Abstract
Ocimum plants are of great interest to traditional medicine in the history of several civilizations, particularly in terms of chronic human diseases. Essential oils obtained from this genus have also been used as therapeutic agents. In this present work, two plants of the Ocimum species from Djibouti, Ocimum basilicum L. and Ocimum americanum L., were subjected to hydrodistillation to obtain their essential oils. Gas chromatography-mass spectrometry was performed to determine the chemical composition of both essential oils. Linalool (41.2%) and estragole (30.1%) are the major compounds among the 37 compounds that have been identified in the essential oil of Ocimum basilicum L. (EOOB), and carvotanacetol (38.4%) and estragole (27.5%) are the main compounds among the 42 compounds that have been identified in the essential oil of Ocimum americanum L. (EOOA). Morever, the cytotoxic activity of EOs was evaluated against 13 human cancer cell lines (K562, A549, HCT116, PC3, U87-MG, MIA-Paca2, HEK293, NCI-N87, RT4, U2OS, A2780, MRC -5 and JIMT-T1) using a luminescence spectrophotometric method; hence, the oils showed significant cytotoxic activities. The antibacterial activities of the oils were assayed on five Gram-positive bacteria (Staphylococcus aureus, Enterococcus faecalis, Streptococcus agalactiae, Staphylococcus epidermidis and Corynebacterium sp.) and seven Gram-negative bacteria (Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Shigella sonnei, Salmonella enterica sv. Typhimurium and Enterobacter cloacae) by agar disc diffusion methods. Both essential oils exhibited moderate activities against Streptococcus agalactiae, and thus each has an activity against Pseudomonas aeruginosa for EOOB and against Enterobacter cloacae for EOOA, while the others are negative. The findings of this work showed the promising anticancer effects of both oils against total cell lines with a potential for use as natural alternatives to synthetic drugs; however, there was only an antibacterial effect against Streptococcus agalactiae.
1. Introduction
The global demand for essential oils has currently risen in several industrial sectors [1,2,3,4]. Essential oils are part of the extract of aromatic plants that are distributed in all countries of the world, where they are considered an imperative element of traditional medicine systems [5,6]. Essential oils are extracted mainly by steam distillation, but can also be prepared using a variety of contemporary techniques that include managing a number of processes, including milling, solvent, temperature, pressure, humidity, etc. [7].
Essential oils are volatile liquids; they are clear, sometimes colored and soluble in organic solvents. They are complex mixtures of terpenoids, including sesquiterpene and monoterpene, and their oxygenated derivatives; thus, they can also incorporate a variety of other molecules such as acids, oxides, and nitrogenous and sulfur derivatives [8]. Various factors influence the chemical compositions of EOs, such as their geographical location, the seasonal period in which they are collected, the composition of the soil and the method of cultivation, storage and the method of oil extraction according to the operations described above [9,10]. They are generally recognized as a promising product in their use as bioactive compounds either alone or in synergy with other chemical and biological compounds in targeted formulations. The scientific interest in research concerning essential oils is due to their numerous medical properties, particularly anticancer properties, which are demonstrated by various mechanisms, including cancer prevention mechanisms, as well as by acting on the established tumor cell according to various reactions: antimutagenic, detoxification, antiproliferative, etc. [11,12,13,14,15].
In this context, the genus Ocimum, a member of the Lamiaceae family comprising more than 150 species, which grows widely and is distributed in temperate regions of the world, is considered an important source of essential oils with a therapeutic potential, especially as antioxidants and antimicrobials, and has also been explored. Furthermore, the essential oil composition of Ocimum species and their chemotypes/morphotypes are variable, with monoterpenoids and phenyl derivatives as the predominant components [16,17].
Thus, the aim of this work was:
- first: evaluate the chemical composition of Djiboutian essential oils including Ocimum basilicum L. (EOOB) and Ocimum americanum L. (EOOA) by GC-MS analysis;
- second: determine the possible cytotoxicity of the two essential oils against 13 cell lines: K562, A549, HCT116, PC3, U87-MG, MIA-Paca2, HEK293, NCI-N87, RT4, U2OS, A2780, MRC -5 and JIMT-T1;
- third: conduct microbial investigations of the two essential oils against 12 bacteria: Gram + (Staphylococcus aureus, Enterococcus faecalis, Streptococcus agalactiae, Staphylococcus epidermidis and Corynebacterium sp.) and Gram—(Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Shigella sonnei, Salmonella enterica sv., Typhimurium and Enterobacter cloacae).
2. Materials and Methods
2.1. Plant Material and Essential Oils Preparation
EOOB and EOOA from the entire aerial parts of Ocimum basilicum L. and Ocimum americanum were harvested, respectively, in Ambouli (11°33′29.3″ N 43°08′47.7″ E) and in Day (11°45′07.1″ N 42°41′50.1″ E), Djibouti and were obtained by steam distillation. The process of this operation is based on heating for 3 h of the balloon, which contains water with vegetable matter (200 g of the plant in 1 L of water), and the water vaporizes. This steam breaks the plant cells, releasing the molecules of interest. The most volatile of them are carried away with the steam. This is then cooled in a condenser to obtain two phases: the aqueous phase and organic phase, which contains the essential oil. After distillation, both oils were directly stored in a refrigerator at 4 °C in the Medicinal Research Institute, Djibouti Research and Study Center (CERD).
The dates of collection of the two plants were March 2018, and the identification was made by a team of botanists under the direction of Dr. Fatouma Mohamed Abdoul-Latif (CERD, Djibouti). The plants Ocimum basilicum L. and Ocimum americanum L. are classified in an herbarium with the respective access numbers OB2-2018 and OA5-2018.
2.2. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was performed using a gas chromatograph coupled to a flame ionization detector (FID) (Agilent 7820 Technologies, Santa Clara, CA, USA). The equipment components and operating conditions are shown in Table 1 [18]. Two approaches were used to identify the components: First, by contrasting their GC retention indices (RI), which were calculated in relation to the retention times of a series of n-alkanes using linear interpolation, with those of real compounds or data from the literature. Another technique compares spectra from commercial mass spectral libraries to those from our personal collection via computer matching [19,20].

Table 1.
Equipment components and operating conditions of GC-MS.
2.3. Antibacterial Test
The antibacterial activity was studied by determining the effect of essential oil by the appearance of a zone of inhibition against Gram-positive and Gram-negative bacteria (Table 2) using the agar diffusion method [21,22,23].

Table 2.
The strains used during the antibacterial test.
The bacteria were cultured in Mueller-Hinton (MH) broth at 37 °C for 24 h. A certain volume of microbial inoculum (20 μL of 5.105 CFU/mL) was spread over the entire agar surface of the Petri dish. Then, a small hole with a diameter of approximately 6 mm was punched with a sterile cork borer, and 50 μL each of the EOOB and EOOA essential oils were poured into the wells, separately, with 5% concentrations in DMSO. The Petri dishes were incubated for 24 h at 37 °C; then, the antimicrobial activity was evaluated according to the visualized zone of inhibition. All the experiments were carried out in triplicate with positive controls (commercial antibiotics) and negative controls (DMSO alone in the wells).
2.4. Cytotoxicity Tests
Ethical approval for this study was granted by the ethics committee of ESTK-USMS (Morocco), and genetic information has been archived under accession number: 2019-1215-0003.
The in vitro cytotoxic activity of the essential oils EOOB and EOOA was made against 13 human cell lines (Table 3) by determining the concentrations of a 50% inhibition of cell proliferation. All cancer cell lines for this test were obtained from international standard collections, and they were cultured according to the instructions recommended in the instructions for use. All cell lines were maintained in their growth media at 37 °C in a humidified atmosphere containing 5% CO2 [24].

