A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Formulations
2.2. Preparation of Powder-Coated Panels
2.3. Electrochemical Measurements
2.4. Characterization Techniques
2.5. Coating Performance Evaluations
3. Results and Discussion
3.1. Coating Properties
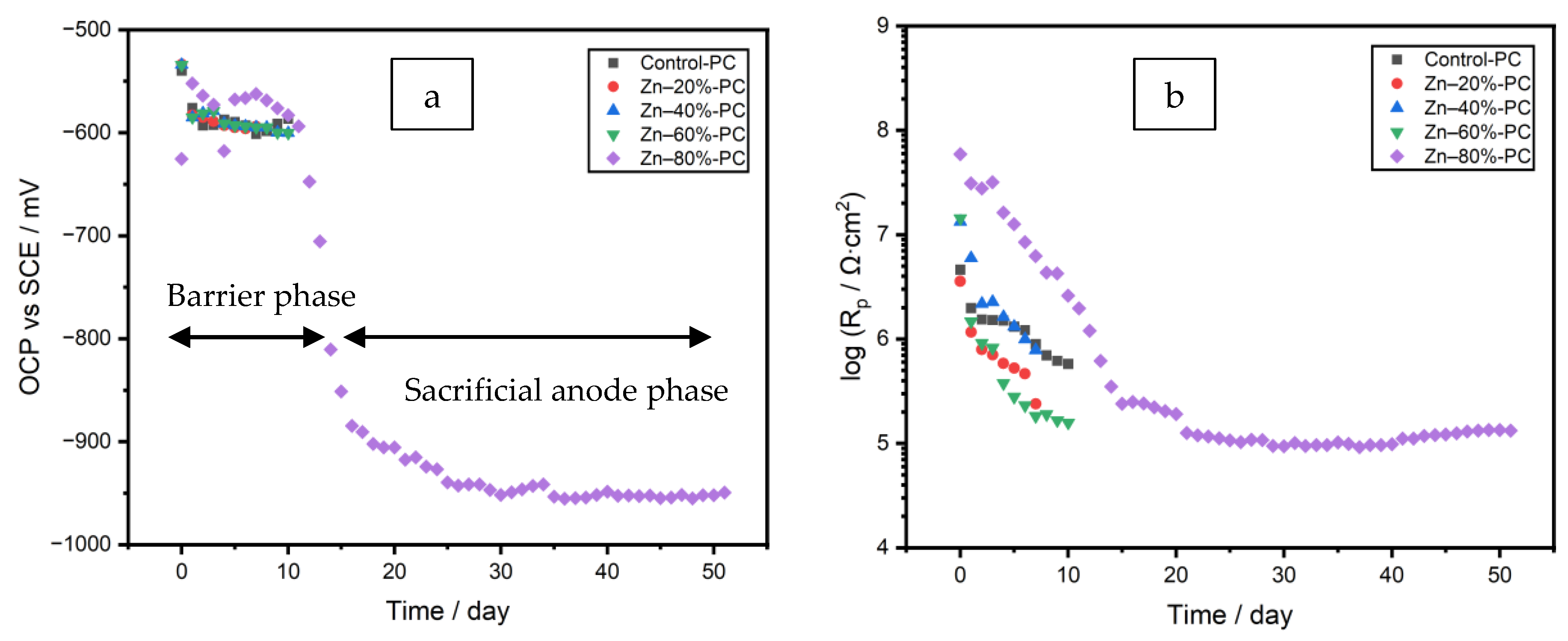
3.2. Evolution of OCP and Rp
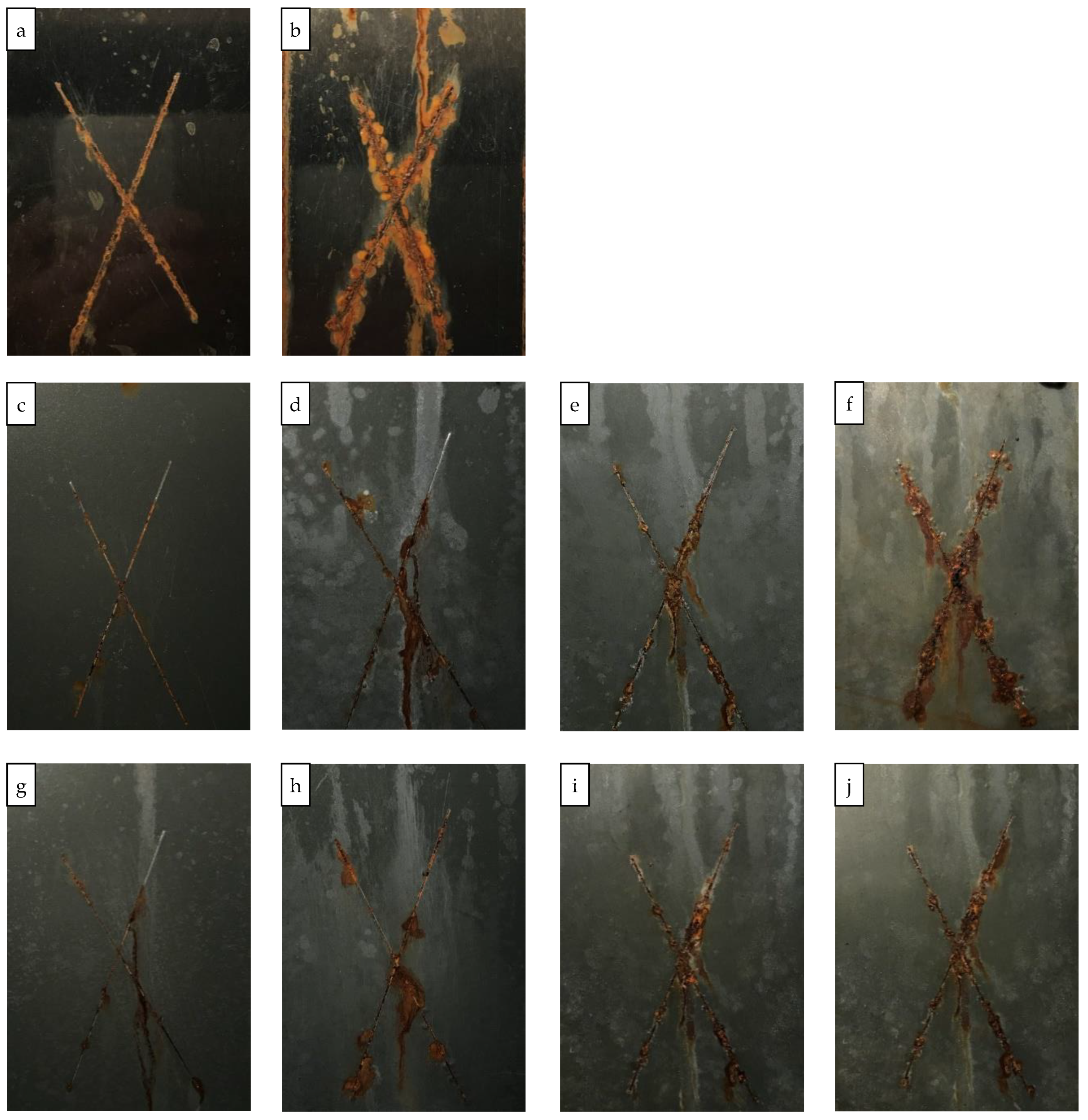
3.3. Identification of Corrosion Products in the Coatings
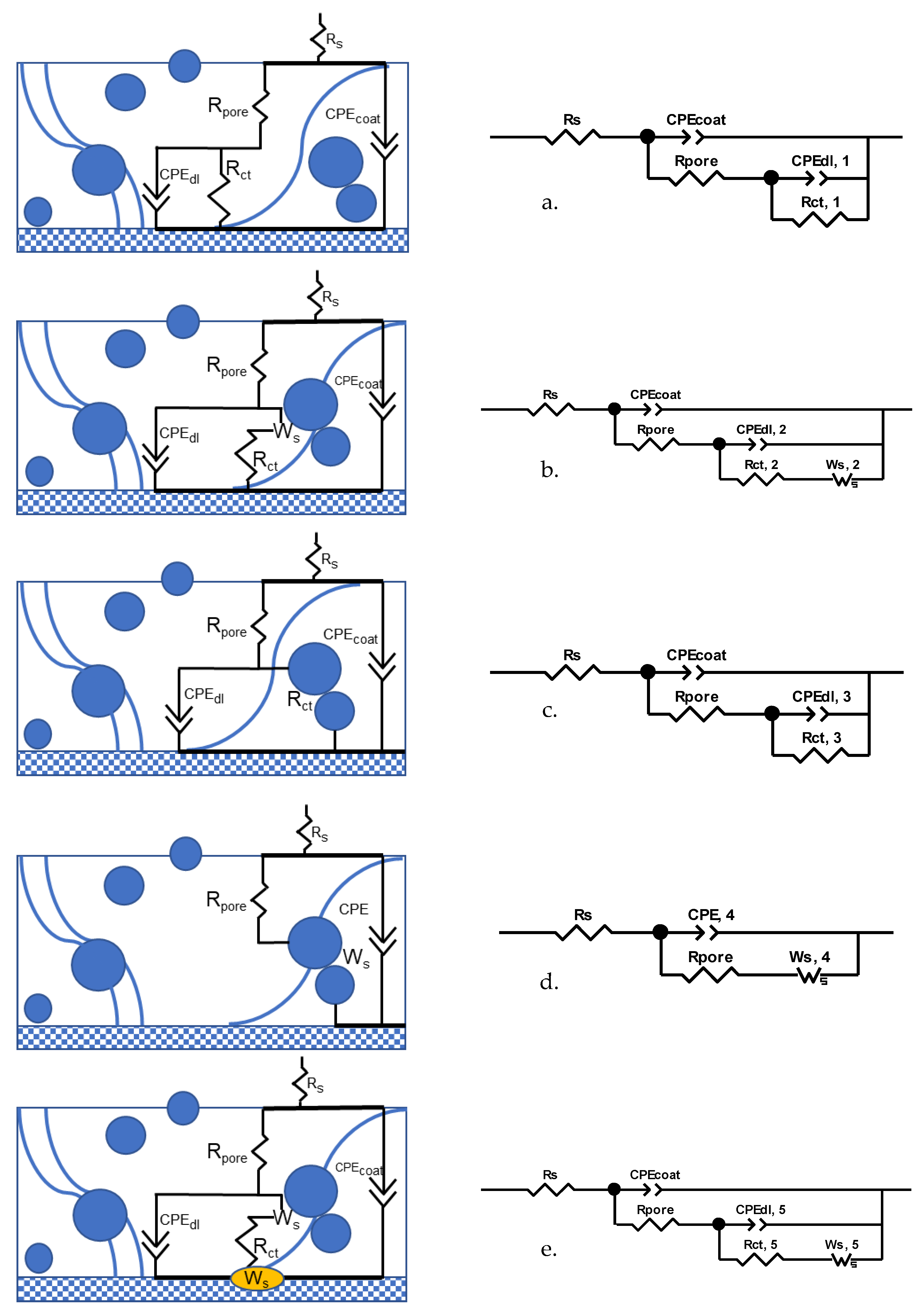
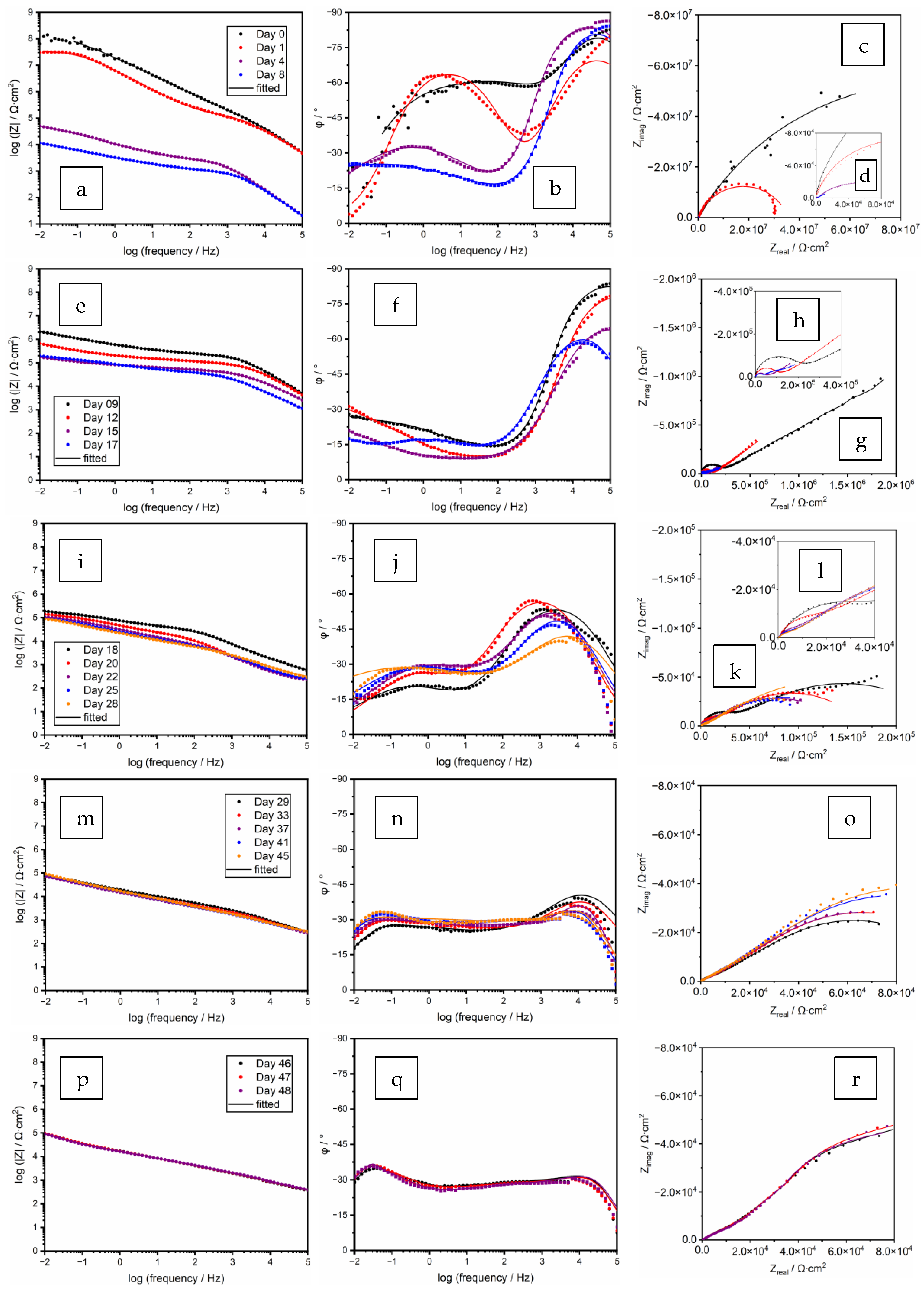
3.4. EIS Equivalent Electrical Circuit Analysis
3.4.1. Stage 1. Initiation of Electrolyte Ingress, Days 0–8
3.4.2. Stage 2. Corrosion of Zinc Particles and Initiation of Galvanic Protection, Days 9–17
3.4.3. Stage 3. Passivation of Zinc Particles, Days 18–28
3.4.4. Stage 4. Re-Activation of Zinc and Diffusion of Zinc Cation, Days 29–45
3.4.5. Stage 5. Localized Corrosion of the Substrate, Days 46–48
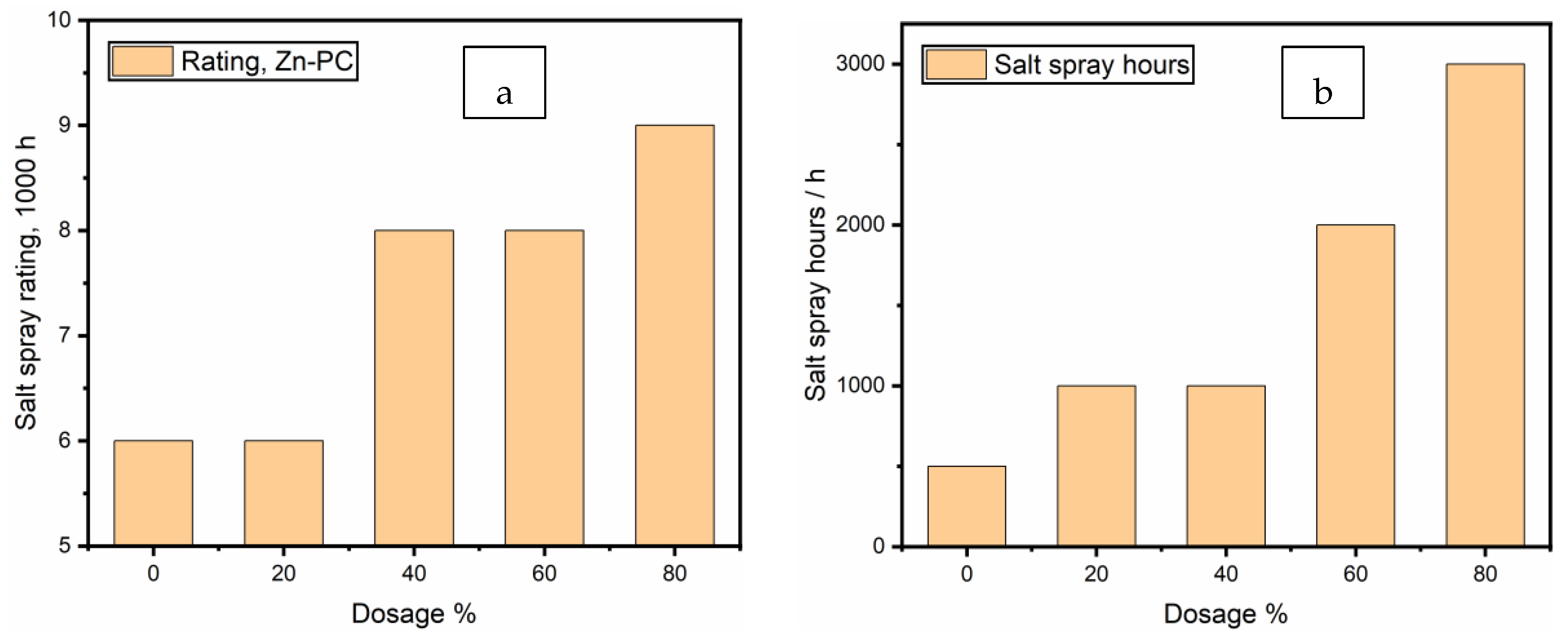
3.5. Neutral Salt-Spray Test Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ReferencesSørensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive Coatings: A Review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, C.; Lin, Q.; Liu, H.; Yao, M. Broad-Beam Laser Cladding of Al-Si Alloy Coating on AZ91HP Magnesium Alloy. Surf. Coat. Technol. 2006, 201, 2701–2706. [Google Scholar] [CrossRef]
- Funke, W. Organic Coatings in Corrosion Protection. In Surface Coatings—2; Springer: Berlin/Heidelberg, Germany, 1988; pp. 107–135. [Google Scholar] [CrossRef]
- Li, W.; Franco, D.C.; Yang, M.S.; Zhu, X.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Comparative Study of the Performances of Al(OH)3 and BaSO4 in Ultrafine Powder Coatings. Processes 2019, 7, 316. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, H. Ultrafine Powder Coatings: An Innovation. Powder Coat. 2005, 16, 39–47. [Google Scholar]
- Turner, S.; Baskir, J.; Nunez, C. Powder Coatings: A Technology Review. In Pollution Prevention Review; Spring: Berlin/Heidelberg, Germany, 1999; pp. 7–21. [Google Scholar]
