| 3 | Shape of elements | Triangular |
| 4 | Number of elements | 927 |
| 5 | Edge elements | 115 |
| 6 | Vertex elements | 8 |
| 7 | Minimum orthogonal quality | 0.8364 |
| 8 | Average element quality | 0.974 |
| 9 | Element area ratio | 0.2703 |
| 10 | Mesh area | 0.408 m2 |
| 11 | Maximum growth rate | 1.619 |
| 12 | Average growth rate | 1.074 |
| 13 | Curvature factor | 0.3 |
| Sr. No | Varying Parameter | Values |
|---|---|---|
| 1 | Feed flow rate (L/min) | 1 |
| 2 | ||
| 3 | ||
| 2 | Initial arsenic concentration (mol/m3) | 2.7 (200 ppb) |
| 4.0 (300 ppb) | ||
| 5.3 (400 ppb) | ||
| 3 | Bed depth (cm) | 40 |
| 60 | ||
| 80 |
| Factors | Symbol | Levels | ||
|---|---|---|---|---|
| Low Level (−1) | Intermediate Level (0) | High Level (1) | ||
| Bed depth (cm) | A | 40 | 60 | 80 |
| Initial As conc. (mmol/m3) | B | 2.7 | 4.0 | 5.3 |
| Feed flow rate (L/min) | C | 1 | 2 | 3 |
| Run | A: Bed Depth (cm) | B: Initial As Conc. (mol/m3) | C: Feed Flow Rate (L/min) | As Removal Efficiency (%) | Bed Saturation Time (mins) |
|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 71.58 | 3050 |
| 2 | 0 | 1 | 1 | 62.88 | 2100 |
| 3 | −1 | −1 | 0 | 56.64 | 2700 |
| 4 | 0 | −1 | −1 | 99.56 | 6550 |
| 5 | −1 | 0 | 0 | 3.55 | 1750 |
| 6 | 0 | 0 | −1 | 99.37 | 4000 |
| 7 | −1 | −1 | 1 | 44.04 | 2500 |
| 8 | 1 | 1 | 1 | 100 | 3100 |
| 9 | 1 | −1 | −1 | 99.99 | 10,750 |
| 10 | 1 | 0 | −1 | 99.99 | 6750 |
| 11 | 1 | 1 | −1 | 99.99 | 4800 |
| 12 | 1 | 0 | 0 | 99.99 | 5150 |
| 13 | 0 | −1 | 1 | 99.44 | 4350 |
| 14 | 0 | −1 | 0 | 99.35 | 5450 |
| 15 | 1 | 1 | 0 | 99.99 | 3950 |
| 16 | −1 | 1 | 1 | 0 | 1100 |
| 17 | −1 | 1 | 0 | 0 | 1200 |
| 18 | −1 | 1 | −1 | 0 | 1350 |
| 19 | −1 | 0 | −1 | 13.82 | 1900 |
| 20 | 1 | −1 | 1 | 99.99 | 7100 |
| 21 | −1 | 0 | 1 | 0.32 | 1550 |
| 22 | 0 | 0 | 1 | 95.83 | 2900 |
| 23 | 1 | −1 | 0 | 99.98 | 8550 |
| 24 | 0 | 0 | 0 | 98.53 | 3250 |
| 25 | 1 | 0 | 1 | 99.98 | 4450 |
| 26 | 0 | 1 | 0 | 91.05 | 2450 |
| 27 | 0 | 1 | −1 | 97.37 | 2800 |
| R1: As Removal Efficiency | ||||||
| Source | SS | df | Mean Square | F-Value | p-Value | |
| Model | 39,780.58 | 9 | 4420.06 | 54.81 | <0.0001 | Significant |
| A: bed depth | 27,849.99 | 1 | 27,849.99 | 345.34 | <0.0001 | |
| B: initial As concentration | 2232.15 | 1 | 2232.15 | 27.68 | <0.0001 | |
| C: feed flow rate | 222.94 | 1 | 222.94 | 2.76 | 0.1147 | |
| AB | 2207.48 | 1 | 2207.48 | 27.37 | <0.0001 | |
| AC | 99.28 | 1 | 99.28 | 1.23 | 0.2826 | |
| BC | 1.17 | 1 | 1.17 | 0.0145 | 0.9057 | |
| A2 | 6971.75 | 1 | 6971.75 | 86.45 | <0.0001 | |
| B2 | 195.01 | 1 | 195.01 | 2.42 | 0.1384 | |
| C2 | 0.8165 | 1 | 0.8165 | 0.0101 | 0.9210 | |
| Residual | 1370.96 | 17 | 80.64 | |||
| Cor total | 41,151.55 | 26 | ||||
| R2: Bed Saturation Time | ||||||
| Model | 1.466 × 108 | 9 | 1.629 × 107 | 244.33 | <0.0001 | Significant |
| A: bed Depth | 7.813 × 107 | 1 | 7.813 × 107 | 1171.83 | <0.0001 | |
| B: initial As concentration | 4.402 × 107 | 1 | 4.402 × 107 | 660.33 | <0.0001 | |
| C: feed flow rate | 9.102 × 106 | 1 | 9.102 × 106 | 136.53 | <0.0001 | |
| AB | 8.250 × 106 | 1 | 8.250 × 106 | 123.75 | <0.0001 | |
| AC | 3.521 × 106 | 1 | 3.521 × 106 | 52.81 | <0.0001 | |
| BC | 1.172 × 106 | 1 | 1.172 × 106 | 17.58 | 0.0006 | |
| A2 | 2.963 × 105 | 1 | 2.963 × 105 | 4.44 | 0.0502 | |
| B2 | 2.022 × 106 | 1 | 2.022 × 106 | 30.33 | <0.0001 | |
| C2 | 89,629.63 | 1 | 89,629.63 | 1.34 | 0.2623 | |
| Residual | 1.133 × 106 | 17 | 66,669.39 | |||
| Cor Total | 1.477 × 108 | 26 | ||||
| Response | Regression Model | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|
| R1: As removal efficiency | −105.861 + 9.87085A − 63990.6B − 9.73762C + 508.093AB + 0.143817AC − 233.545BC − 0.0852188A2 + 3.20023 × 106B2 − 0.368892C2 | 0.9667 | 0.949 | 0.9152 |
| R2: bed saturation time | 2033.11 + 216.045A − 2.38616 × 106B − 512.523C + 31,061.9AB − 27.0833AC + 234,135 BC + 0.555556A2 + 3.25894 × 108B2 + 122.222C2 | 0.9923 | 0.9883 | 09764 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solangi, Z.A.; Bhatti, I.; Qureshi, K. A Combined CFD-Response Surface Methodology Approach for Simulation and Optimization of Arsenic Removal in a Fixed Bed Adsorption Column. Processes 2022, 10, 1730. https://doi.org/10.3390/pr10091730
Solangi ZA, Bhatti I, Qureshi K. A Combined CFD-Response Surface Methodology Approach for Simulation and Optimization of Arsenic Removal in a Fixed Bed Adsorption Column. Processes. 2022; 10(9):1730. https://doi.org/10.3390/pr10091730
Chicago/Turabian StyleSolangi, Zulfiqar Ali, Inamullah Bhatti, and Khadija Qureshi. 2022. "A Combined CFD-Response Surface Methodology Approach for Simulation and Optimization of Arsenic Removal in a Fixed Bed Adsorption Column" Processes 10, no. 9: 1730. https://doi.org/10.3390/pr10091730