Table 3.
The origins of human cancer cell lines.
Cell viability was determined by a luminescent assay according to the manufacturer’s instructions (Promega, Madison, WI, USA). For IC50 determination, the cells were seeded in 96-well plates (3 × 103 cells/well) containing 90 μL of growth medium. After 24 h of culture, the cells were treated with the tested compounds at 8 different final concentrations (10; 5; 1; 0.5; 0.1; 0.05; 0.01 and 0.005 μg/mL). Each concentration was obtained from serial dilutions in culture medium starting from the stock solution. Control cells were treated with the vehicle. Experiments were performed in triplicate.
After 72 h of incubation, 100 μL of CellTiter Glo Reagent were added for 15 min before recording luminescence with a spectrophotometric plate reader PolarStar Omega (BMG LabTech). The dose-response curves were plotted with Graph Prism software, and the IC50 values were calculated using the Graph Prism software from polynomial curves (four- or five-parameter logistic equations) [25].
2.5. Statistical Analysis
The statistical analysis of the values obtained from the determination of yields, antibacterial activity and cytotoxicity were carried out by a Type A evaluation of standard uncertainty with the Student test (t < 0.05).
Principal component analyses (PCA) and the functioning of the correlations were carried out on the basis of a correlation matrix calculated on the average cytotoxic activity data. XLSTAT was used for all calculations.
3. Results
3.1. Chemical Composition
The results of the gas chromatographic analysis coupled with the mass spectrometry of the essential oils of the plants studied are shown in Table 4. Chromatographic analyses of essential oils made it possible to identify 37 compound for EOOB, which represent 98.8%, and 42 compounds for EOOA, which represent 100%. The analysis of the results given in the table of characterization showed all of the following results: in the first identification of EOOB, Linalool (41.2%) and estragole (30.1%) were detected as majority compounds; moreover, other compounds such as α-Bergamotene (7.5%), τ-cadinol (3,7%) and δ-cadinene (2.3%) exhibited moderate concentrations. In the second, EOOA was characterized by the presence of carvotanacetol (38.4%) and estragole (27.5%) as main compounds, and α-Bergamotene (9%), τ-cadinol (5.1%), δ-cadinene (2.5%) and elemene (2.1%) were detected as moderate concentrations. We note that 27 compounds were detected in both essential oils.

Table 4.
Chemical composition of the EOOB and EOOA.
3.2. Antibacterial Activity
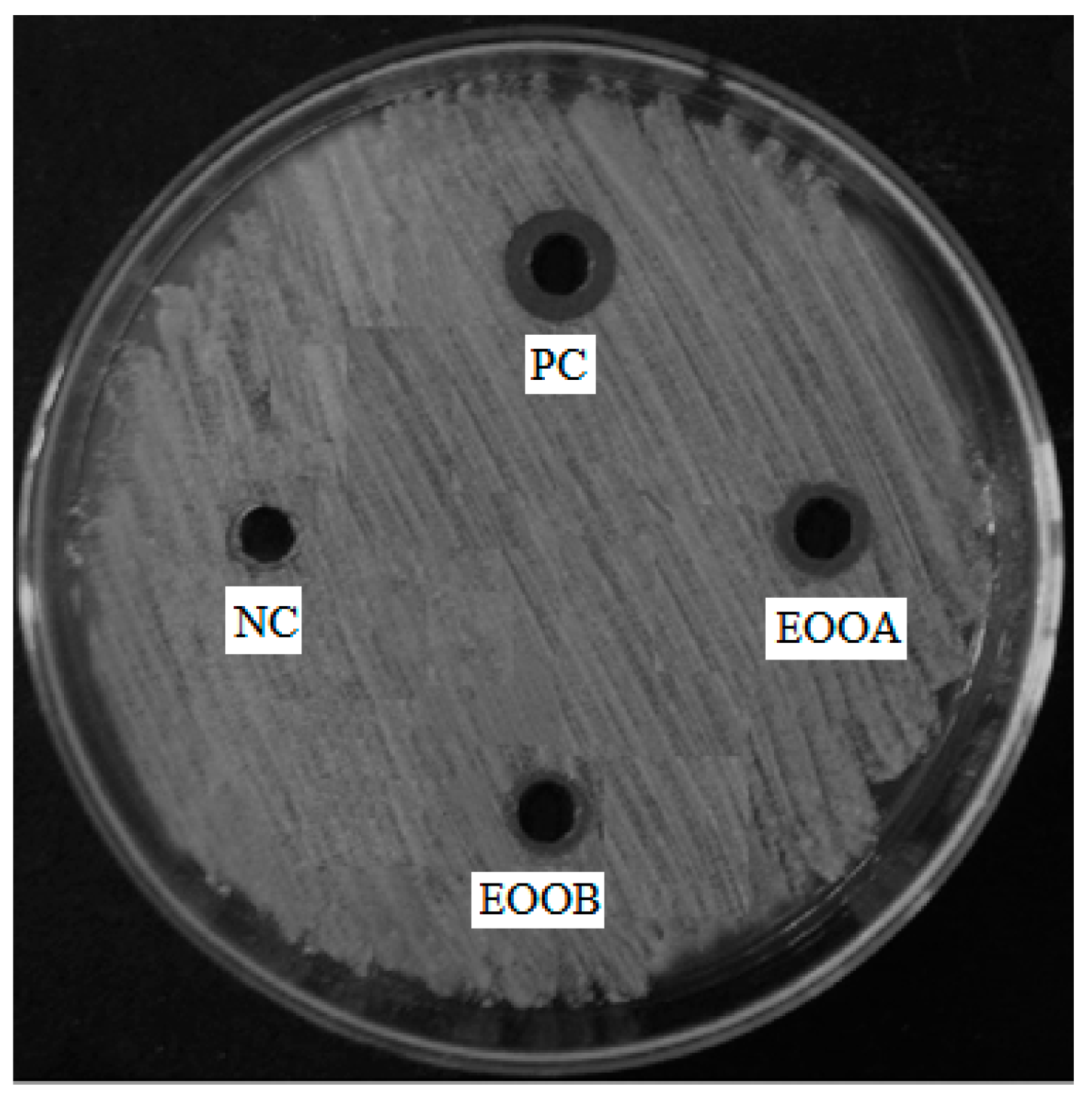
The results of the bacterial activities of two essential oils EOOB and EOOA against the bacteria selected above are mentioned in Table 5. According to this table, both EOOB and EOOA show significant activity against Streptococcus agalactiae (Figure 1); however, these two essential oils show no activity against the following strains: Staphylococcus aureus, Enterococcus faecalis, Staphylococcus epidermidis, Corynebacterium sp., Escherichia coli, Klebsiella pneumoniae, Acinetobacter baumannii, Shigella sonnei, Salmonella enterica sv. Typhimurium. in addition, EOOB has an activity against Pseudomonas aeruginosa and EOOA has an activity against Enterobacter cloacae.

Table 5.
Antibacterial activity of the EOOB and EOOA. (+): inhibition; (−): no inhibition.