- Li, W.; Franco, D.C.; Yang, M.S.; Zhu, X.; Zhang, H.; Shao, Y.; Zhang, H.; Zhu, J. Investigation of the Performance of ATH Powders in Organic Powder Coatings. Coatings 2019, 9, 110. [Google Scholar] [CrossRef]
- Crapper, G. Powder Coatings. In Polymer Science: A Comprehensive Reference; Elsevier: Amsterdam, The Netherlands, 2012; Volume 10, pp. 541–566. ISBN 9780080878621. [Google Scholar]
- Farshchi, N.; Gedan-Smolka, M. Polyurethane Powder Coatings: A Review of Composition and Characterization. Ind. Eng. Chem. Res. 2020, 59, 15121–15132. [Google Scholar] [CrossRef]
- Upadhyay, V.; Harkal, U.D.; Webster, D.C.; Bierwagen, G.P. Preliminary Investigation of the Impact of Polymer Composition on Electrochemical Properties of Coatings as Determined by Electrochemical Impedance Spectroscopy. J. Coat. Technol. Res. 2013, 10, 865–878. [Google Scholar] [CrossRef]
- Spyrou, E. Powder Coatings: Chemistry and Technology, 3rd edition; Vincentz Network GmbH & Co KG: Hannover, Germany, 2012; ISBN 978-3-86630-824-4. [Google Scholar]
- Farrell, R. Powder Coatings. Met. Finish. 2010, 108, 100–107. [Google Scholar] [CrossRef]
- De Lange, P. A History of Powder Coatings. Ind. Paint Powder 2004, 80, 23–27. [Google Scholar]
- Streitberger, H.-J.; Goldschmidt, A. Processing of Powder Coatings. In Basics of Coating Technology; Vincentz Network GmbH & Co KG: Hannover, Germany, 2018. [Google Scholar]
- Marchebois, H.; Savall, C.; Bernard, J.; Touzain, S. Electrochemical Behavior of Zinc-Rich Powder Coatings in Artificial Sea Water. Electrochim. Acta 2004, 49, 2945–2954. [Google Scholar] [CrossRef]
- Touzain, S. Accelerated Aging of Zinc-Rich Powder Coatings Containing Different Conductive Pigments; CSC Publishing: St. Paul, MN, USA, 2009; pp. 1–6. [Google Scholar]
- Marchebois, H.; Joiret, S.; Savall, C.; Bernard, J.; Touzain, S. Characterization of Zinc-Rich Powder Coatings by EIS and Raman Spectroscopy. Surf. Coat. Technol. 2002, 157, 151–161. [Google Scholar] [CrossRef]
- Meroufel, A.; Touzain, S. EIS Characterisation of New Zinc-Rich Powder Coatings. Prog. Org. Coat. 2007, 59, 197–205. [Google Scholar] [CrossRef]
