Preparation and Phosphorus Removal Performance of Zr–La–Fe Ternary Composite Adsorbent Embedded with Sodium Alginate
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Zr–La–Fe Ternary Adsorbent Embedded with Sodium Alginate
2.3. Sample Characterization
2.4. Experimental Methods
2.5. Thermodynamic and Kinetic Fitting of Adsorption
2.5.1. Thermodynamic Fitting of Adsorption
2.5.2. Adsorption Kinetics Fitting
3. Results and Discussion
3.1. XRD and FT-IR Characterization
3.2. FT-IR Characterization Results and Analysis
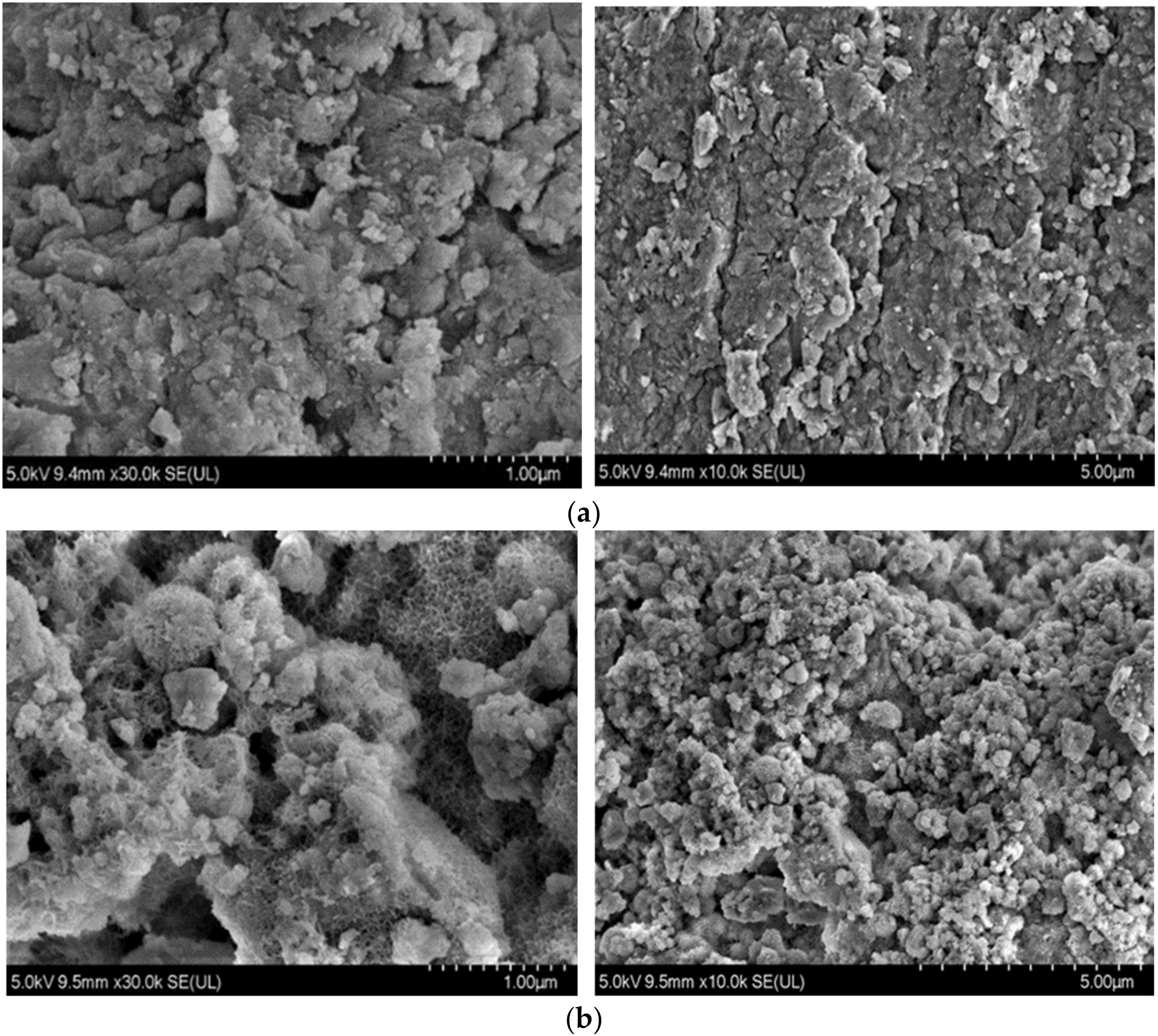
3.3. SEM Characterization Results and Analysis
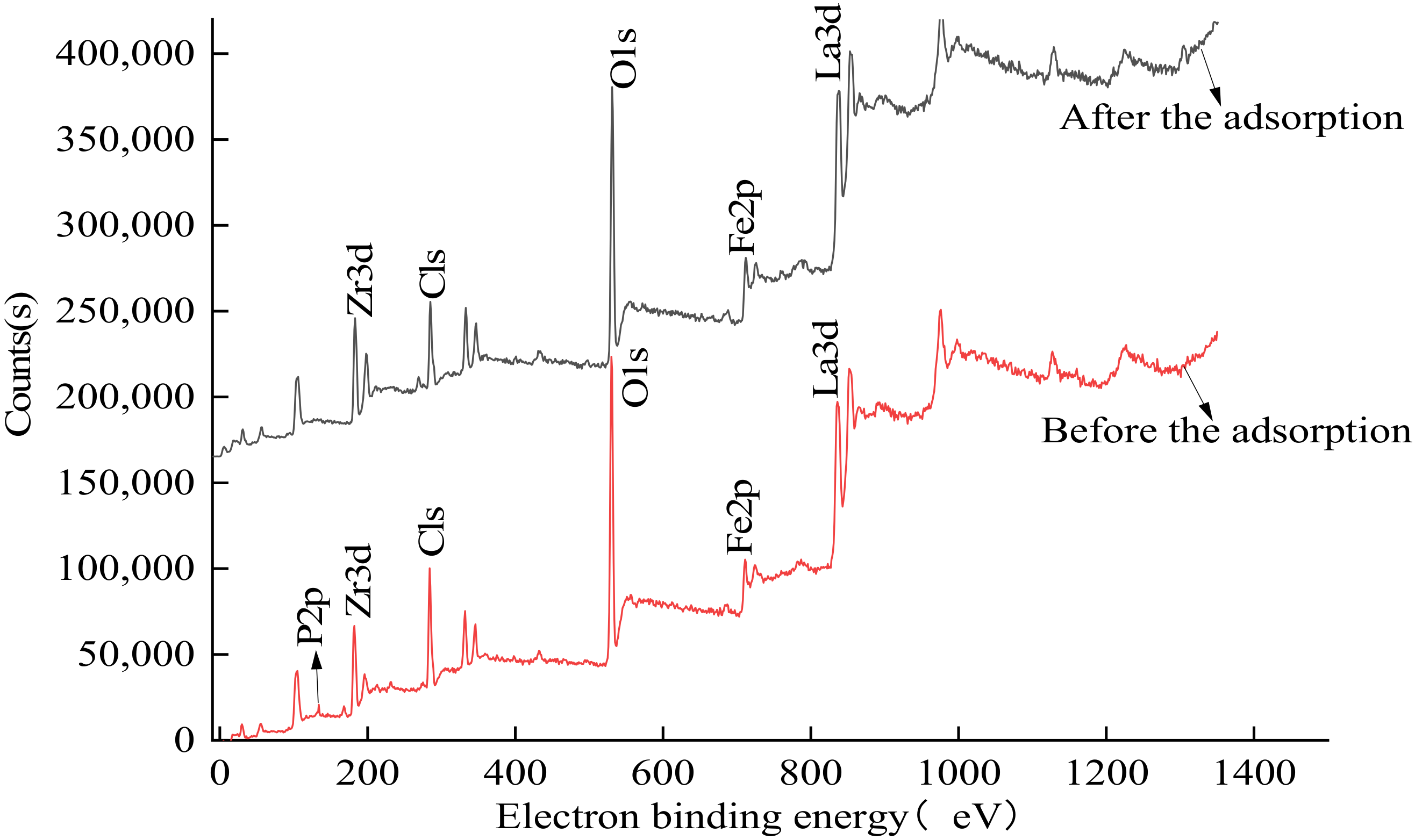
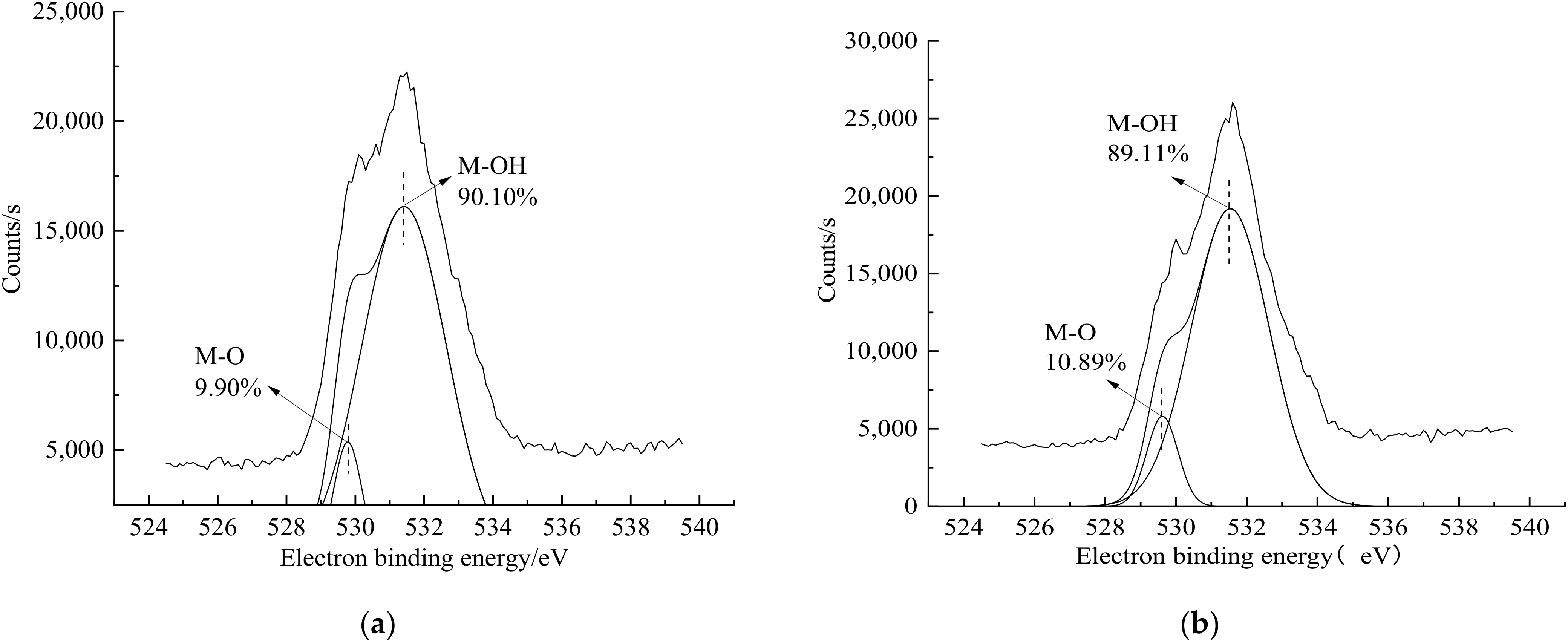
3.4. XPS Characterization Results and Analysis
3.5. Point-of-Zero-Charge Test
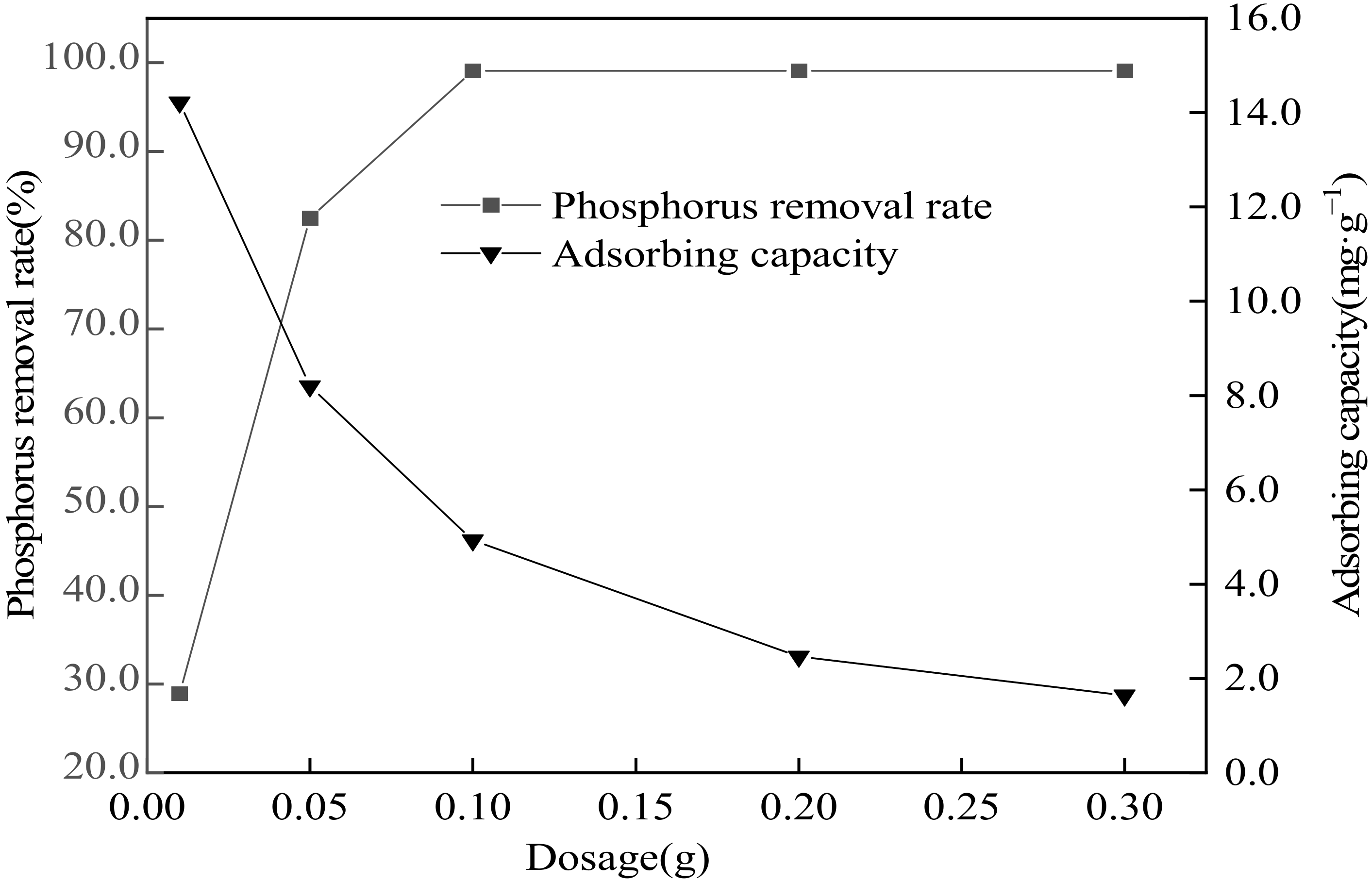
3.6. Influence of Adsorbent Dosage on Phosphorus Removal Performance
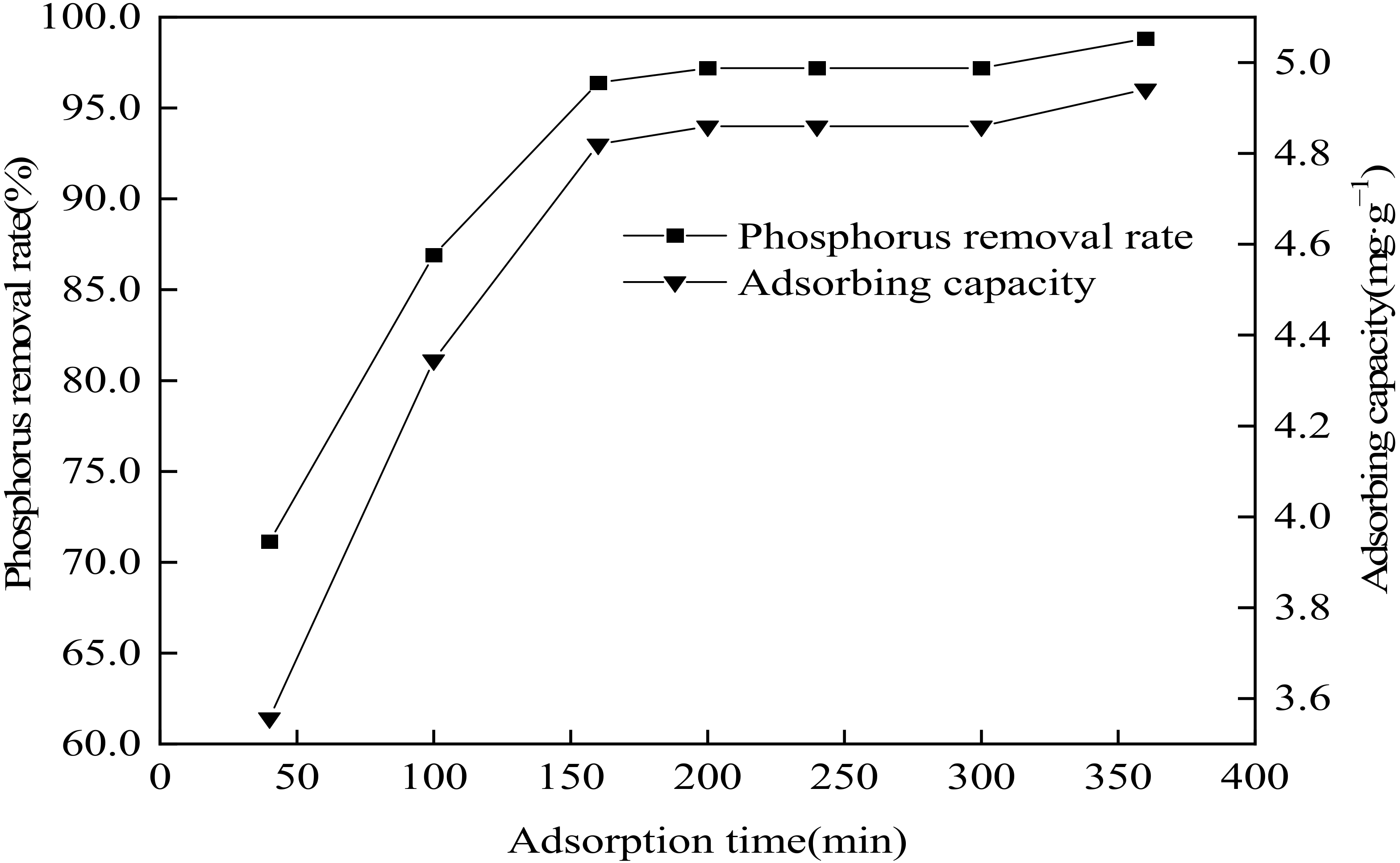
3.7. Influence of Reaction Time on Phosphorus Removal Performance
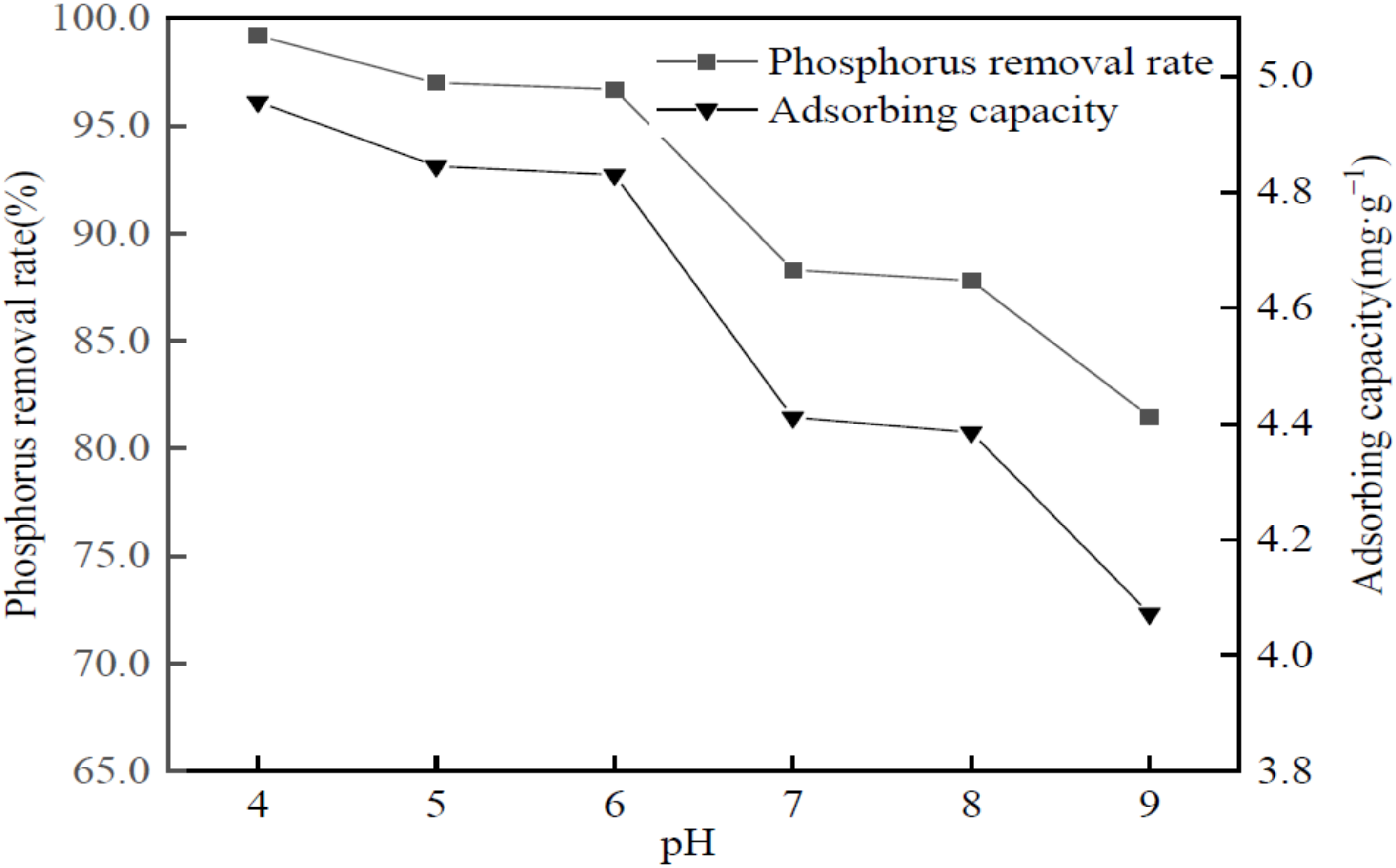
3.8. Influence of pH on Phosphorus Removal Performance of Phosphorus-Containing Wastewater
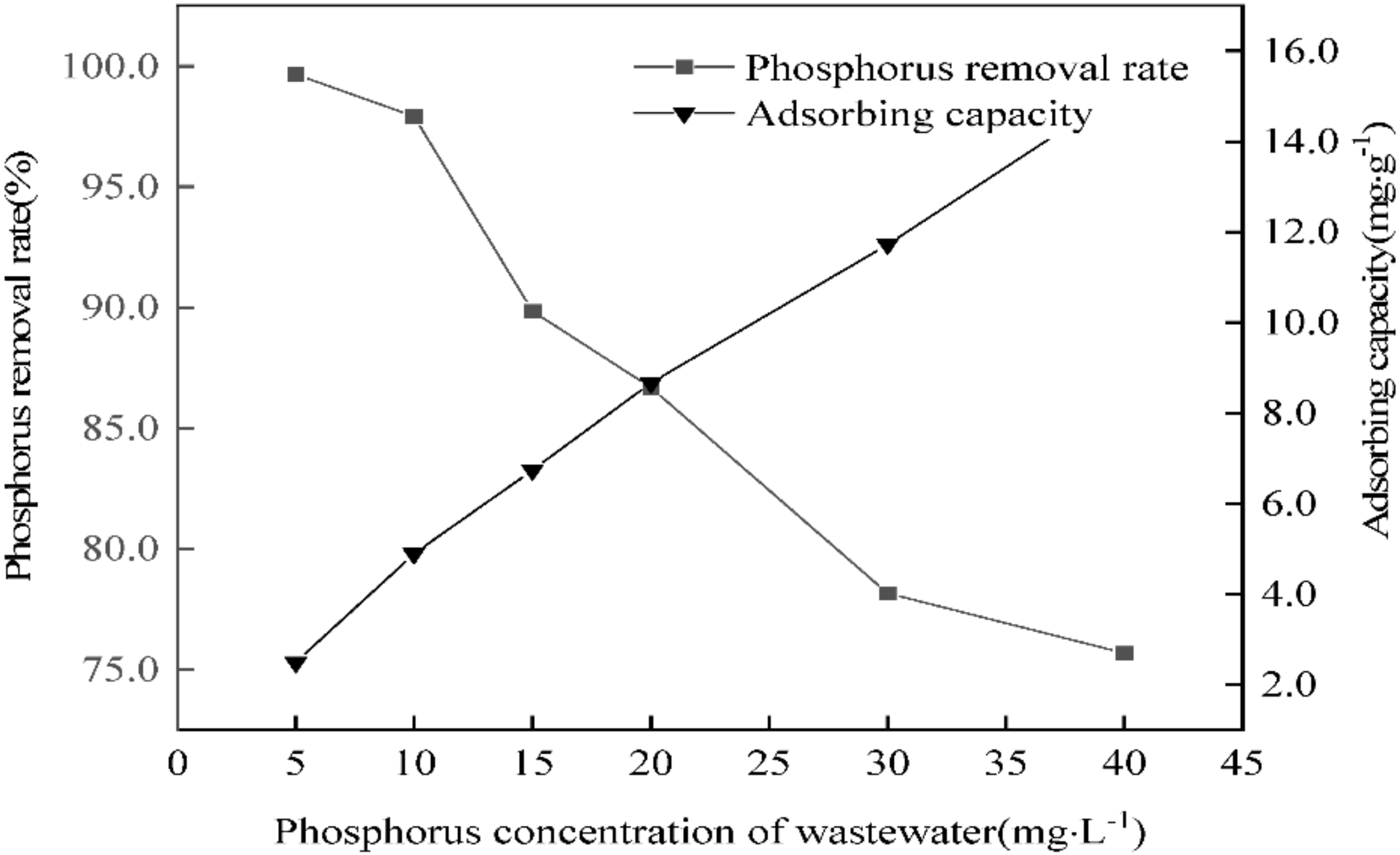
3.9. Influence of Initial Phosphorus Concentration of Wastewater on Phosphorus Removal Performance
3.10. Orthogonal Adsorption Experiment