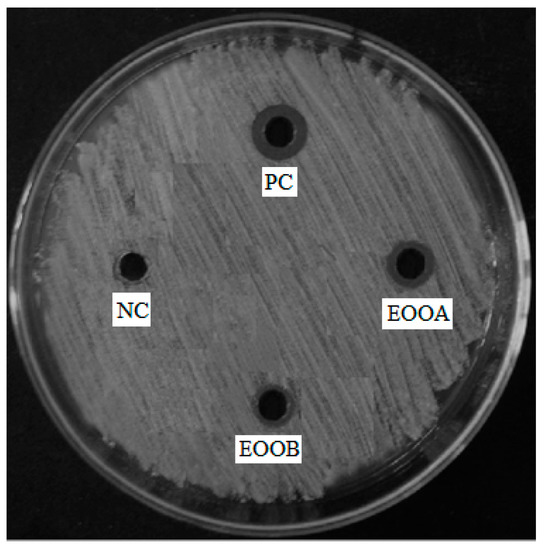
Figure 1.
Antibacterial activity of EOOB, EOOA, negative control (NC) and positive control (PC: penicillin) against Streptococcus agalactiae.
3.3. Cytotoxicity Activity
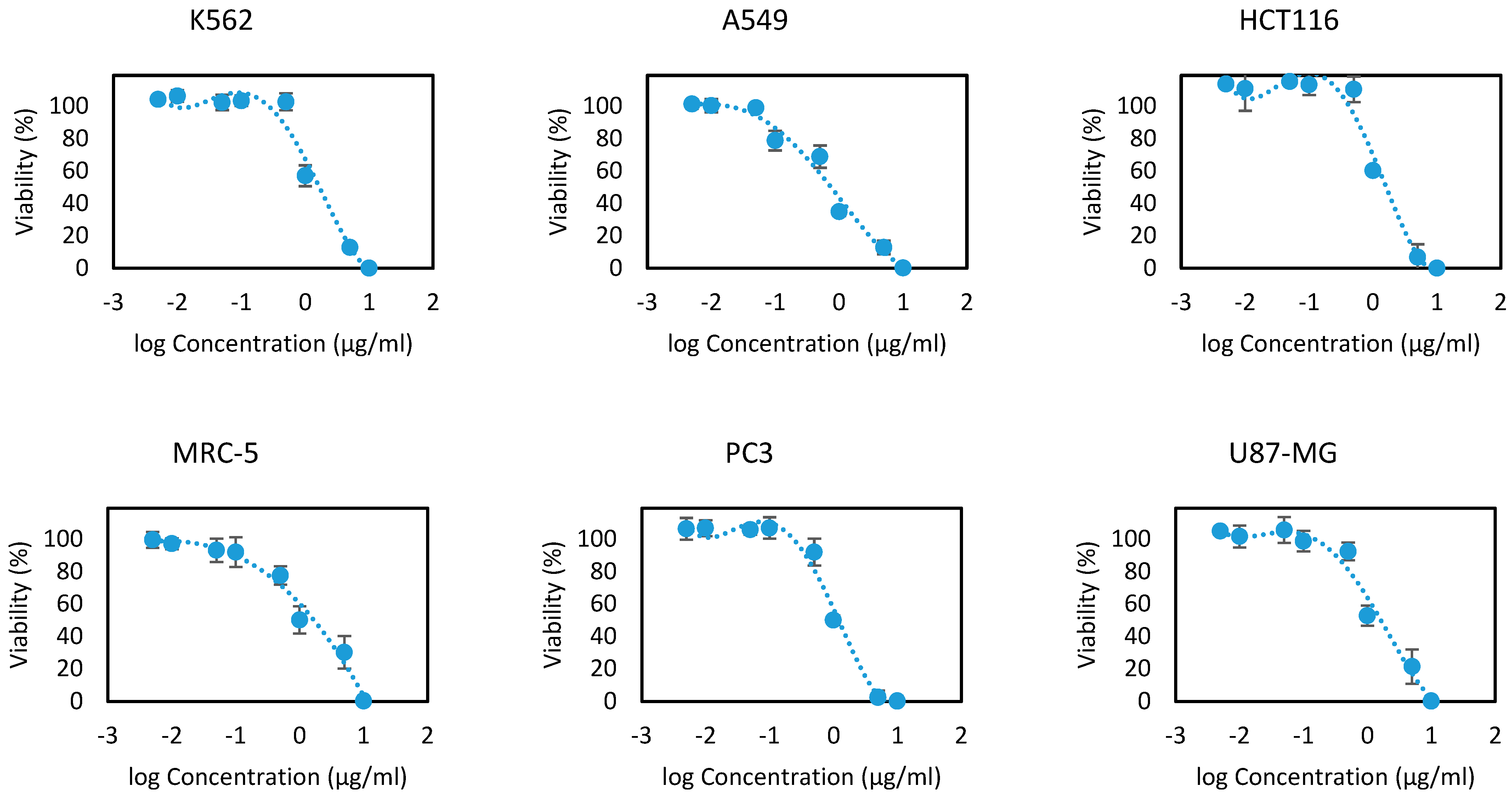
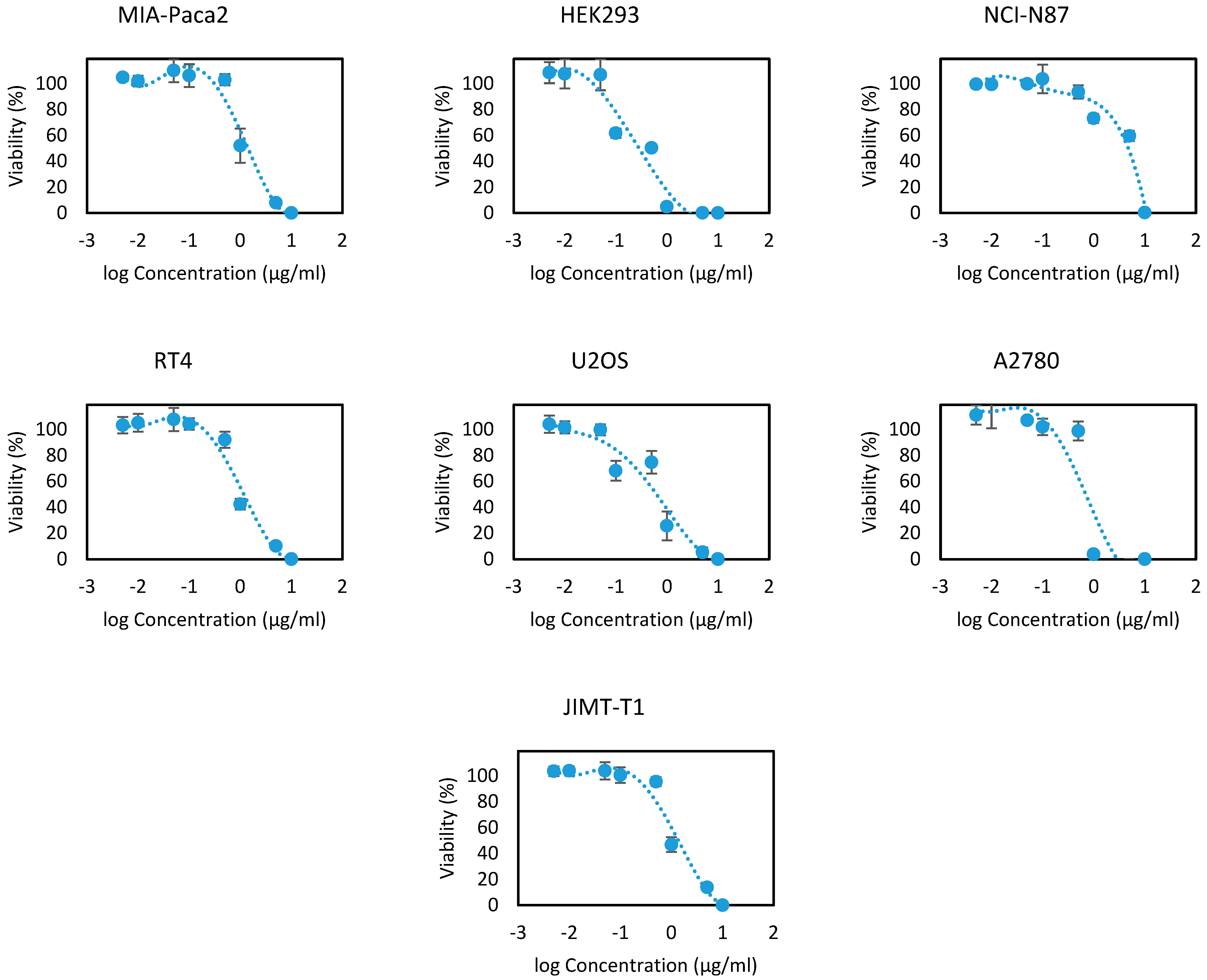
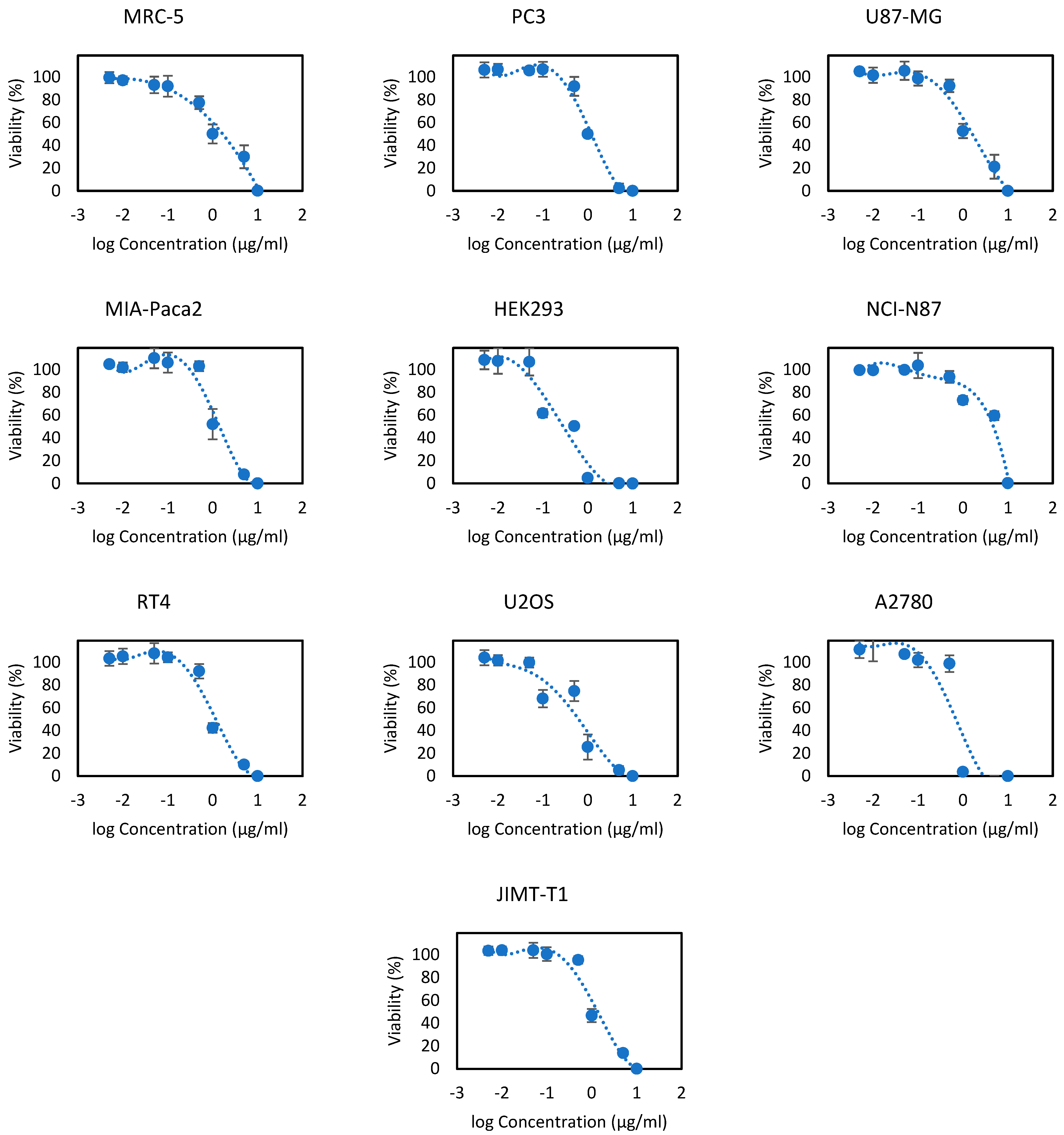
EOOB and EOOA essential oils were evaluated on the 13 human cell lines at different concentrations ranging between 10, 5, 1, 0.5, 0.1, 0.05, 0.01 and 0.005 µg/mL. The results are presented in Figure 2 and Figure 3 as the cell viability