- Schaefer, K.; Miszczyk, A. Improvement of Electrochemical Action of Zinc-Rich Paints by Addition of Nanoparticulate Zinc. Corros. Sci. 2013, 66, 380–391. [Google Scholar] [CrossRef]
- Giúdice, C.; Benftez, J.C.; Linares, M.M. Zinc-Rich Epoxy Primers Based on Lamellar Zinc Dust. JOCCA Surf. Coat. Int. 1997, 80, 279–284. [Google Scholar] [CrossRef]
- Meroufel, A.; Deslouis, C.; Touzain, S. Electrochemical and Anticorrosion Performances of Zinc-Rich and Polyaniline Powder Coatings. Electrochim. Acta 2008, 53, 2331–2338. [Google Scholar] [CrossRef]
- Abreu, C.M.; Izquierdo, M.; Keddam, M.; Nóvoa, X.R.; Takenouti, H. Electrochemical Behaviour of Zinc-Rich Epoxy Paints in 3% NaCl Solution. Electrochim. Acta 1996, 41, 2405–2415. [Google Scholar] [CrossRef]
- SSPC. Coating Standard No. 20 Zinc-Rich Coating (Type I-Inorganic, and Type II-Organic); The Society for Protective Coatings: Pittsburgh, PA, USA, 2019. [Google Scholar]
- Jagtap, R.N.; Patil, P.P.; Hassan, S.Z. Effect of Zinc Oxide in Combating Corrosion in Zinc-Rich Primer. Prog. Org. Coat. 2008, 63, 389–394. [Google Scholar] [CrossRef]
- Pereyra, A.M.; Giudice, C.A. Shaped for Performance. Eur. Coat. J. 2007, 9, 40. [Google Scholar]
- Wang, H.; Huang, S.L.; Zuo, Y.J.; Zhou, T.; Zhang, L.R. Corrosion Resistance of Lamellar Aluminium Pigments Coated by SiO2 by Sol-Gel Method. Corros. Sci. 2011, 53, 161–167. [Google Scholar] [CrossRef]
- Kalendová, A. Effects of Particle Sizes and Shapes of Zinc Metal on the Properties of Anticorrosive Coatings. Prog. Org. Coat. 2003, 46, 324–332. [Google Scholar] [CrossRef]
- ASTM B117-16 Standard Practice for Operating Salt Spray (Fog) Apparatus; ASTM International: West Conshohocken, PA, USA, 2016.
- Tsai, C.H.; Mansfeld, F. Determination of Coating Deterioration with EIS: Part II. Development of a Method for Field Testing of Protective Coatings. Corrosion 1993, 49, 726–737. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Ferreira, M.G.S. Ranking High-Quality Paint Systems Using EIS. Part II: Defective Coatings. Corros. Sci. 2003, 45, 123–138. [Google Scholar] [CrossRef]
- Margarit-Mattos, I.C.P. EIS and Organic Coatings Performance: Revisiting Some Key Points. Electrochim. Acta 2020, 354, 136725. [Google Scholar] [CrossRef]
