An Effective Method for Working Fluid Design of Organic Rankine Cycle
Abstract
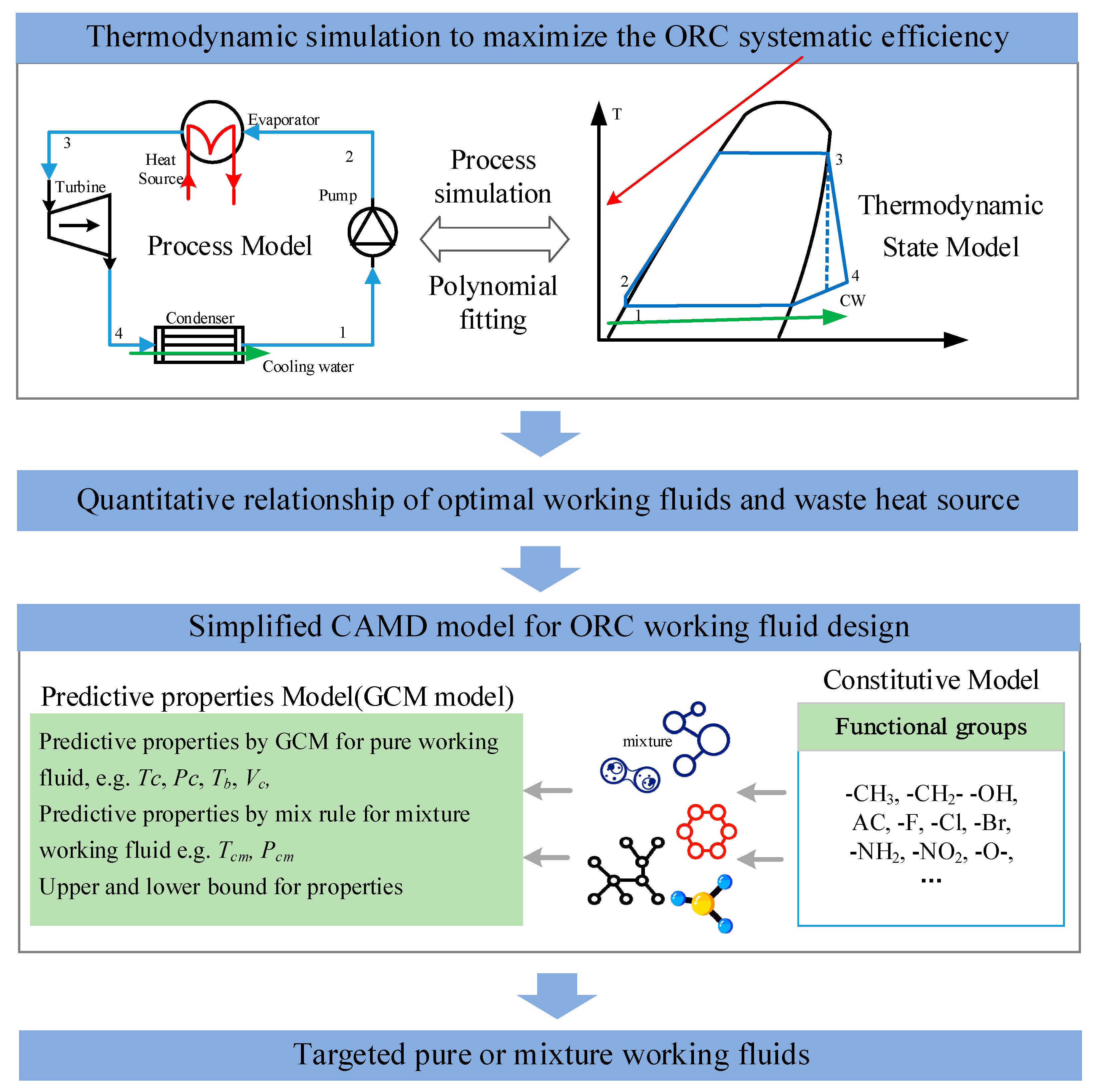
:1. Introduction
2. Design of Mixture Working Fluid for ORC
2.1. Quantitative Rules for the Selection of Pure Working Fluid
- Rule 1: The optimal working fluid satisfies the “double-pinch rules”.