versus logarithms of concentrations. From these concentration-viability curves, the 50% inhibition concentrations (IC50) were determined, as shown in Table 6. All the values that were found prove the efficacy of EOOB and EOOA against cancer cells, and these values are in several cases better than the standards used.
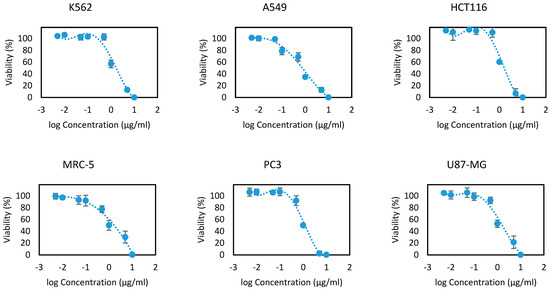
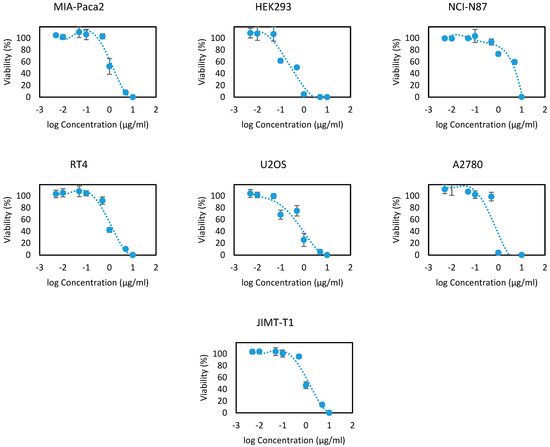
Figure 2.
Cytotoxicity curves of the essential oil of EOOB.
Figure 3.
Cytotoxicity curves of the essential oil of EOOA.

Table 6.
IC50 (ng/mL) values for EOOB and EOOA.
