1. Introduction
Carbon dioxide, the main cause of the greenhouse effect, has attracted much attention in recent years. Thermal power plants that consume large amounts of coal are one of the main causes of the greenhouse effect, but it is difficult to replace them with alternative power plants due to their high energy production efficiency. For this reason, the removal process of flue gas including carbon dioxide from coal-fired power plants has been widely studied [
1].
The International Energy Agency (IEA) reported that CO
2 emissions dropped by 5.8% during the COVID-19 pandemic, and coal-based CO
2 emissions declined by 0.6 Gt CO
2. However, coal-based electricity generation contributes 35% of production, which is still larger than renewables, oil, and nuclear [
2]. Further, they have identified that Carbon Capture and Storage (CCS) technology will be essential to meet the global goal of limiting global temperature increase. At least 90% of CO
2 could be captured from the power plant and energy industries [
3]. More than 80% of energy comes from the combustion of fossil fuels, which contributes to global warming. However, after CO
2-capturing processes, CO
2 can be turned into fuels such as methanol, formic acid, dimethyl carbonate, and methyl formate [
4]. There are various ways to capture carbon dioxide, including absorption, adsorption, membrane, cryogenic, and CO
2/O
2 combustion. Absorption is divided into two methods: chemical and physical absorption. Chemical absorption uses an aqueous solution, such as of amines or sodium hydroxide and sodium carbonate. Absorption has the advantage that the solvent is regenerated and returned to a continuous carbon dioxide removal process [
5]. CCS technology still faces some challenges, however. Large scale CCS has a high energy demand, leading to high operating costs. Optimization of the process is required to reduce energy demands and cost [
6]. Various CO
2 capture processes have been developed with amine solvents, such as MEA (monoethanolamine). However, MEA has drawbacks: the high solvent cost, thermal degradation, and low CO
2 absorption efficiency. Therefore, NH
3 has been identified as a new absorption solvent. Comparisons of amines and ammonia as CO
2 capture solvents have been undertaken. By theoretical values, NH
3 can capture carbon dioxide twice as effectively as MEA [
7]. Dave et al. [
8] showed that 5 wt% ammonia solvent has at least 23% to 29% lower reboiler duty than 30 wt% amine solvent in carbon dioxide capture.
Shanbaz et al. [
9] simulated the CO
2 capture process with a CaO solvent in syngas production from steam gasification. Riva et al. [
10] simulated the post-combustion CO
2 capture process by chemical absorption using ionic liquid. Sinaki et al. [
11] simulated CO
2 capture with the MEA solvent from the post-combustion of mazut using Aspen Hysys. Darde et al. [
12] simulated the Chilled Ammonia Process (CAP) with the Extended UNIQUAC model. They focused on absorption at low temperatures to increase CO
2 loading. Li et al. [
13] simulated the inter-heating process and rich-split process to reduce reboiler heat duty at a large-scale coal-fired power station. Niu et al. [
14] studied the CO
2 capture process with aqueous ammonia using the eNRTL model, and simulated the CO
2 capture system and NH
3 abatement system. Ishaq et al. [
15] simulated the post-combustion CO
2 capture process with aqueous ammonia and a split flow arrangement using the eNRTL model. They compared the results of the absorber height and split fraction, which affects reboiler heat duty. Mathias et al. [
16] conducted an analysis and quantitative evaluation on chilled ammonia processes (CAP) with a thermodynamics analysis and process simulation. The analysis can provide a way to predict operational problems. The evaluation revealed how the process performance changes as operating conditions change, and identified the optimal conditions to operate the process. Song et al. [
17] studied a carbon dioxide capture process with PTSA (Pressure–Temperature Swing Adsorption). In the study, an advanced PTSA method was used which involved an integrated chemical heat transformer and pressure recovery. An advanced PTSA method was simulated with PRO/II software, and it resulted in 40% energy savings when compared to conventional PTSA methods. The carbon dioxide removal rates and energy consumption of recent carbon dioxide removal processes are summarized in
Table 1.