- Marchebois, H.; Touzain, S.; Joiret, S.; Bernard, J.; Savall, C. Zinc-Rich Powder Coatings Corrosion in Sea Water: Influence of Conductive Pigments. Prog. Org. Coat. 2002, 45, 415–421. [Google Scholar] [CrossRef]
- Asemani, H.R.; Ahmadi, P.; Sarabi, A.A.; Eivaz Mohammadloo, H. Effect of Zirconium Conversion Coating: Adhesion and Anti-Corrosion Properties of Epoxy Organic Coating Containing Zinc Aluminum Polyphosphate (ZAPP) Pigment on Carbon Mild Steel. Prog. Org. Coat. 2016, 94, 18–27. [Google Scholar] [CrossRef]
- Park, J.H.; Yun, T.H.; Kim, K.Y.; Song, Y.K.; Park, J.M. The Improvement of Anticorrosion Properties of Zinc-Rich Organic Coating by Incorporating Surface-Modified Zinc Particle. Prog. Org. Coat. 2012, 74, 25–35. [Google Scholar] [CrossRef]
- Huang, S.; Kong, G.; Yang, B.; Zhang, S.; Che, C. Effects of Graphene on the Corrosion Evolution of Zinc Particles in Waterborne Epoxy Zinc-Containing Coatings. Prog. Org. Coat. 2020, 140, 105531. [Google Scholar] [CrossRef]
- Scully, J.R. Electrochemical Impedance of Organic-Coated Steel: Correlation of Impedance Parameters with Long-Term Coating Deterioration. J. Electrochem. Soc. 1989, 136, 979–990. [Google Scholar] [CrossRef]
- Hack, H.P.; Scully, J.R. Defect Area Determination of Organic Coated Steels in Seawater Using the Breakpoint Frequency Method. J. Electrochem. Soc. 1991, 138, 233–238. [Google Scholar] [CrossRef]
- Scully, J.R.; Hensley, S.T. Lifetime Prediction for Organic Coatings on Steel and a Magnesium Alloy Using Electrochemical Impedance Methods. Corrosion 1994, 50, 705–716. [Google Scholar] [CrossRef]
- Tröltzsch, U.; Kanoun, O. Generalization of Transmission Line Models for Deriving the Impedance of Diffusion and Porous Media. Electrochim. Acta 2012, 75, 347–356. [Google Scholar] [CrossRef]
- Walter, G.W. A Critical Review of d.c. Electrochemical Tests for Painted Metals. Corros. Sci. 1986, 26, 39–47. [Google Scholar] [CrossRef]
- Bierwagen, G. Next Generation of Aircraft Coatings Systems. J. Coat. Technol. 2001, 73, 45–52. [Google Scholar] [CrossRef]