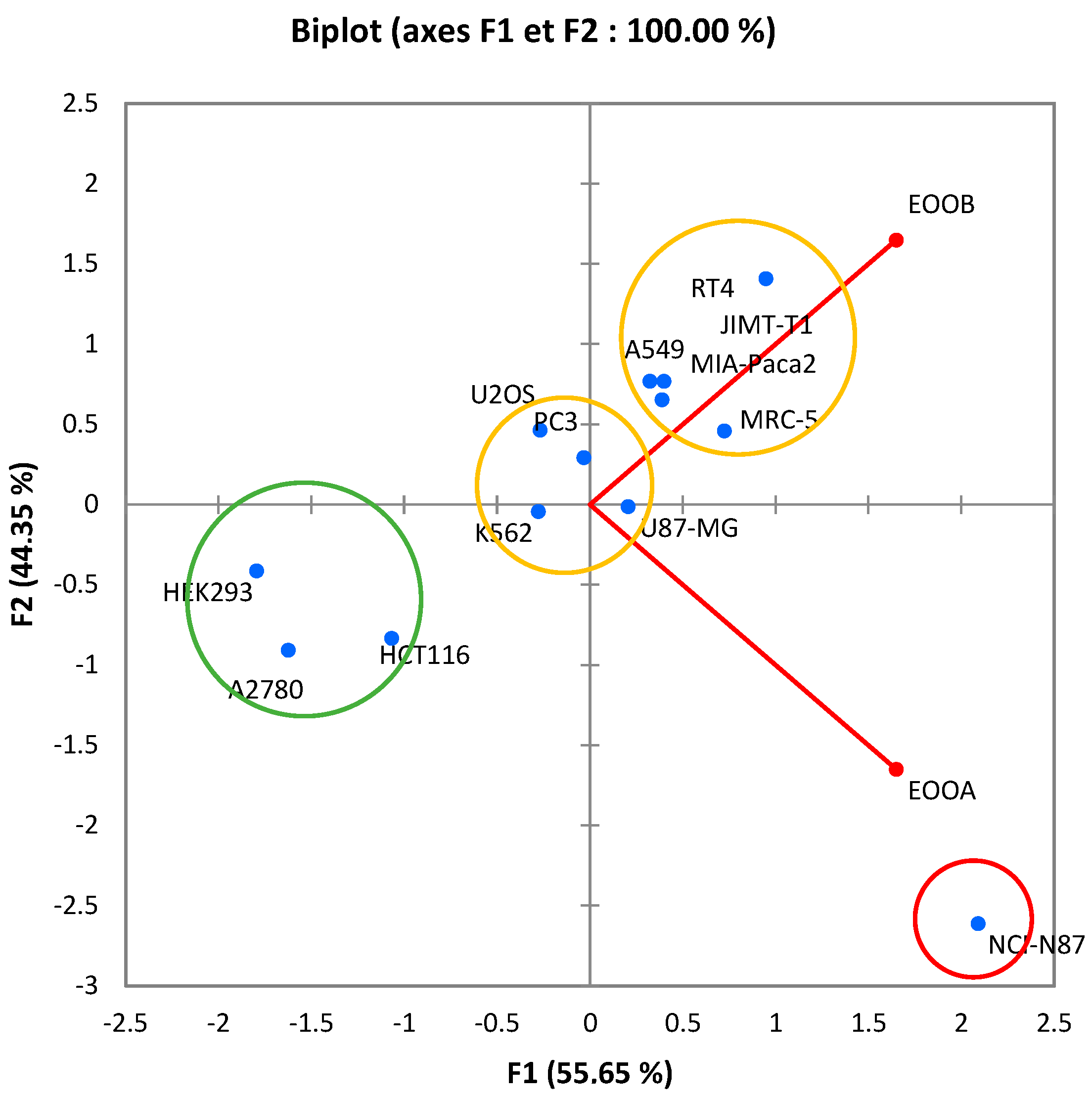
The principal component analysis (PCA) of the inhibition concentrations of 50% of both essential oils (Figure 4) makes it possible to provide important information, corresponding to a total of 100% distributed successively over 55.65% of the F1 axis and over 44.35% of the F2 axis.
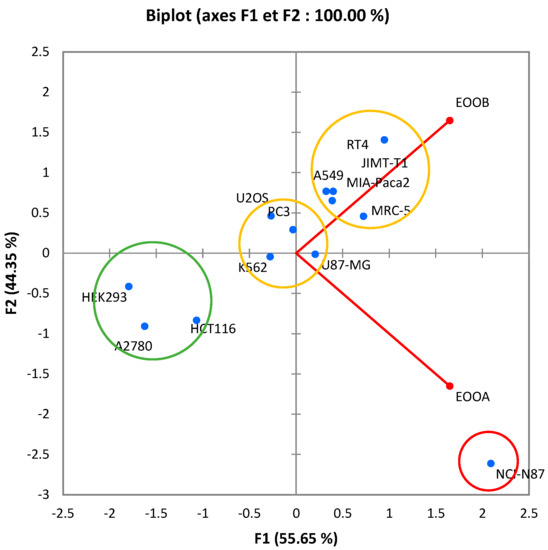
Figure 4.
Biplot of correlation between samples tested and cancer cell lines.
The near-right angle formed by the two essential oils (θ~90°) is not correlated, which means that the effects of EOOB and EOOA are independent.
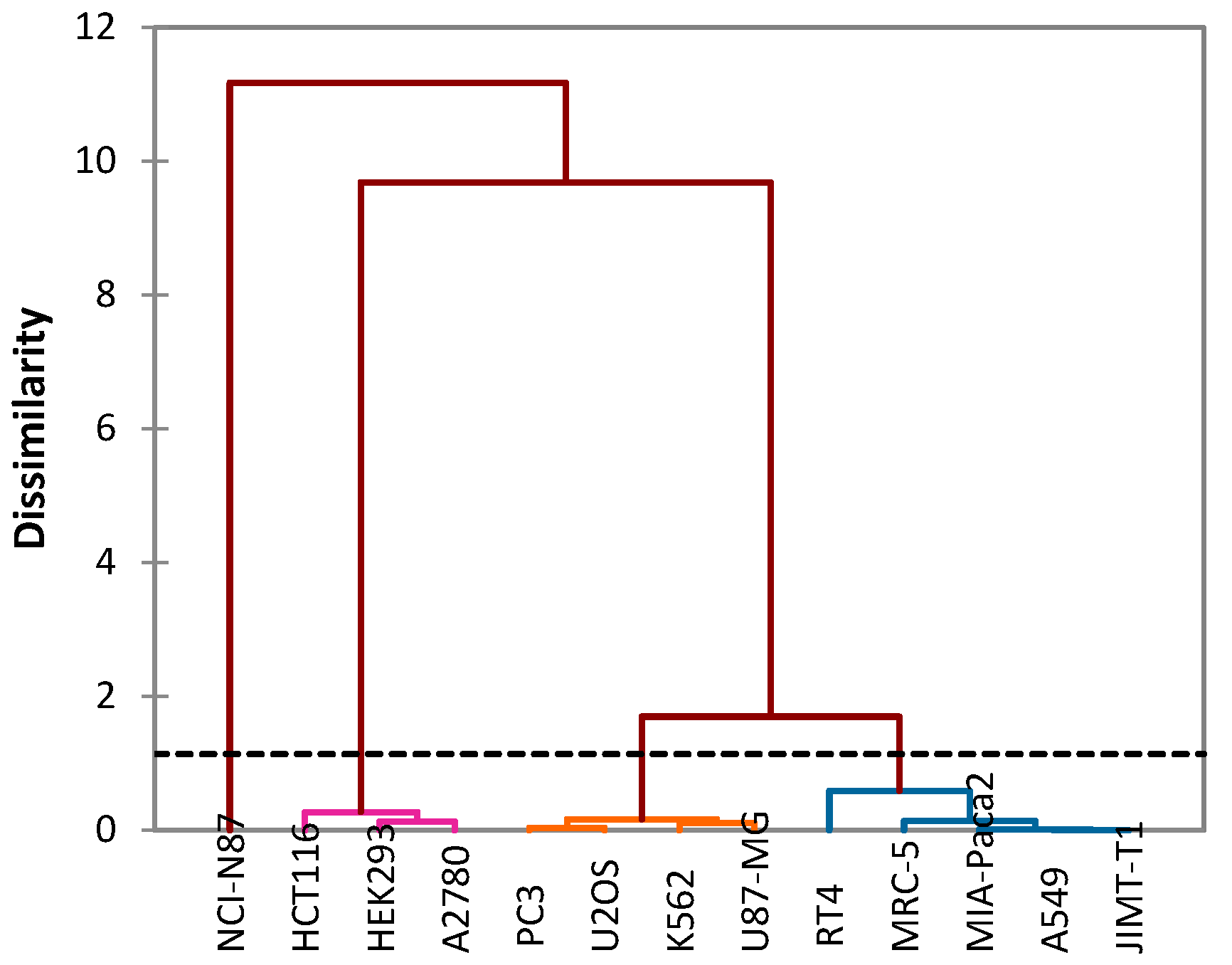
In addition, this analysis makes it possible to group the effect of oils against cancer cell lines into four groups (Figure 5):
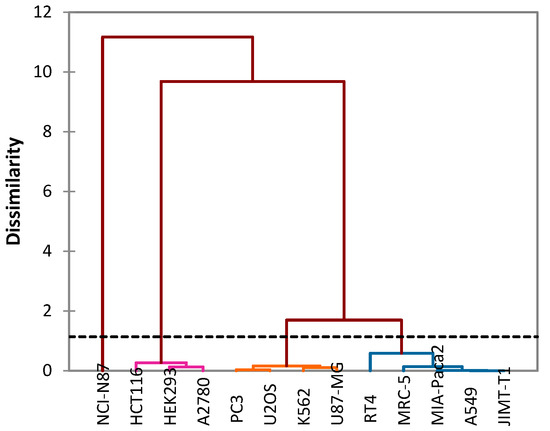
Figure 5.