- Rule 2: The optimal pure working fluid can be determined via a linear relation between the critical temperature of the working fluid and the inlet temperature of the heat source.where is the optimal critical temperature, TWH,in is the waste heat inlet temperature and a and b are constants. It is worthy of noting that this relation is simple and significantly useful for the selection and even design of the working fluids of ORC, because the complex ORC process and thermodynamic model can be omitted; the optimal critical temperature can be determined by only given waste heat inlet temperature [26,28]
- Rule 3: The working fluid with higher critical pressure is preferred when its critical temperatures is similar.
- Rule 4: The ratio of the critical temperature and critical pressure of the optimal working, Pc/Tc should not be more than one.
2.2. Simplified CAMD Model for the Design of Working Fluid
2.2.1. Objective Function
2.2.2. Constraints
3. Case Study
3.1. Fundamental Data
3.2. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pasinato, H.D. Working fluid dependence on source temperature for organic Rankine cycles. J. Energ. Resour. Technol. 2020, 142, 012103. [Google Scholar] [CrossRef]
- Chen, H.J.; Goswami, D.Y.; Stefanakos, E.K. A review of thermodynamic cycles and working fluids for the conversion of low-grade heat. Renew. Sust. Energ. Rev. 2010, 14, 3059–3067. [Google Scholar] [CrossRef]
- Deng, S.; Su, W.; Zhao, L. A neural network for predicting normal boiling point of pure refrigerants using molecular groups and a topological index. Int. J. Refrig. 2016, 63, 63–71. [Google Scholar] [CrossRef]
- Odele, O.; Macchietto, S. Computer aided molecular design: A novel method for optimal solvent selection. Fluid Phase Equilibr. 1993, 82, 47–54. [Google Scholar] [CrossRef]
- Linke, P.; Papadopoulos, A.I.; Seferlis, P. Systematic methods for working fluid selection and the design, integration and control of organic Rankine cycles—A review. Energies 2015, 8, 4755–4801. [Google Scholar] [CrossRef]
- Papadopoulos, A.I.; Stijepovic, M.; Linke, P. On the systematic design and selection of optimal working fluids for Organic Rankine Cycles. Appl. Therm. Eng. 2010, 30, 760–769. [Google Scholar] [CrossRef]
- Palma-Flores, O.; Flores-Tlacuahuac, A.; Canseco-Melchor, G. Optimal molecular design of working fluids for sustainable low-temperature energy recovery. Comput. Chem. Eng. 2015, 72, 334–349. [Google Scholar] [CrossRef]
- Palma-Flores, O.; Flores-Tlacuahuac, A.; Canseco-Melchorb, G. Simultaneous molecular and process design for waste heat recovery. Energy 2016, 99, 32–47. [Google Scholar] [CrossRef]
- Lampe, M.; Stavrou, M.; Bucker, H.; Gross, J.; Bardow, A. Simultaneous optimization of working fluid and process for organic Rankine cycles using PC-SAFT. Ind. Eng. Chem. Res. 2014, 53, 8821–8830. [Google Scholar] [CrossRef]
- Schilling, J.; Lampe, M.; Gross, J.; Bardow, A. 1-stage CoMT-CAMD: An approach for integrated design of ORC process and working fluid using PC-SAFT. Chem. Eng. Sci. 2016, 159, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Lampe, M.; Stavrou, M.; Schilling, J.; Sauer, E.; Gross, J.; Bardow, A. Computer-aided molecular design in the continuous-molecular targeting framework using group-contribution PC-SAFT. Comput. Chem. Eng. 2015, 81, 278–287. [Google Scholar] [CrossRef]
- White, M.T.; Oyewunmi, O.A.; Haslam, A.J.; Markides, C.N. Industrial waste-heat recovery through integrated computer-aided working-fluid and ORC system optimisation using SAFT-γ Mie. Energ. Convers. Manag. 2017, 150, 851–869. [Google Scholar] [CrossRef]
- Schilling, J.; Tillmanns, D.; Lampe, M.; Hopp, M.; Gross, J.; Bardow, A. From molecules to dollars: Integrating molecular design into thermo-economic process design using consistent thermodynamic modeling. Mol. Syst. Des. Eng. 2017, 2, 301–320. [Google Scholar] [CrossRef]