- Oliveira, C.G.; Ferreira, M.G.S. Ranking High-Quality Paint Systems Using EIS. Part I: Intact Coatings. Corros. Sci. 2003, 45, 123–138. [Google Scholar] [CrossRef]
- Gowri, S.; Balakrishnan, K. The Effect of the PVC/CPVC Ratio on the Corrosion Resistance Properties of Organic Coatings. Prog. Org. Coat. 1994, 23, 363–377. [Google Scholar] [CrossRef]
- Ding, R.; Zheng, Y.; Yu, H.; Li, W.; Wang, X.; Gui, T. Study of Water Permeation Dynamics and Anti-Corrosion Mechanism of Graphene/Zinc Coatings. J. Alloys Compd. 2018, 748, 481–495. [Google Scholar] [CrossRef]
- Hussain, A.K.; Seetharamaiah, N.; Pichumani, M.; Chakra, C.S. Research Progress in Organic Zinc Rich Primer Coatings for Cathodic Protection of Metals—A Comprehensive Review. Prog. Org. Coat 2021, 153, 106040. [Google Scholar] [CrossRef]
- Jiang, Q.; Miao, Q.; Liang, W.P.; Ying, F.; Tong, F.; Xu, Y.; Ren, B.L.; Yao, Z.J.; Zhang, P.Z. Corrosion Behavior of Arc Sprayed Al-Zn-Si-RE Coatings on Mild Steel in 3.5 Wt% NaCl Solution. Electrochim. Acta 2014, 115, 644–656. [Google Scholar] [CrossRef]
- Rosalbino, F.; Scavino, G.; Macciò, D.; Saccone, A. Influence of the Alloying Component on the Corrosion Behaviour of Zinc in Neutral Aerated Sodium Chloride Solution. Corros. Sci. 2014, 89, 286–294. [Google Scholar] [CrossRef]
- Misawa, T.; Hashimoto, K.; Shimodaira, S. The Mechanism of Formation of Iron Oxide and Oxyhydroxides in Aqueous Solutions at Room Temperature. Corros. Sci. 1974, 14, 131–149. [Google Scholar] [CrossRef]
- Poursaee, A.; Hansson, C.M. Reinforcing Steel Passivation in Mortar and Pore Solution. Cem. Concr. Res. 2007, 37, 1127–1133. [Google Scholar] [CrossRef]
- Li, W.; Brown, B.; Young, D.; Nešić, S. Investigation of Pseudo-Passivation of Mild Steel in CO2 Corrosion. Corrosion 2014, 70, 294–302. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, A.S.; Causse, N.; Musiani, M.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Determination of Water Uptake in Organic Coatings Deposited on 2024 Aluminium Alloy: Comparison between Impedance Measurements and Gravimetry. Prog. Org. Coat. 2017, 112, 93–100. [Google Scholar] [CrossRef]