Cluster analysis of the studied and tested cancer cells.
- Group I: Includes a single cancer cell line NCI-N87, from which essential oils show moderate IC50 values.
- Group II: Includes three cancer cell lines: HCT116, HEK293 and A2780. This group contains significant IC50 values of both essential oils.
- Group III: Includes four cancer cell lines: K562, PC3, U87-MG and U2OS.
- GROUP IV: Includes five cancer cell lines: A549, MIA-Paca2, RT4, MRC-5 and JIMT-T1.
The last two groups III and IV present significant IC50 values of EOOA compared to EOOB.
4. Discussions
The essential oils of different species of the genus Ocimum have a great added value in different industrial sectors due to their notable biological properties in several scientific studies, which are generally linked to their chemical compositions rich in several types of molecules, such as: terpene hydrocarbons, terpenoids, monoterpenes alcohol, phenol aromatics, etc. [26,27,28].
The two selected species Ocimum basilicum L. and Ocimum americanum L. from Djibouti were chosen according to ethnobotanical criteria, biomass availability and traditional use in herbal medicine (Hypoglycemic, antispasmodic, analgesic and hypotensive, antipyretic, anticancer) [29,30]. The extraction by hydrodistillation of the two species gives, respectively, essential oils EOOB and EOOA; additionally, the chemical composition of these two essential oils provided the identification of tens of compounds including Estragole, which is a common majority compound between the two oils, as well Linalool, a second major compound of EOOB, and Carvotanacetol, a second major compound of EOOA. We note that the other six (6) compounds in common were identified with moderate concentrations, and these were Camphor, Bornyl acetate, Elemene, α-bergamotene, δ-cadinene and τ-cadinol. The results of the chemical composition of the two oils are consistent with the results obtained by several studies (Table 7) [31,32,33,34,35]. The composition of essential oils is roughly varied depending on several factors: location, weather conditions, growing conditions, seasonal variations, harvest period and the process of obtaining the oil (grinding, type extraction, preservation, etc.) [36,37,38]

Table 7.
Investigation of chemical composition of EOOB and EOOA.
Regarding the biological properties of different species of the genus Ocimum, several studies have been carried out that aim at one or more in vitro and in vivo evaluations [39,40,41,42]. Our work targeted two in vitro activities. The first test focused on antibacterial effects against clinical strains, and the second test targeted the cytotoxicity of against cancer lines. The antibacterial activity does not show good results except for the positive results noted for the two oils EOOB and EOOA against Streptococcus agalactiae, as well as against Pseudomonas aeruginosa for EOOB and against Enterobacter cloacae for EOOA. Recently, it has been shown that essential oils of the genus Ocimum do not have a great antibacterial efficacy against bacteria of medical origin [43,44]. On the other hand, the cytotoxicity tests of the two essential oils EOOB and EOOA provide encouraging results following the evaluation of thirteen (13) human cancer lines: K562, A549, HCT116, PC3, U87-MG, MIA-Paca2, HEK293, NCI-N87, RT4, U2OS, A2780, MRC-5, and JIMT-T1. These results bring one back to the existence of estragole as a major compound in EOOB and EOOA. Based on previous work, estragole has the potential to inhibit the growth of cancer cells, and hence estragole is a promoter compound that may have many benefits in the treatment of cancer [45,46,47]; additionally, it is known to have genotoxic effects, which may at least partially explain the findings of the current study [48,49].
5. Conclusions
The essential oils of Ocimum basilicum L. and Ocimum americanum L. inhibited the growth of thirteen (13) human cancer cells: K562, A549, HCT116, PC3, U87-MG, MIA-Paca2, HEK293, NCI-N87, RT4, U2OS, A2780, MRC-5 and JIMT-T1. According to inhibition concentrations of 50% (IC50), the essential oils that are the topic of this study exhibit a significant anticancer activity in vitro. Additionally, both oils have moderate antibacterial characteristics, particularly against Streptococcus agalactiae, Pseudomonas aeruginosa and Enterobacter cloacae. These suggest that essential oil could be a potential source of pharmaceuticals. Other more in-depth studies on the cytotoxicity in cells and the mechanisms of antitumor activity in vitro and/or in vivo would be important to better understand the role of the chemical compounds and biological activities of essential oils of the genus Ocimum in terms of their safe application.