3.11. Orthogonal Verification of the Treatment Effect of Composite Adsorbent on Actual Urban Domestic Sewage
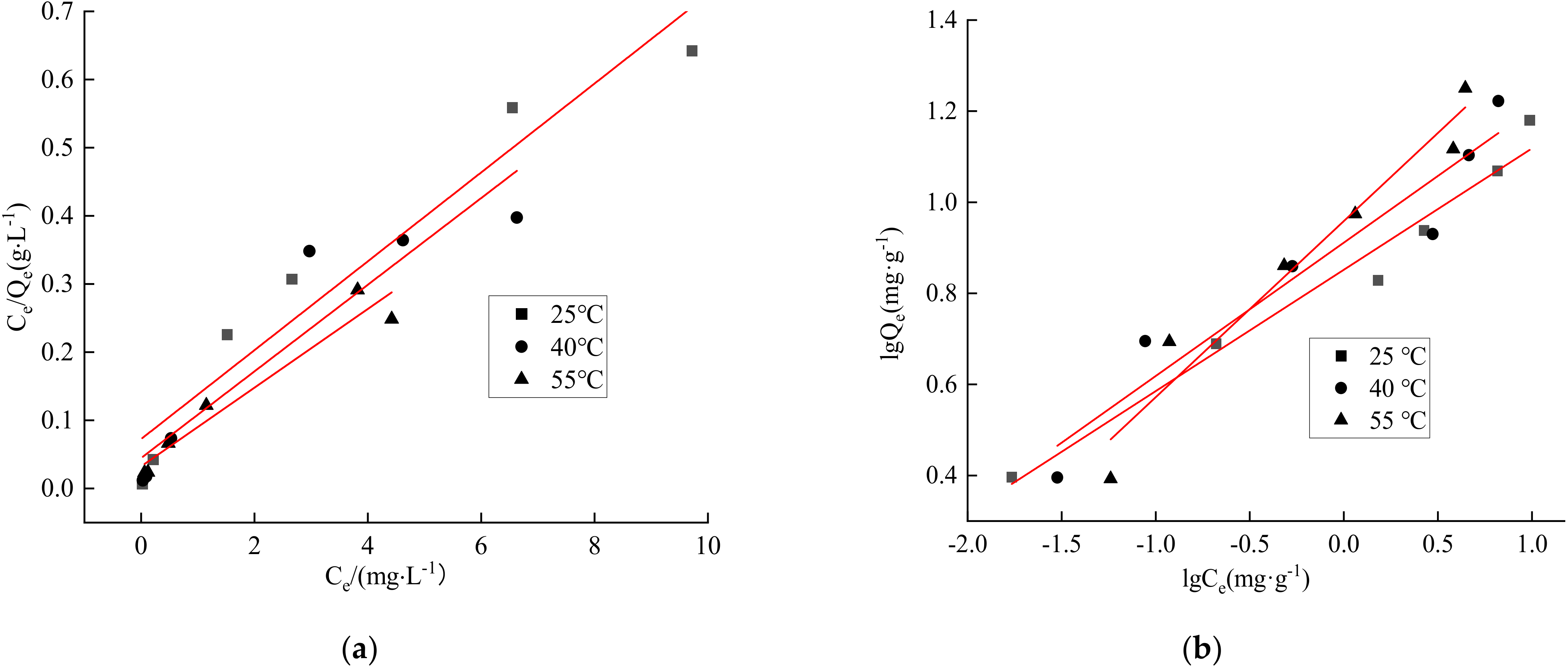
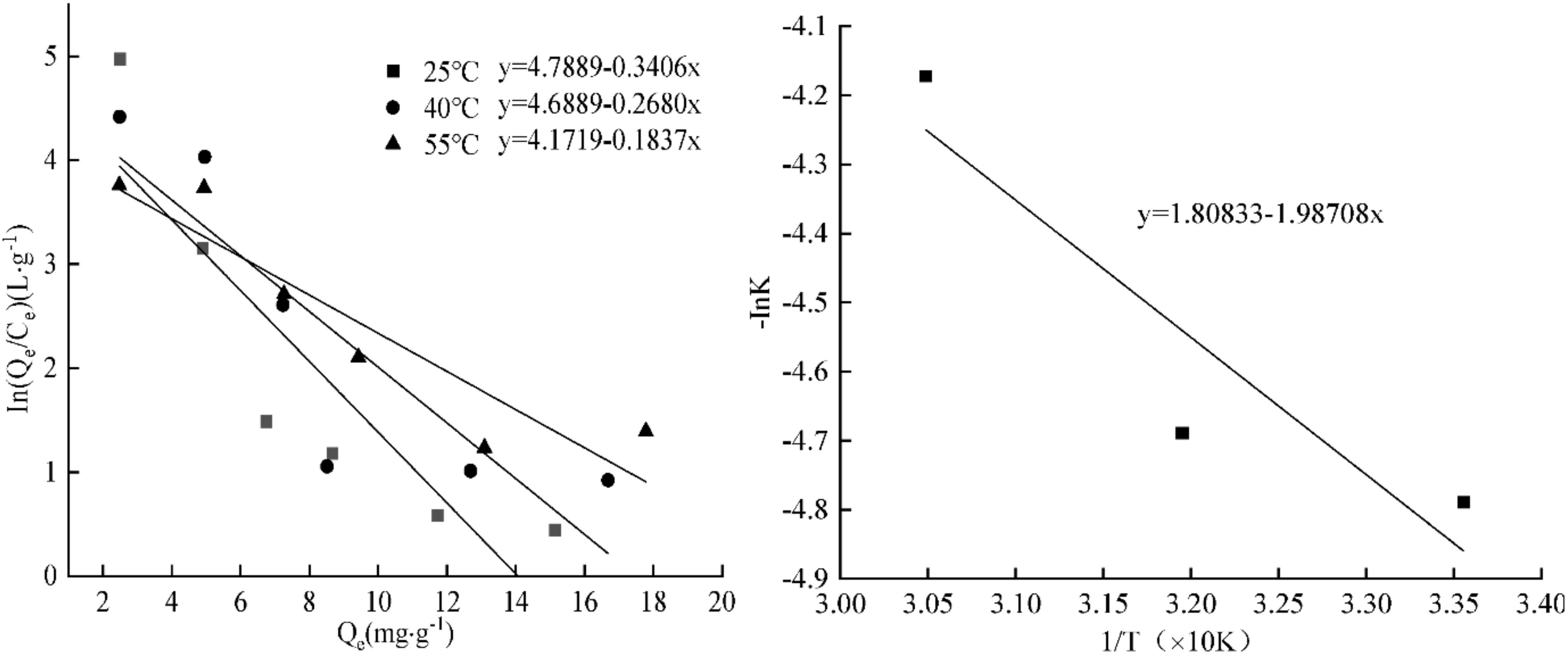
3.12. Results of Adsorption Isotherm Fitting
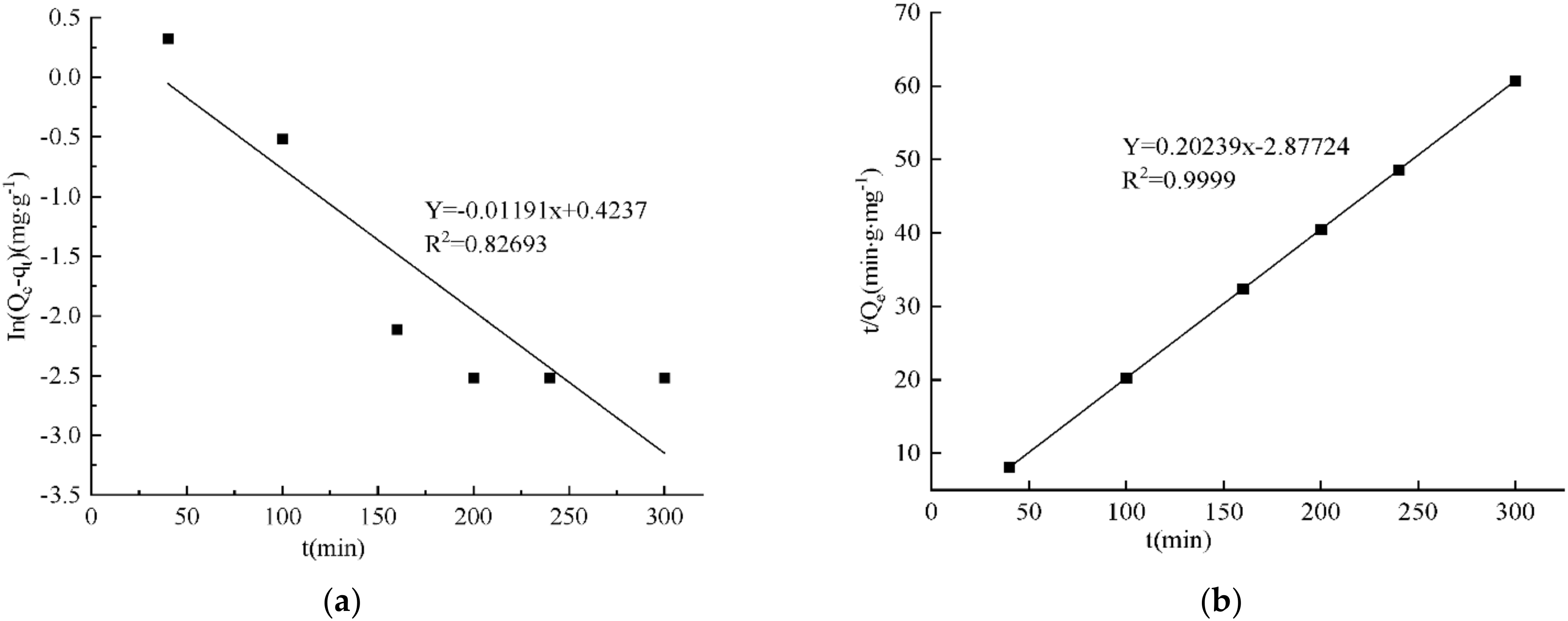
3.13. Results of Adsorption Kinetics Fitting
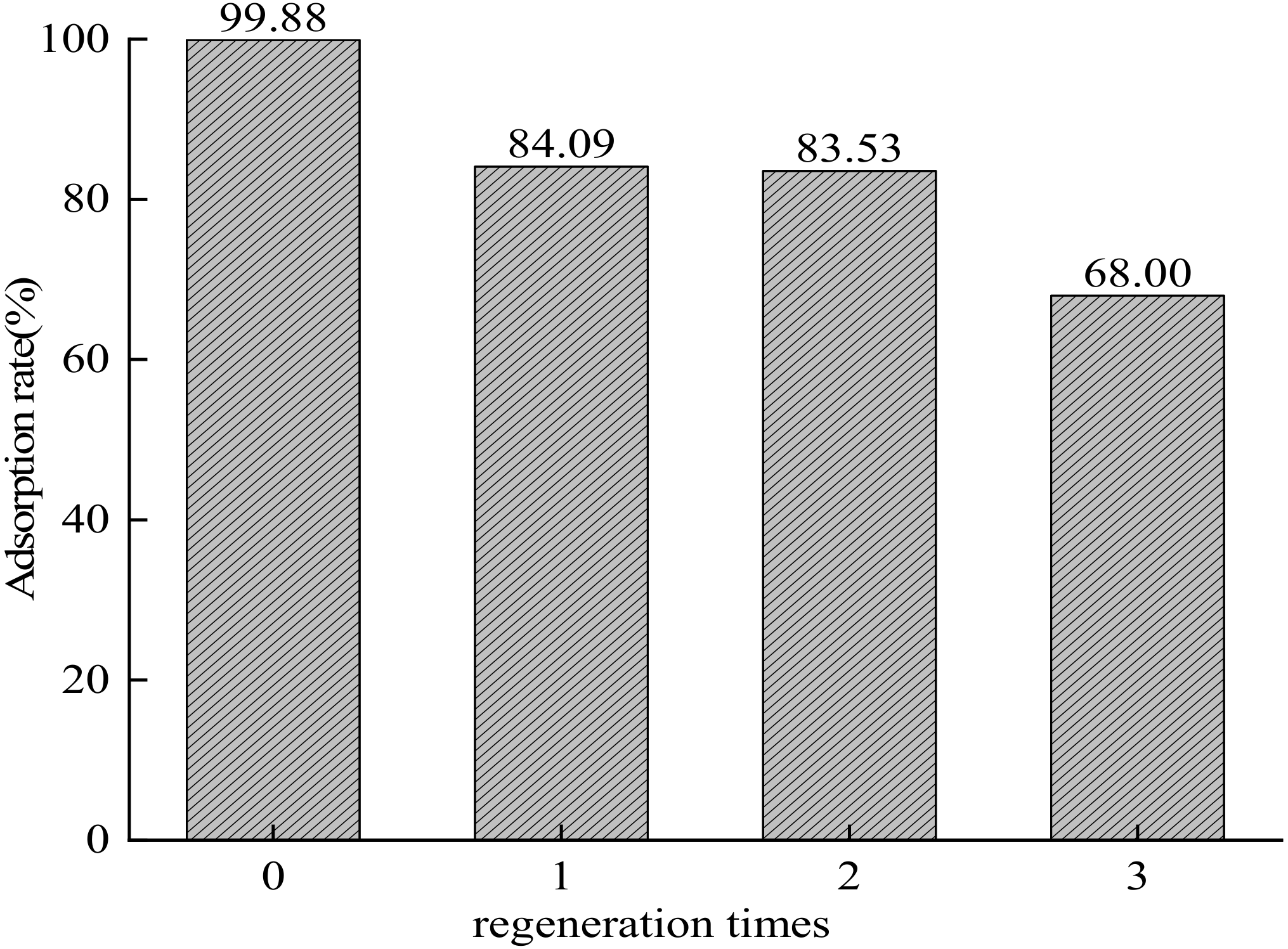
3.14. Regeneration Performance of Composite Adsorbent
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, J.-O.; Chung, J. Implementing chemical precipitation as a pretreatment for phosphorus removal in membrane bioreactor-based municipal wastewater treatment plants. KSCE J. Civ. Eng. 2014, 18, 956–963. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Z.; Wang, H.; Yan, Q. Optimization and reaction kinetics analysis for phosphorus removal in struvite precipitation process. Water Environ. Res. 2020, 92, 1162–1172. [Google Scholar] [CrossRef] [PubMed]
- Ochoa, C.; Hernández, M.A.; Bayona, O.L.; Camargo, H.A.; Cabeza, I.O.; Candela, A.M. Phosphorus recovery by struvite from anaerobic co-digestion effluents during residual biomass treatment. Biomass Convers. Biorefin. 2020, 11, 261–274. [Google Scholar] [CrossRef]
- Izadi, P.; Eldyasti, A. Design, operation and technology configurations for enhanced biological phosphorus removal (EBPR) process: A review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 561–593. [Google Scholar] [CrossRef]