Ullah et al. [
18] studied a CO
2 capture process with an ammonia solvent, and the RVC (Rich Vapor Compression) process and the CSS (Cold Solvent Split) process were combined to significantly increase energy consumption efficiency when compared to the existing CO
2 capture process. Ullah et al. [
19] studied an additional energy-saving process by combining the RVC process with the LVC (Lean Vapor Compression) process and the CSS process. Jiang et al. [
20] achieved energy savings through the Cold Rich Split process with ammonia. It achieved a 34% reduction effect when compared to the existing ammonia process and a 44% reduction effect compared to the MEA process. A follow-up study in Jiang et al. [
21] proceeded to capture CO
2 in the PZ (Piperazine) + ammonia process by adding PZ to the ammonia-based CO
2 capture process. By applying the CSS process to this process, energy was reduced by 20% when compared to the MEA-based CO
2 capture process. Liu et al. [
22] compared the energy efficiency with the inter-heating process, rich-split process, and combined process (inter-heating + rich split). The combined process showed better energy efficiency than other two processes. These are the studies that increase energy efficiency in CO
2 capture processes by applying split flow.
In this paper, we simulated the split flow CO
2 removal process with an ammonia solvent, applying the eNRTL thermodynamics model in Aspen Plus to find the operating conditions to improve the consumption of reboiler heat duty, as shown in
Table 1.
Case Study 1 analyzed the carbon dioxide capture process of a cement plant to verify the eNRTL model, and Case Study 2 simulated the carbon dioxide capture process by changing various process operating conditions.
2. Process Design
This simulation was based on flue gas from a 500 MW (megawatt) coal-fired power station as a feed. The specifications of flue gas from the 500 MW coal-fired power station are shown in
Table 2 [
23]. The fixed operating conditions of the carbon dioxide removal process in this study are summarized in
Table 3.
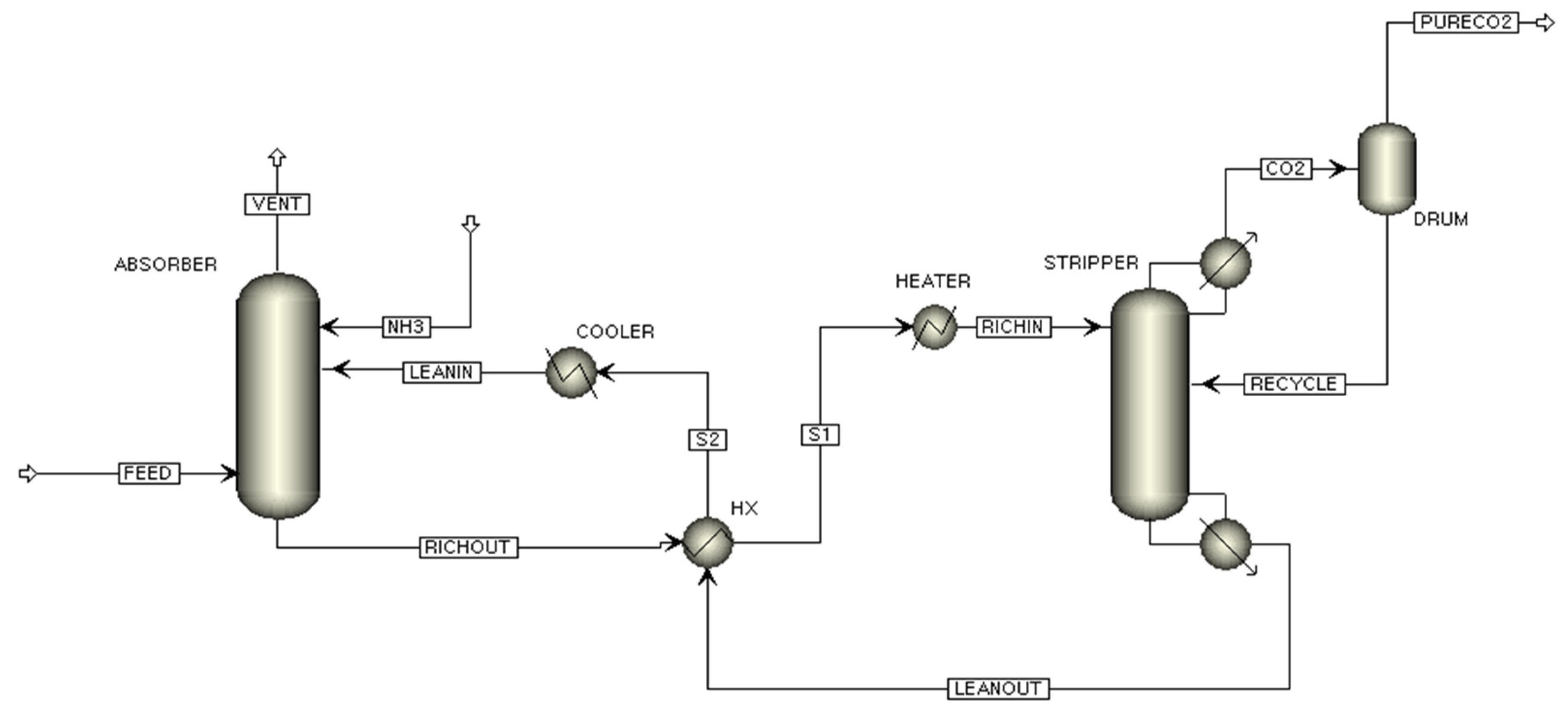
The flowsheet of the conventional flow CO
2 capture process of this study is shown in
Figure 1. The CO
2 capture process can be divided into two parts: the CO
2 capture part and NH
3 abatement part. The flue gas enters the CO
2 absorber and is absorbed by contacting the NH
3 solution. The overhead stream from the CO
2 absorber goes to the NH
3 abatement part to separate N
2 and NH
3. The separated NH
3 stream is recycled to the CO
2 capture part and is mixed with the CO
2 lean stream. An absorber bottom stream, which is called the rich stream, goes to the CO
2 stripper to remove CO
2 from the absorbed NH