Author Contributions
Conceptualization, F.M.A.-L. and T.A.; methodology, A.E.; software, A.M., M.N., A.R., A.A. and J.B.; validation, F.M.A.-L. and T.A.; formal analysis, A.E. and J.B.; investigation, F.M.A.-L., A.M.; resources, F.M.A.-L.; data curation, F.M.A.-L.; writing—original draft preparation, A.A.; writing—review and editing, F.M.A.-L.; visualization, T.A.; supervision, A.A.; project administration, F.M.A.-L.; funding acquisition, F.M.A.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All the data from this research are mentioned in this paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Obame-Engonga, L.C.; Abdoul-Latif, F.M.; Ondo, J.P.; Sima-Obiang, C.; Ngoua-Meye-Misso, R.L.; Traoré, A. Phytochemical screening, antioxidant and antibacterial activities of Guibourtia ehie and Syzygium rowlandii medicinal plants from Gabon. Int. J. Curr. Res. 2017, 9, 56354–56360. [Google Scholar]
- Gharby, S.; Asdadi, A.; Ibourki, M.; Hamdouch, A.; Ainane, T.; Hassani, L.A.I. Chemical characterization of the essential oil from aerial parts of Lavandula rejdalii Upson & Jury, a medicinal plant endemic to Morocco. J. Essent. Oil Bear. Plants 2020, 23, 1422–1427. [Google Scholar]
- Abdoul-Latif, F.M.; Ainane, A.; Merito, A.; Ainane, T. Chemical composition and biological activities of essential oils from Djibouti. J. Anal. Sci. Appl. Biotechnol. 2022, 4, 1–9. [Google Scholar] [CrossRef]
- Ainane, A.; Abdoul-Latif, F.M.; Mohamed, J.; Attahar, W.; Ouassil, M.; Shybat, Z.L.; El Yaacoubi, A.; Ainane, T. Behaviour desorption study of the essential oil of Cedrus atlantica in a porous clay versus insecticidal activity against Sitophilus granarius: Explanation of the phenomenon by statistical studies. Int. J. Metrol. Qual. Eng. 2021, 12, 12. [Google Scholar] [CrossRef]
- Ainane, A.; Abdoul-Latif, F.M.; Abdoul-Latif, T.M.; Ainane, T. Evaluation of biological activities of two essential oils as a safe environmental bioinsecticides: Case of Eucalyptus globulus and Rosmarinus officinalis. Przegląd Nauk. Inżynieria i Kształtowanie Środowiska 2020, 29, 544–556. [Google Scholar] [CrossRef]
- Ainane, T.; Elkouali, M.; Ainane, A.; Talbi, M. Moroccan traditional fragrance based essential oils: Preparation, composition and chemical identification. Pharma Chem. 2014, 6, 84–89. [Google Scholar]
- Gali-Muhtasib, H.; Hilan, C.; Khater, C. Traditional uses of Salvia libanotica (East Mediterranean sage) and the effects of its essential oils. J. Ethnopharmacol. 2000, 71, 513–520. [Google Scholar] [CrossRef]
- Ouassil, M.; Abdoul-Latif, F.M.; Am, A.; Attahar, W.; Ainane, A.; Ainane, T. Chemical composition of bay laurel and rosemary essential oils from Morocco and their antifungal activity against fusarium strains. Pharmacologyonline 2021, 2, 426–433. [Google Scholar]
- Elabboubi, M.; Bennani, L.; Ainane, A.; Charaf, S.; Bouhadi, M.; Elkouali, M.; Talbi, M.; Cherroud, S.; Ainane, T. Treatment of mycoses by essential oils: Mini Review. J. Anal. Sci. Appl. Biotechnol. 2019, 1, 35–40. [Google Scholar]
- Abdoul-Latif, F.M.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Essential Oils of Tagetes minuta and Lavandula coronopifolia from Djibouti: Chemical Composition, Antibacterial Activity and Cytotoxic Activity against Various Human Cancer Cell Lines. Int. J. Plant Biol. 2022, 13, 315–329. [Google Scholar] [CrossRef]
- Shybat, Z.L.; Abdoul-Latif, F.M.; Mohamed, J.; Ainane, A.; Ainane, T. Antifungal activity of the essential oil of morrocan myrtle (Myrtus communis L.): Application in agriculture. Pharmacologyonline 2021, 2, 485–491. [Google Scholar]
- Abdoul-latif, F.M.; Ainane, A.; Abdoul-latif, T.M.; Ainane, T. Chemical study and evaluation of insectical properties of African Lippia citriodora essential oil. J. Biopestic. 2020, 13, 119–126. [Google Scholar]
- Campana, R.; Tiboni, M.; Maggi, F.; Cappellacci, L.; Cianfaglione, K.; Morshedloo, M.R.; Frangipani, E.; Casettari, L. Comparative Analysis of the Antimicrobial Activity of Essential Oils and Their Formulated Microemulsions against Foodborne Pathogens and Spoilage Bacteria. Antibiotics 2022, 11, 447. [Google Scholar] [CrossRef] [PubMed]
- Eddabbeh, F.-E.; Mohamed Abdoul-Latif, F.; Ainane, A.; Ejjabraoui, M.; Ainane, T. The composition of the essential oil and the antimicrobialand antifunqal activities of Tetraclinis articulata (Vahl) masters from the Moroccan central plateau (Morocco). Pharmacologyonline 2021, 2, 458–464. [Google Scholar]
- Morshedloo, M.R.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Maggi, F. Chemical composition, antioxidant activity and cytotoxicity on tumour cells of the essential oil from flowers of Magnolia grandiflora cultivated in Iran. Nat. Prod. Res. 2017, 31, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Adholeya, A.; Conlan, X.A.; Cahill, D.M. Acidic potassium permanganate chemiluminescence for the determination of antioxidant potential in three cultivars of Ocimum basilicum. Plant Foods Hum. Nutr. 2016, 71, 72–80. [Google Scholar] [CrossRef]
- Wossa, S.W.; Rali, T.; Leach, D.N. Atile chemical constituents of three Ocimum species (Lamiaceae) from Papua New Guinea. South Pac. J. Nat. Appl. Sci. 2008, 26, 25–27. [Google Scholar] [CrossRef]
- Abdoul-Latif, F.M.; Ainane, A.; Oumaskour, K.; Boujaber, N.; Mohamed, J.; Ainane, T. In vitro antidiabetic activity of essential oil of two species of Artemisia: Artemisia heba-alba asso and Artemisia ifranensis. Pharmacologyonline 2021, 3, 812–820. [Google Scholar]
- Ainane, A.; Cherroud, S.; El Kouali, M.; Abba, E.H.; Ainane, T. Chemical compositions, insecticidal and antimicrobial activities of two moroccan essential oils of Citrus limonum and Syzygium aromaticum. Pharmacol. J. 2020, 30, 190–199. [Google Scholar]
- Ainane, T.; Mohamed Abdoul-Latif, F.; Ouassil, M.; Am, A.; Ainane, A. Antagonistic antifungal activities of Mentha suaveolens and Artemisia absinthium essential oils from Morocco. Pharmacologyonline 2021, 2, 470–478. [Google Scholar]
- Elmi, A.; Spina, R.; Risler, A.; Philippot, S.; Mérito, A.; Duval, R.E.; Abdoul-Latif, F.M.; Laurain-Mattar, D. Evaluation of antioxidant and antibacterial activities, cytotoxicity of Acacia seyal Del bark extracts and isolated compounds. Molecules 2020, 25, 2392. [Google Scholar] [CrossRef] [PubMed]
- Saviano, A.M.; Lourenço, F.R. Measurement uncertainty estimation based on multiple regression analysis (MRA) and Monte Carlo (MC) simulations—Application to agar diffusion method. Measurement 2018, 115, 269–278. [Google Scholar] [CrossRef]
- Elmi, A.; Spina, R.; Abdoul-Latif, F.M.; Yagi, S.; Fontanay, S.; Risler, A.; Laurain-Mattar, D. Rapid screening for bioactive natural compounds in Indigofera caerulea Rox fruits. Ind. Crops Prod. 2018, 125, 123–130. [Google Scholar] [CrossRef]