- Matsuura, N.; Masakke, Y.; Karthikeyan, S.; Kanazawa, S.; Honda, R.; Yamamoto-Ikemoto, R.; Konstantinidis, K.T. Metagenomic insights into the effect of sulfate on enhanced biological phosphorus removal. Appl. Microbiol. Biotechnol. 2021, 105, 2181–2193. [Google Scholar] [CrossRef]
- Banu, J.R.; Merrylin, J.; Kavitha, S.; Kannah, R.Y.; Selvakumar, P.; Gopikumar, S.; Sivashanmugam, P.; Do, K.-U.; Kumar, G. Trends in Biological Nutrient Removal for the Treatment of Low Strength Organic Wastewaters. Curr. Pollut. Rep. 2021, 7, 1–30. [Google Scholar] [CrossRef]
- Khalil, A.K.A.; Dweiri, F.; Almanassra, I.W.; Chatla, A.; Atieh, M.A. Mg-Al Layered Double Hydroxide Doped Activated Carbon Composites for Phosphate Removal from Synthetic Water: Adsorption and Thermodynamics Studies. Sustainability 2022, 14, 6991. [Google Scholar] [CrossRef]
- Huang, C.; Tang, C.; Wu, Q.; Zhu, Q. Magnetic MnFe2O4/ZnFe-LDH for Enhanced Phosphate and Cr (VI) Removal from Water. Environ. Sci. Pollut. Res. 2022, 29, 59224–59234. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Q.; Ji, F.; Jiang, L.; Liu, C.; Shen, Q.; Liu, Q. Phosphate removal performances of layered double hydroxides (LDH) embedded polyvinyl alcohol/lanthanum alginate hydrogels. Chem. Eng. J. 2021, 430, 132754. [Google Scholar] [CrossRef]