3 solution. The lean stream from the bottom of the CO
2 stripper is fed to the top of the CO
2 absorber. A flowsheet of the split flow CO
2 capture process is shown in
Figure 2. The difference of this system from the conventional CO
2 capture process is the side stream from the CO
2 stripper, which is fed into the CO
2 absorber. Using the split-flow process has the advantage of reducing energy usage in the stripper [
24,
25].
The electrolyte Non-Random Two-Liquid (eNRTL) thermodynamics model helps to predict ionic species in liquid phases as a function of the equilibrium of CO
2 solubility and temperature [
26]. Dash et al. [
27] carried out an experiment on CO
2 solubility in AMP (2-amino-2-methyl-1-propanol) using the eNRTL model. The model theory was developed to correlate with and predict the vapor–liquid equilibrium (VLE) for CO
2 in aqueous AMP. The model’s prediction has shown good agreement with the experimental data. The model prediction of eNRTL thermodynamics was conducted by Yu et al. [
28], based on post-combustion capture using aqueous ammonia. The prediction showed the technical feasibility of the process at a low ammonia concentration. In this study, the eNRTL model was applied to the split flow CO
2-capturing process with the Aspen Plus simulation. Niu et al. [
29] conducted a pilot plant at the laboratory scale for CO
2 capture by aqueous ammonia. In addition, an Aspen Plus simulation was performed, and the simulation results and the experimental results showed good agreement.
Table 3.
Fixed operating conditions [
30].
Table 3.
Fixed operating conditions [
30].
| Parameter | Value |
|---|
| Absorber | |
| Feed temperature/pressure (flue gas) | 50 °C/1.0133 bar |
| Number of stages | 6 |
| Pressure | 1.0133 bar |
| Stripper | |
| Number of stages | 20 |
| Pressure | 1.0133 bar |
| Ammonia solution | |
| NH3 solution temperature/pressure | 25 °C/1.0133 bar |
| NH3 mass fraction | 7% |
The CO
2 capture process using aqueous amine solution has already been researched previously. Due to disadvantages such as thermal degradation and the high absorbent cost of amine solvents, Ciferno et al. [
31] showed that 7 wt% aqueous ammonia can be used in the CO
2 capture process, and compared the cost and energy with 30 wt% MEA. The qualitative comparison of amines and ammonia is shown in
Table 4 [
32]. Ammonia solvents have twice the CO
2 capture capacity and half the regeneration energy compared to amine solvents. In addition, it has the advantages of low thermal degradation and low absorbent cost; however, it also has the disadvantage of high volatility and the need for a regeneration process. For the above reasons, this paper used aqueous ammonia solution for the CO
2 capture process.