- Aborehab, N.M.; Elnagar, M.R.; Waly, N.E. Gallic acid potentiates the apoptotic effect of paclitaxel and carboplatin via overexpression of Bax and P53 on the MCF-7 human breast cancer cell line. J. Biochem. Mol. Toxicol. 2021, 35, e22638. [Google Scholar] [CrossRef] [PubMed]
- Pecnard, S.; Provot, O.; Levaique, H.; Bignon, J.; Askenatzis, L.; Saller, F.; Hamze, A. Cyclic bridged analogs of isoCA-4: Design, synthesis and biological evaluation. Eur. J. Med. Chem. 2021, 209, 112873. [Google Scholar] [CrossRef]
- Gurav, T.P.; Dholakia, B.B.; Giri, A.P. A glance at the chemodiversity of Ocimum species: Trends, implications, and strategies for the quality and yield improvement of essential oil. Phytochem. Rev. 2021, 21, 879–913. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Adetunji, C.O.; Olaniyan, O.T.; Ojo, S.K.; Samuel, M.O.; Temitayo, B.T.; Contreras, M.d. Antimicrobial, Antioxidant and Other Pharmacological Activities of Ocimum Species: Potential to Be Used as Food Preservatives and Functional Ingredients. Food Rev. Int. 2021, 1–31. [Google Scholar] [CrossRef]
- Kumar, R.; Saha, P.; Lokare, P.; Datta, K.; Selvakumar, P.; Chourasia, A. A Systemic Review of Ocimum sanctum (Tulsi): Morphological Characteristics, Phytoconstituents and Therapeutic Applications. Int. J. Res. Appl. Sci. Biotechnol. 2022, 9, 221–226. [Google Scholar] [CrossRef]
- Hassan-Abdallah, A.; Merito, A.; Hassan, S.; Aboubaker, D.; Djama, M.; Asfaw, Z.; Kelbessa, E. Medicinal plants and their uses by the people in the Region of Randa, Djibouti. J. Ethnopharmacol. 2013, 148, 701–713. [Google Scholar] [CrossRef]
- Vieira, R.F.; Simon, J.E. Chemical characterization of basil (Ocimum spp.) found in the markets and used in traditional medicine in Brazil. Econ. Bot. 2000, 54, 207–216. [Google Scholar] [CrossRef]
- Farouk, A.; Fikry, R.; Mohsen, M. Chemical composition and antioxidant activity of Ocimum basilicum L. essential oil cultivated in Madinah Monawara, Saudi Arabia and its comparison to the Egyptian chemotype. J. Essent. Oil Bear. Plants 2016, 19, 1119–1128. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Guedri, M.M.; Ahlem, Z.; Mariem, B.A.; Romdhane, M.; Okla, M.; Al-Hashimi, A.; Elgawad, H.A. Essential Oil Composition and Antioxidant and Antifungal Activities of Two Varieties of Ocimum basilicum L. (Lamiaceae) at Two Phenological Stages. Agronomy 2022, 12, 825. [Google Scholar]
- Mondello, L.; Zappia, G.; Cotroneo, A.; Bonaccorsi, I.; Chowdhury, J.U.; Yusuf, M.; Dugo, G. Studies on the essential oil-bearing plants of Bangladesh. Part VIII. Composition of some Ocimum oils O. basilicum L. var. purpurascens, O. sanctum L. green, O. sanctum L. purple, O. americanum L., citral type, O. americanum L., camphor type. Flavour Fragr. J. 2002, 17, 335–340. [Google Scholar] [CrossRef]
- de Almeida, I.; Alviano, D.S.; Vieira, D.O.; Alves, P.B.; Blank, A.F.; Lopes, A.H.; Rosa, M.D.S.S. Antigiardial activity of Ocimum basilicum essential oil. Parasitol. Res. 2007, 101, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Sabo, V.A.; Knezevic, P. 3Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Ainane, T.; Abdoul-Latif, F.M.; Shybat, Z.L.; Mohamed, J.; Ainane, A. Antifungal activity of essential oil of Pistacia atlantica against Ascochyta rabiei and its correlation with antioxidant activity. Pharmacologyonline 2021, 3, 829–837. [Google Scholar]
- Mohamed Abdoul-Latif, F.; Elmi, A.; Merito, A.; Nour, M.; Risler, A.; Ainane, A.; Bignon, J.; Ainane, T. Chemical Analysis of Essential Oils of Cymbopogon schoenanthus (L.) Spreng. and Nepeta azurea R.Br. ex Benth from Djbouti, In-Vitro Cytotoxicity against Cancer Cell Lines and Antibacterial Activities. Appl. Sci. 2022, 12, 8699. [Google Scholar] [CrossRef]
- Pandey, A.K.; Singh, P.; Tripathi, N.N. Chemistry and bioactivities of essential oils of some Ocimum species: An overview. Asian Pac. J. Trop. Biomed. 2014, 4, 682–694. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S.; Trchounian, A. Chemical composition and some biological activities of the essential oils from basil Ocimum different cultivars. BMC Complementary Altern. Med. 2017, 17, 1–8. [Google Scholar] [CrossRef]
- Jakovljević, D.; Stanković, M.; Warchoł, M.; Skrzypek, E. Basil (Ocimum L.) cell and organ culture for the secondary metabolites production: A review. Plant Cell Tissue Organ Cult. 2022, 149, 61–79. [Google Scholar] [CrossRef]
- Bharati, A.C.; Yadav, P.K.; Pandey, S.; Wal, P.; Sagar, M.K.; Kumar, A. Chemistry, Biological Activities, and Uses of Basil Seed Gum. In Gums, Resins and Latexes of Plant Origin: Chemistry, Biological Activities and Uses; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–17. [Google Scholar]
- Habyarimana, T.; Cyuzuzo, P.; Yamukujije, C.; Yadufashije, C.; Niyonzima, F.N. Phytochemical and Antimicrobial Activity of Ocimum suave Against Selected Human Pathogenic Bacteria. J. Drug Deliv. Ther. 2022, 12, 123–128. [Google Scholar] [CrossRef]
- Sneha, K.; Narayanankutty, A.; Job, J.T.; Olatunji, O.J.; Alfarhan, A.; Famurewa, A.C.; Ramesh, V. Antimicrobial and larvicidal activities of different ocimum essential oils extracted by ultrasound-assisted hydrodistillation. Molecules 2022, 27, 1456. [Google Scholar] [CrossRef] [PubMed]
- Talbi, M.; Saadali, B.; Boriky, D.; Bennani, L.; Elkouali, M.; Ainane, T. Two natural compounds–a benzofuran and a phenylpropane–from Artemisia dracunculus. J. Asian Nat. Prod. Res. 2016, 18, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Levorato, S.; Dominici, L.; Fatigoni, C.; Zadra, C.; Pagiotti, R.; Moretti, M.; Villarini, M. In vitro toxicity evaluation of estragole-containing preparations derived from Foeniculum vulgare Mill. (fennel) on HepG2 cells. Food Chem. Toxicol. 2018, 111, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Monien, B.H.; Sachse, B.; Niederwieser, B.; Abraham, K. Detection of N-acetyl-S-[3′-(4-methoxyphenyl) allyl]-l-Cys (AMPAC) in human urine samples after controlled exposure to fennel tea: A new metabolite of estragole and trans-anethole. Chem. Res. Toxicol. 2019, 32, 2260–2267. [Google Scholar] [CrossRef]
- Kalantari, H.; Galehdari, H.; Zaree, Z.; Gesztelyi, R.; Varga, B.; Haines, D.; Juhasz, B. Toxicological and mutagenic analysis of Artemisia dracunculus (tarragon) extract. Food Chem. Toxicol. 2013, 51, 26–32. [Google Scholar] [CrossRef]
- Zeni, V.; Benelli, G.; Campolo, O.; Giunti, G.; Palmeri, V.; Maggi, F.; Canale, A. Toxics or lures? Biological and behavioral effects of plant essential oils on tephritidae fruit flies. Molecules 2021, 26, 5898. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).