- Bouvet, G.; Nguyen, D.D.; Mallarino, S.; Touzain, S. Analysis of the Non-Ideal Capacitive Behaviour for High Impedance Organic Coatings. Prog. Org. Coat. 2014, 77, 2045–2053. [Google Scholar] [CrossRef]
- Thomas, S.; Birbilis, N.; Venkatraman, M.S.; Cole, I.S. Self-Repairing Oxides to Protect Zinc: Review, Discussion and Prospects. Corros. Sci. 2013, 69, 11–22. [Google Scholar] [CrossRef]
- Mouanga, M.; Berçot, P.; Rauch, J.Y. Comparison of Corrosion Behaviour of Zinc in NaCl and in NaOH Solutions. Part I: Corrosion Layer Characterization. Corros. Sci. 2010, 52, 3984–3992. [Google Scholar] [CrossRef]
- Shi, H.; Liu, F.; Han, E.H. The Corrosion Behavior of Zinc-Rich Paints on Steel: Influence of Simulated Salts Deposition in an Offshore Atmosphere at the Steel/Paint Interface. Surf. Coat. Technol. 2011, 205, 4532–4539. [Google Scholar] [CrossRef]
- Shirehjini, F.T.; Danaee, I.; Eskandari, H.; Zarei, D. Effect of Nano Clay on Corrosion Protection of Zinc-Rich Epoxy Coatings on Steel 37. J. Mater. Sci. Technol. 2016, 32, 1152–1160. [Google Scholar] [CrossRef]
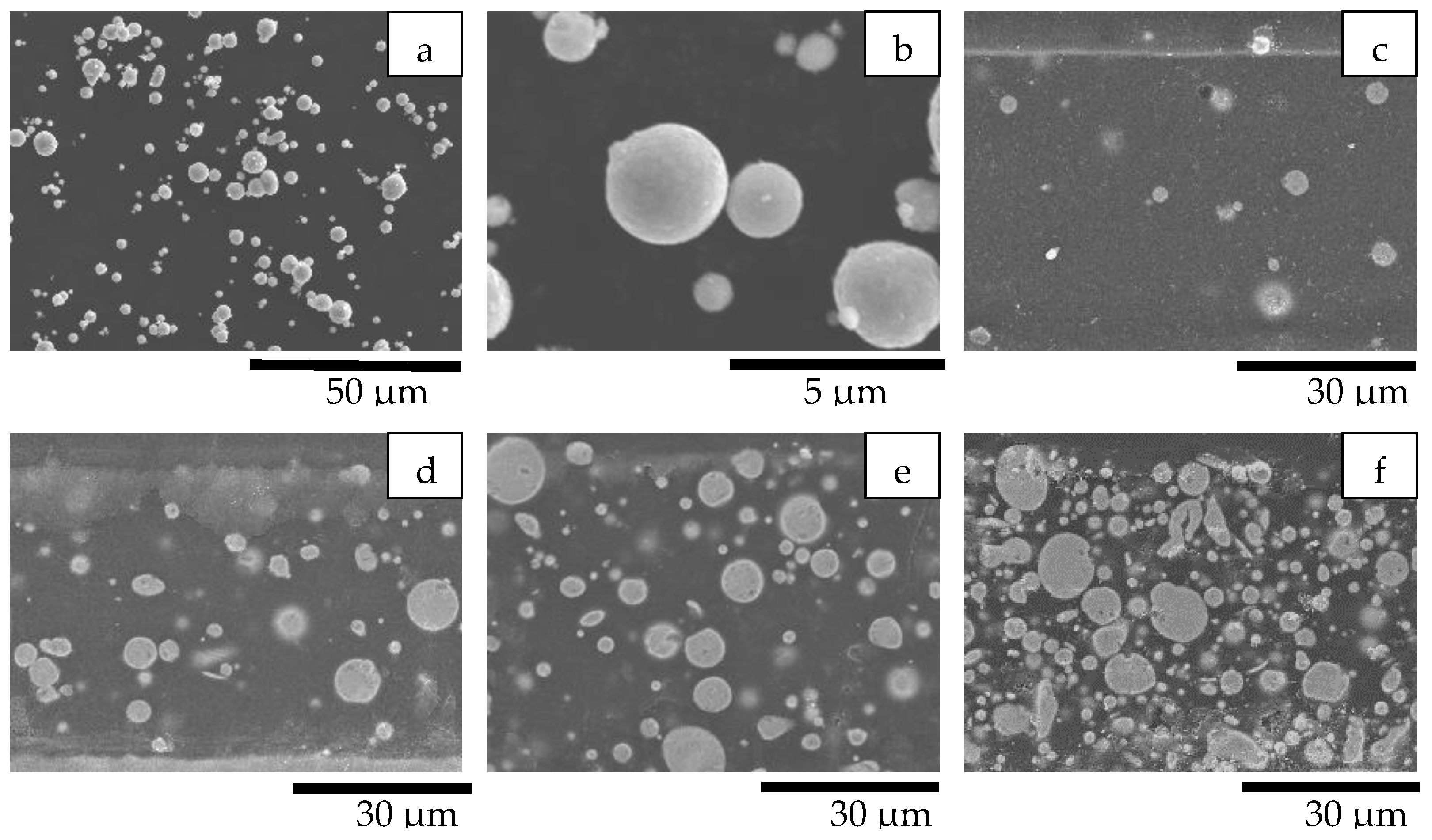
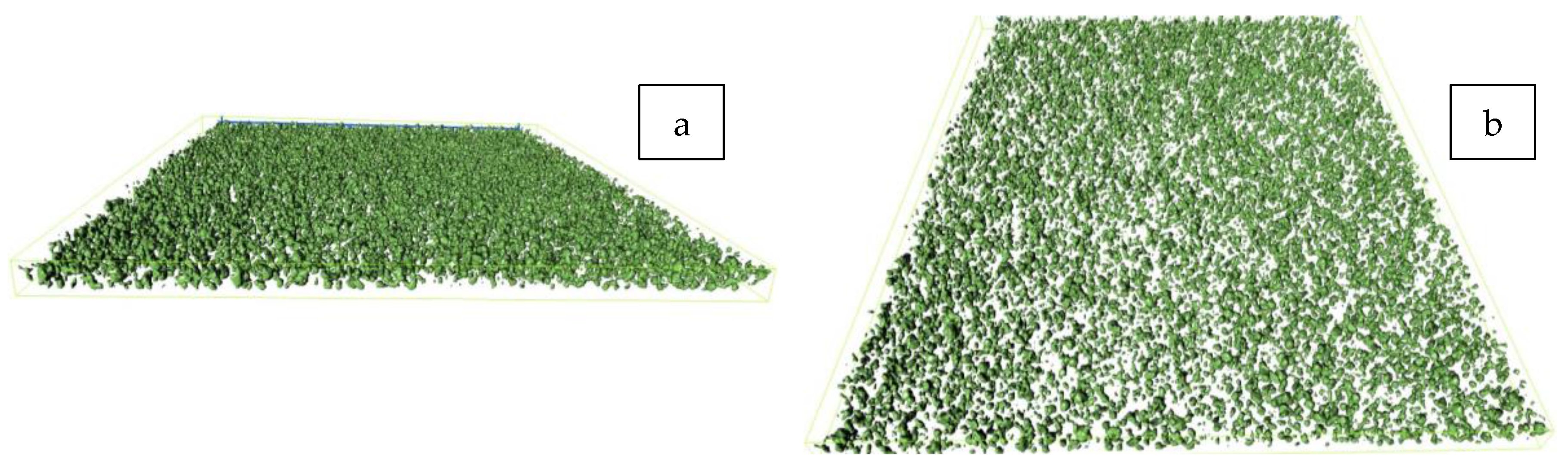
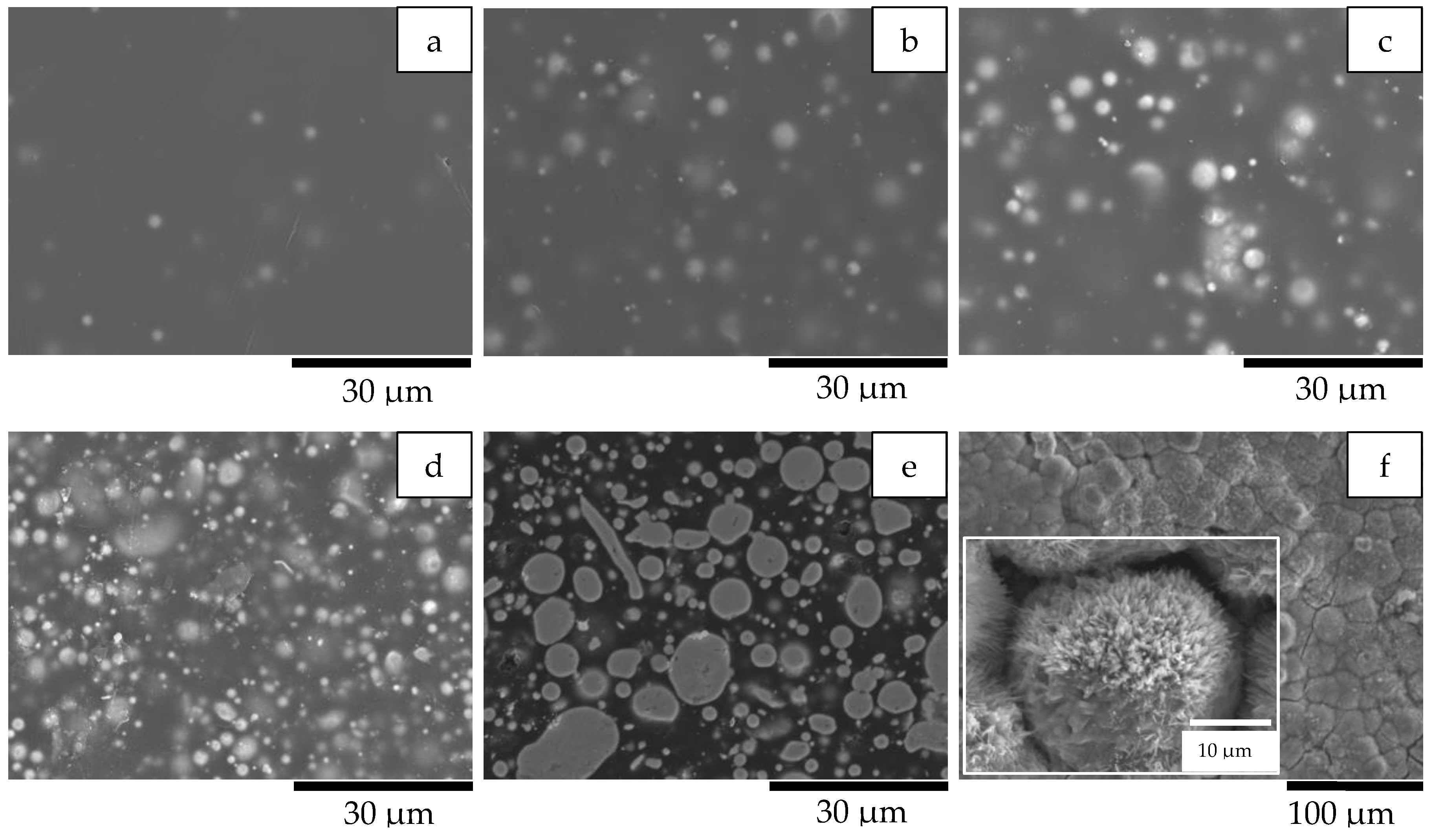
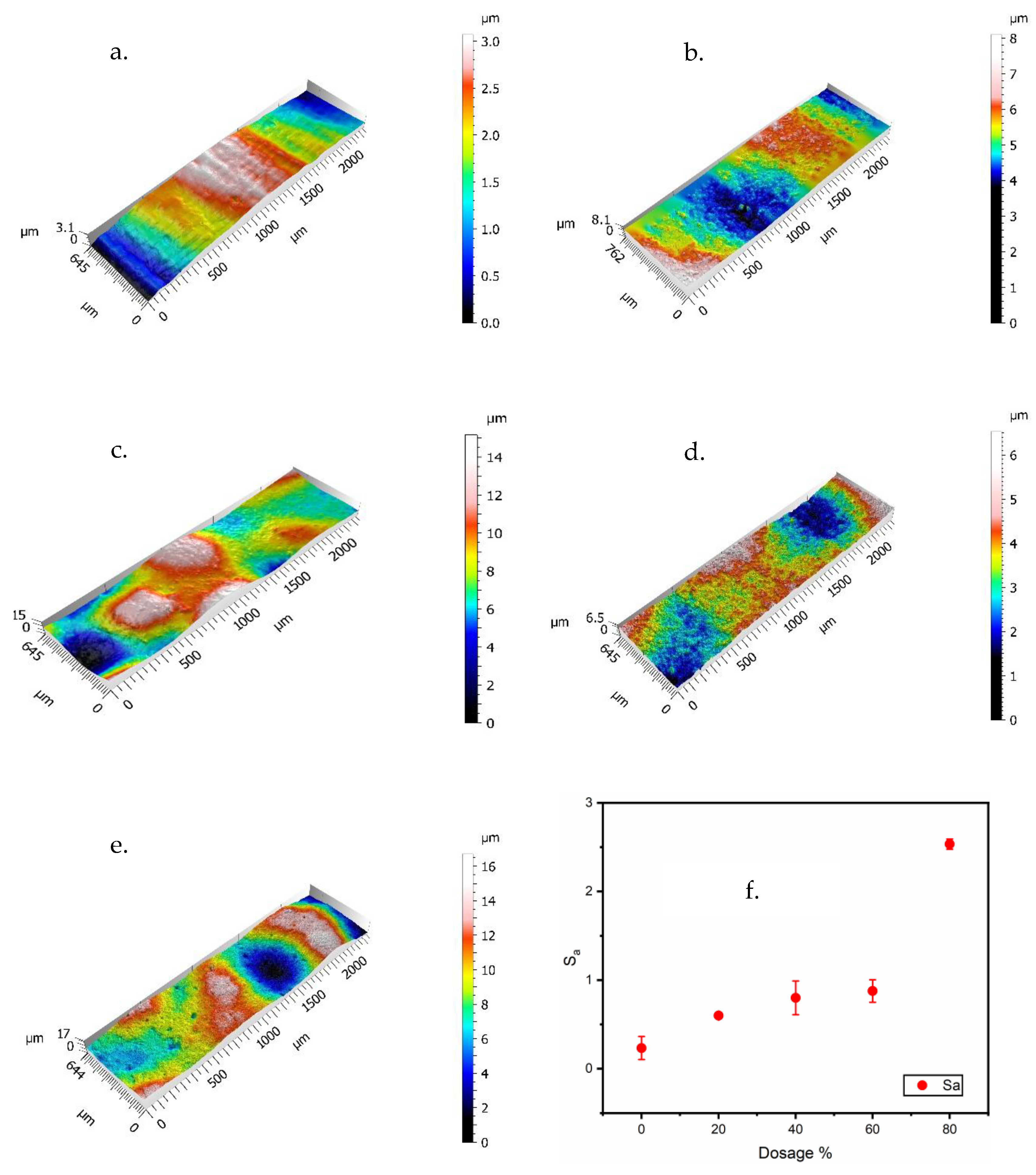


| Formula Code | Zinc Powder/wt% | Binder Content/wt% |
|---|---|---|
| Control-PC | 0.0 | 100.0 |
| Zn–20%-PC | 20.0 | 80.0 |
| Zn–40%-PC | 40.0 | 60.0 |
| Zn–60%-PC | 60.0 | 40.0 |
| Zn–80%-PC | 80.0 | 20.0 |
| Component | Composition | Content/wt% |
|---|---|---|
| Resin | carboxylated polyester | 90.8 |
| Curing agent | TGIC | 6.8 |
| Flow and leveling agent | polyacrylate | 1.6 |
| Degassing agent | benzoin | 0.8 |
| Measurements | Instruments | ASTM standards |
|---|---|---|
| Adhesion | Elcometer 107 cross hatch cutter (Elcometer Limited, Manchester, UK) | D3359–09 |
| Impact resistance | Elcometer 1615 variable impact tester (Elcometer Limited, Manchester, UK) | D2794–93 (Reapproved 2010) |
| Pencil hardness | BYK 5800 pencil hardness tester (BYK Gardner USA, Wallingford, CT, USA) | D3363–05 (Reapproved 2011) |
| Surface quality | Rhopoint IQ 20/60 gloss meter/goniophotometer (Rhopoint Instruments Ltd., St Leonards, UK) | Specular gloss, D523–14 distinctness-of-image (DOI), D5767–18 Reflection haze, D4039–09 (Reapproved 2015) |