The simulation was carried out assuming that the operating conditions of the process included atmospheric pressure, except for salt formation. The limitations of this simulation are that the purity of the pure CO2 stream is slightly low, about 96%, and an abatement process is required due to the limitation of ammonia emission into the atmosphere, however, the recycle stream was not included in the simulation because the process was not designed.
4. Results
The simulation presented here determined the operating conditions when the CO
2 removal process using aqueous ammonia has a CO
2 removal rate of 90%. Based on the above various operating conditions, such as the operating conditions of stripping column, conditions of the aqueous ammonia solvent and the flow rate of side stream, the reboiler heat duty produced a result of 1298.98 kJ/kgCO
2 when the CO
2 removal rate was 90% and 1705.35 kJ/kgCO
2 at 95% removal rate. The operating conditions of the process are shown in
Table S4.
Table S4 is included in
supplementary materials.
This paper compared the energy consumption per kgCO
2 of conventional flow and split flow at the same CO
2 removal rate to show that the split flow process is the improved process. Ishaq et al. [
15] and Bae et al. [
37] showed that split flow has a lower reboiler heat duty than conventional flow. The results of this paper were similar to the results of the previous studies. Therefore, the simulation applied split flow and compared the results. We compared the energy consumption of the conventional flow and split flow process with a rate of 90% and 95%, respectively. For 90% carbon dioxide removal, split flow had a 10.24% lower reboiler heat duty than the conventional flow process when the flow rate of side stream is 5 kmol/s. This figure represents an operating cost savings of
$838,457 per year, calculated as a utility cost of Aspen Plus. At 95% carbon dioxide removal, split flow had a 7.14% lower reboiler heat duty than conventional flow when the flow rate was the same as the 90% removal rate. The results showed that the split flow process had a lower reboiler duty than the conventional flow process at both carbon dioxide removal rates. The results are summarized in
Table 13. In addition, this paper compared the preheater outlet temperature to reduce the operating cost of preheater. When the temperature of the stream entering the stripping column is lower than the temperature of the stripping column top product stream, the total duty is constant, but when it is high, additional costs are incurred by using cooling water. For this reason, it is advantageous to reduce operating costs by setting the temperature of the preheater installed in front of the stripping column to 80 °C or less.
The simulation results were compared to other papers using aqueous ammonia with a 90% CO
2 removal rate. The energy consumption per kgCO
2 was improved when compared to Darde et al. [
12] and Ishaq et al. [
15].
5. Conclusions
The simulation of the CO2 capture process of flue gas from a 500 MW coal-fired power station was performed with various operating conditions with aqueous ammonia solution using the eNRTL model. Operating conditions were determined by changing the values of the reboiler duty, reflux ratio, and temperature and mass fraction of ammonia solution according to the CO2 removal rate. The CO2 removal rate increased as the reboiler duty increased, and the reflux ratio showed the opposite trend. It was confirmed that the use of a 7% ammonia mass fraction resulted in energy savings by comparing the reboiler heat duty at same carbon dioxide removal rate. The comparison of reboiler heat duty through the temperature change of ammonia solution was performed. The reboiler heat duty was the lowest when other conditions were the same at room temperature of 25 °C, and at other temperature conditions, heating duty or cooling duty was required, so 25 °C was an appropriate operating condition. By changing the temperature of the pre-heater of the CO2 stripper, we found that setting the temperature to lower than 80 °C, in terms of the process operating cost, was beneficial. The operating conditions at a 90% carbon dioxide removal rate and 90% removal rate were found as a result. The conventional flow process and split flow process showed an energy consumption difference when the carbon dioxide removal rate was 90%, which persisted if the carbon dioxide removal rate was 95% by changing the flow rate of the side stream. When these results were observed by changing the flow rate of the side stream of the stripping column, the difference in energy consumption peaked at 5 kmol/s of flow rate, and then the difference decreased as it increased. It was confirmed that the split flow is more beneficial in terms of energy than the conventional flow, based on the same results as the previous research results. As a result of the study, the operating conditions of the process aiming for the carbon dioxide removal rate of 95% were confirmed, and 10.24% and 7.14% energy savings were obtained in the process with the removal rate of 90% and 95% compared to the conventional flow process, respectively. We developed operating conditions for the split-flow CO2 capture process with a reduced energy consumption per kgCO2 by at least 26.19% and up to 36.63%, compared to other papers, with a 90% CO2 removal rate.