- Alagha, O.; Manzar, M.S.; Zubair, M.; Anil, I.; Mu’Azu, N.D.; Qureshi, A. Comparative Adsorptive Removal of Phosphate and Nitrate from Wastewater Using Biochar-MgAl LDH Nanocomposites: Coexisting Anions Effect and Mechanistic Studies. Nanomaterials 2020, 10, 336. [Google Scholar] [CrossRef] [Green Version]
- Parasana, N.; Shah, M.; Unnarkat, A. Recent advances in developing innovative sorbents for phosphorus removal—Perspective and opportunities. Environ. Sci. Pollut. Res. 2022, 29, 38985–39016. [Google Scholar] [CrossRef]
- Xu, Z.; Zhong, Y.; Wang, Y.; Song, X.; Huang, W. Removal performance and mechanism of phosphorus by different Fe-based layered double hydroxides. Environ. Sci. Pollut. Res. 2022, 1–11. [Google Scholar] [CrossRef]
- Yuan, J.; Zhu, Y.; Wang, J.; Liu, Z.; He, M.; Zhang, T.; Li, P.; Qiu, F. Facile Modification of Biochar Derived from Agricultural Straw Waste with Effective Adsorption and Removal of Phosphorus from Domestic Sewage. J. Inorg. Organomet. Polym. Mater. 2021, 31, 3867–3879. [Google Scholar] [CrossRef]
- Duan, H.; Zhang, L.; Wang, Y.; Liu, Y.; Wang, Y. Phosphate removal from aqueous solution by Fe–La binary (hydr)oxides: Characterizations and mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 62662–62676. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, C.; Wen, K.; Han, R. Adsorption of phosphate from aqueous solution by lanthanum modified macroporous chelating resin. Korean J. Chem. Eng. 2020, 37, 766–775. [Google Scholar] [CrossRef]