| Neutral salt spray | MX-9204 (Associated Environmental Systems, Acton, MA, USA) | B117–16 D1654–08 |
| Degree of rusting | Visual inspection | D610–08 (Reapproved 2019) |
| Time | CPEcoat | Rpore | CPEdl, 1 | Rct, 1 | χ2 | ||
|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | |
| 0 | 5.73 × 10−10 | 0.958 | 1.39 × 105 | 1.39 × 10−8 | 0.649 | 1.89 × 108 | 3.66 × 10−3 |
| 1 | 2.49 × 10−9 | 0.847 | 1.54 × 105 | 3.24 × 10−8 | 0.767 | 3.55 × 107 | 3.53 × 10−3 |
| 4 | 5.35 × 10−8 | 0.970 | 5.45 × 105 | 1.71 × 10−7 | 0.476 | 2.31 × 107 | 4.15 × 10−4 |
| 8 | 4.87 × 10−10 | 0.966 | 1.86 × 105 | 7.97 × 10−7 | 0.344 | 1.61 × 107 | 2.31 × 10−4 |
| Time | CPEcoat | Rpore | CPEdl, 2 | Rct, 2 | Ws, 2, RD | Ws, 2, TD | Ws, 2, P | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | Ω∙cm2∙sP | s | ||
| 9 | 5.97 × 10−10 | 0.954 | 1.12 × 105 | 1.23× 10−6 | 0.320 | 1.39 × 107 | 3.93 × 109 | 6.28 × 103 | 0.977 | 1.45 × 10−4 |
| 12 | 1.16 × 10−9 | 0.941 | 7.48 × 102 | 1.01 × 10−6 | 0.368 | 9.24 × 104 | 2.24 × 106 | 3.43 × 104 | 0.412 | 1.21 × 10−4 |
| 15 | 9.87 × 10−9 | 0.880 | 8.87 × 102 | 8.52 × 10−6 | 0.260 | 1.10 × 105 | 2.07 × 106 | 1.79 × 105 | 0.468 | 1.14 × 10−4 |
| 17 | 2.85 × 10−8 | 0.851 | 1.23 × 103 | 5.81 × 10−6 | 0.439 | 1.52 × 105 | 4.18 × 105 | 6.13 × 104 | 0.482 | 1.43 × 10−4 |
| Time | CPEcoat | Rpore | CPEdl, 3 | Rct, 3 | χ2 | ||
|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | |
| 18 | 2.86 × 10−7 | 0.692 | 4.24 × 104 | 9.25 × 10−6 | 0.480 | 2.13 × 105 | 1.07 × 10−3 |
| 20 | 6.59 × 10−7 | 0.735 | 2.34 × 104 | 1.07 × 10−5 | 0.531 | 1.44 × 105 | 1.84 × 10−3 |
| 22 | 7.27 × 10−7 | 0.728 | 8.92 × 103 | 1.38 × 10−5 | 0.473 | 1.41 × 105 | 2.13 × 10−3 |
| 25 | 8.58 × 10−5 | 0.715 | 6.58 × 103 | 1.89 × 10−5 | 0.443 | 1.59 × 105 | 1.83 × 10−3 |
| 28 | 1.15 × 10−6 | 0.637 | 5.14 × 103 | 2.44 × 10−5 | 0.386 | 3.30 × 105 | 1.24 × 10−3 |
| Time | Rpore | CPE, 4 | Ws, 4, RD | Ws, 4, TD | Ws, 4, P | χ2 | |
|---|---|---|---|---|---|---|---|
| Days | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2∙sP | s | ||