- Park, Y.; Gorman, C.; Ford, E. Lanthanum carbonate nanofibers for phosphorus removal from water. J. Mater. Sci. 2020, 55, 5008–5020. [Google Scholar] [CrossRef]
- Nakarmi, A.; Moreira, R.; Bourdo, S.E.; Watanabe, F.; Toland, A.; Viswanathan, T. Removal and Recovery of Phosphorus from Contaminated Water Using Novel, Reusable, Renewable Resource-Based Aluminum/Cerium Oxide Nanocomposite. Water Air Soil Pollut. 2020, 231, 559. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Yu, X.; Zheng, P. A novel Fe(II)-Ca synergistic phosphorus removal process: Process optimization and phosphorus recovery. Environ. Sci. Pollut. Res. 2017, 25, 1543–1550. [Google Scholar] [CrossRef]
- Banu, R.J.; Do, K.U.; Yeom, I.T. Phosphorus removal in low alkalinity secondary effluent using alum. Int. J. Environ. Sci. Technol. 2007, 5, 93–98. [Google Scholar] [CrossRef]
- Liu, B.; Lou, S.; Zeng, Y.; Qin, Y.; Zhang, W.; Zhang, L.; Liu, X. High-efficiency adsorption of phosphate by Fe-Zr-La tri-metal oxide composite from aqueous media: Performance and mechanism. Adv. Powder Technol. 2021, 32, 4587–4598. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, J.P. Key factors for optimum performance in phosphate removal from contaminated water by a Fe–Mg–La tri-metal composite sorbent. J. Colloid Interface Sci. 2015, 445, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, L.; Chen, J.P. Adsorption of fluoride by Fe–Mg–La triple-metal composite: Adsorbent preparation, illustration of performance and study of mechanisms. Chem. Eng. J. 2015, 262, 839–846. [Google Scholar] [CrossRef]
- Lǚ, J.; Liu, H.; Liu, R.; Zhao, X.; Sun, L.; Qu, J. Adsorptive removal of phosphate by a nanostructured Fe–Al–Mn trimetal oxide adsorbent. Powder Technol. 2013, 233, 146–154. [Google Scholar] [CrossRef]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. J. Am. Chem. Soc. 1916, 38, 2221–2295. [Google Scholar] [CrossRef]
- Freundlich, H. Uber die adsorption in lasungen. J. Z. Phys. Chem. 1906, 57, 385–470. [Google Scholar] [CrossRef]
- Yang, H.; Luo, B.; Zhang, Y.; Zhou, B.; Ahmed, S.M.; Liu, H.; Liu, X.; He, Y.; Xia, S. Study of Humic Acid Adsorption Character on Natural Maifan Stone: Characterization, Kinetics, Adsorption Isotherm, and Thermodynamics. ACS Omega 2020, 5, 7683–7692. [Google Scholar] [CrossRef]
- Zubair, M.; Manzar, M.S.; Suleiman, M.A.; Fernandes, D.P.; Meili, L.; Al Bin Essa, W.; Al-Adam, H.; AlGhamdi, J.M.; Mu’Azu, N.D.; Haladu, S.A.; et al. Production of magnetic biochar-steel dust composites for enhanced phosphate adsorption. J. Water Process Eng. 2022, 47, 102793. [Google Scholar] [CrossRef]



| Test Number | pH | Initial Concentration (mg·L−1) | Adsorbent Dosage (g) | Adsorption Time (min) | Removal Rate (%) |
|---|---|---|---|---|---|
| 1 | 5 | 5 | 0.05 | 100 | 77.80 |
| 2 | 5 | 10 | 0.1 | 160 | 86.50 |
| 3 | 5 | 15 | 0.2 | 200 | 99.60 |
| 4 | 6 | 5 | 0.1 | 200 | 98.20 |
| 5 | 6 | 10 | 0.2 | 100 | 98.50 |
| 6 | 6 | 15 | 0.05 | 160 | 64.13 |
| 7 | 7 | 5 | 0.2 | 160 | 98.00 |
| 8 | 7 | 10 | 0.05 | 200 | 68.60 |
| 9 | 7 | 15 | 0.1 | 100 | 69.67 |
| K1 | 263.90 | 274.00 | 210.53 | 245.97 | |
| K2 | 260.83 | 253.60 | 254.37 | 248.63 | |
| K3 | 236.27 | 233.40 | 296.10 | 266.40 | |
| k1 | 87.97 | 91.33 | 70.18 | 81.99 | |
| k2 | 86.94 | 84.53 | 84.79 | 82.88 | |
| k3 | 78.76 | 77.80 | 98.70 | 88.80 | |
| R | 9.21 | 13.53 | 28.52 | 6.81 |
| Sewage Quality Parameters | Concentration | Unit |
|---|---|---|
| COD | 321 ± 0.45 | mg·L−1 |
| BOD | 180 ± 0.16 | mg·L−1 |
| NH3-N | 3.26 ± 0.06 | mg·L−1 |
| TP | 4.17 ± 0.04 | mg·L−1 |
| ORP | 365 ± 0.78 | mV |
| pH | 6.87 ± 0.05 | / |
| Conductivity | 985 ± 0.13 | μS/cm |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| Temperature (°C) | a | Qm/(mg·g−1) | R2 | k | 1/n | R2 |
| 25 | 0.07 | 15.3 | 0.9394 | 7.11 | 0.27 | 0.9729 |
| 40 | 0.04 | 15.7 | 0.8792 | 8.15 | 0.29 | 0.9234 |
| 55 | 0.03 | 17.3 | 0.9406 | 9.02 | 0.39 | 0.9513 |