| 29 | 2.53 × 103 | 1.82 × 10−8 | 0.656 | 1.50 × 105 | 4.88 × 103 | 0.324 | 7.90 × 10−4 |
| 33 | 1.63 × 103 | 6.29 × 10−7 | 0.679 | 1.12 × 105 | 6.54 × 103 | 0.340 | 8.18 × 10−4 |
| 37 | 2.01 × 102 | 1.82 × 10−8 | 1.000 | 1.15 × 105 | 8.49 × 103 | 0.337 | 7.96 × 10−4 |
| 41 | 8.58 × 101 | 1.85 × 10−8 | 1.000 | 1.38 × 105 | 9.90 × 103 | 0.345 | 7.29 × 10−4 |
| 45 | 2.71 × 102 | 5.19 × 10−8 | 0.908 | 1.50 × 105 | 1.08 × 104 | 0.347 | 8.29 × 10−4 |
| Time | CPEcoat | Rpore | CPEdl, 5 | Rct, 5 | Ws, 5, RD | Ws, 5, TD | Ws, 5, P | χ2 | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Days | Qcoat/ Ω−1∙cm−2∙sα | αcoat | Ω∙cm2 | Qdl/ Ω−1∙cm−2∙sα | αdl | Ω∙cm2 | Ω∙cm2∙sP | s | ||
| 46 | 2.46 × 10−5 | 0.357 | 7.86 × 101 | 7.61 × 10−9 | 1.000 | 5.72 × 104 | 4.69 × 105 | 2.11 × 103 | 0.64 | 4.24 × 10−4 |
| 47 | 2.45 × 10−5 | 0.355 | 7.39 × 101 | 7.37 × 10−9 | 1.000 | 5.44 × 104 | 5.28 × 105 | 2.57 × 103 | 0.64 | 3.71 × 10−4 |
| 48 | 2.38 × 10−5 | 0.354 | 7.98 × 101 | 6.77 × 10−9 | 1.000 | 4.35 × 104 | 3.65 × 105 | 2.66 × 103 | 0.62 | 3.52 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.S.; Huang, J.; Noël, J.J.; Chen, J.; Barker, I.; Henderson, J.D.; Zhang, H.; Zhang, H.; Zhu, J. A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings. Processes 2022, 10, 1853. https://doi.org/10.3390/pr10091853
Yang MS, Huang J, Noël JJ, Chen J, Barker I, Henderson JD, Zhang H, Zhang H, Zhu J. A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings. Processes. 2022; 10(9):1853. https://doi.org/10.3390/pr10091853
Chicago/Turabian StyleYang, Marshall Shuai, Jinbao Huang, James Joseph Noël, Jian Chen, Ivan Barker, Jeffrey Daniel Henderson, Hui Zhang, Haiping Zhang, and Jesse Zhu. 2022. "A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings" Processes 10, no. 9: 1853. https://doi.org/10.3390/pr10091853
APA StyleYang, M. S., Huang, J., Noël, J. J., Chen, J., Barker, I., Henderson, J. D., Zhang, H., Zhang, H., & Zhu, J. (2022). A Mechanistic Study on the Anti-Corrosive Performance of Zinc-Rich Polyester/TGIC Powder Coatings. Processes, 10(9), 1853. https://doi.org/10.3390/pr10091853