| Thermodynamic Constant | 298 K | 313 K | 328 K |
| ΔG/(kJ·mol−1) | −11.9 | −12.2 | −11.4 |
| ΔH/(kJ·mol−1) | −16.5 | −16.5 | −16.5 |
| ΔS/(J·mol−1·K−1 ) | 15.0 | 15.0 | 15.0 |
| Quasi First Order Dynamics Model | Quasi Second Order Dynamics Model | ||||
|---|---|---|---|---|---|
| Qe/(mg·g−1) | k1/h−1 | R2 | Qe/(mg·g−1) | k2/h−1 | R2 |
| 1.53 | 0.0119 | 0.8269 | 4.94 | 0.0142 | 0.9999 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Liang, H.; Mo, Y.; Wei, Y. Preparation and Phosphorus Removal Performance of Zr–La–Fe Ternary Composite Adsorbent Embedded with Sodium Alginate. Processes 2022, 10, 1761. https://doi.org/10.3390/pr10091761
Li X, Liang H, Mo Y, Wei Y. Preparation and Phosphorus Removal Performance of Zr–La–Fe Ternary Composite Adsorbent Embedded with Sodium Alginate. Processes. 2022; 10(9):1761. https://doi.org/10.3390/pr10091761
Chicago/Turabian StyleLi, Xiuling, Hanyu Liang, Yanling Mo, and Yansong Wei. 2022. "Preparation and Phosphorus Removal Performance of Zr–La–Fe Ternary Composite Adsorbent Embedded with Sodium Alginate" Processes 10, no. 9: 1761. https://doi.org/10.3390/pr10091761
APA StyleLi, X., Liang, H., Mo, Y., & Wei, Y. (2022). Preparation and Phosphorus Removal Performance of Zr–La–Fe Ternary Composite Adsorbent Embedded with Sodium Alginate. Processes, 10(9), 1761. https://doi.org/10.3390/pr10091761