- Schilling, J.; Entrup, M.; Hopp, M.; Gross, J.; Bardow, A. Towards optimal mixtures of working fluids: Integrated design of processes and mixtures for Organic Rankine Cycles. Renew. Sust. Energ. Rev. 2021, 135, 110179. [Google Scholar] [CrossRef]
- van Kleef, L.M.; Oyewunmi, O.A.; Markides, C.N. Multi-objective thermo-economic optimization of organic Rankine cycle (ORC) power systems in waste-heat recovery applications using computer-aided molecular design techniques. Appl. Energy 2019, 251, 112513. [Google Scholar] [CrossRef]
- Luo, X.; Wang, Y.; Liang, J.; Qi, J.; Su, W.; Yang, Z.; Chen, J.; Wang, C.; Chen, Y. Improved correlations for working fluid properties prediction and their application in performance evaluation of sub-critical Organic Rankine Cycle. Energy 2019, 174, 122–137. [Google Scholar] [CrossRef]
- Karunanithi, A.T.; Achenie, L.E.K.; Gani, R. A New Decomposition-Based Computer-Aided Molecular/Mixture Design Methodology for the Design of Optimal Solvents and Solvent Mixtures. Ind. Eng. Chem. Res. 2005, 44, 4785–4797. [Google Scholar] [CrossRef]
- Papadopoulos, A.I.; Stijepovic, M.; Linke, P.; Seferlis, P.; Voutetakis, S. Toward Optimum Working Fluid Mixtures for Organic Rankine Cycles using Molecular Design and Sensitivity Analysis. Ind. Eng. Chem. Res. 2013, 52, 12116–12133. [Google Scholar] [CrossRef]
- Cignitti, S.; Mansouri, S.S.; Woodley, J.M.; Abildskov, J. Systematic Optimization-Based Integrated Chemical Product–Process Design Framework. Ind. Eng. Chem. Res. 2018, 57, 677–688. [Google Scholar] [CrossRef]
- Abadi, G.B.; Kim, K.C. Investigation of organic Rankine cycles with zeotropic mixtures as a working fluid: Advantages and issues. Renew. Sust. Energ. Rev. 2017, 73, 1000–1013. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, K.; Wang, M.; Xu, J. Thermodynamic selection criteria of zeotropic mixtures for subcritical organic Rankine cycle. Energy 2019, 167, 484–497. [Google Scholar] [CrossRef]
- Zhai, H.; An, Q.; Shi, L. Analysis of the quantitative correlation between the heat source temperature and the critical temperature of the optimal pure working fluid for subcritical organic Rankine cycles. Appl. Therm. Eng. 2016, 99, 383–391. [Google Scholar] [CrossRef]
- Aljundi, I.H. Effect of dry hydrocarbons and critical point temperature on the efficiencies of organic Rankine cycle. Renew. Energy 2011, 36, 1196–1202. [Google Scholar] [CrossRef]
- Vivian, J.; Manente, G.; Lazzaretto, A. A general framework to select working fluid and configuration of ORCs for low-to-medium temperature heat sources. Appl. Energy 2015, 156, 727–746. [Google Scholar] [CrossRef]
- Manente, G.; Lazzaretto, A.; Bonamico, E. Design guidelines for the choice between single and dual pressure layouts in organic Rankine cycle (ORC) systems. Energy 2017, 123, 413–431. [Google Scholar] [CrossRef]
- Li, J.; Ge, Z.; Duan, Y.; Yang, Z.; Liu, Q. Parametric optimization and thermodynamic performance comparison of single-pressure and dual-pressure evaporation organic Rankine cycles. Appl. Energy 2018, 217, 409–421. [Google Scholar] [CrossRef]
- Aspen Plus, version V12.1; Aspen Technology, Inc.: Bedford, MA, USA, 2006.
- Tang, J.; Kang, L.; Liu, Y. Automated configuration of organic Rankine cycle system based on process simulations. Energ. Convers. Manag. 2022, 253, 115186. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Liu, L.; Du, J.; Tula, A.K.; Eden, M.; Gani, R. OptCAMD: An optimization-based framework and tool for molecular and mixture product design. Comput. Chem. Eng. 2019, 124, 285–301. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Ning, P.; Cao, H.; Wen, H. Modified Structural Constraints for Candidate Molecule Generation in Computer-Aided Molecular Design. Ind. Eng. Chem. Res. 2018, 57, 6937–6946. [Google Scholar] [CrossRef]
- Lemmon, E.; Huber, M.L.; Mclinden, M.O. NIST Standard Reference Database 23: Reference Fluid Thermodynamic and Transport Properties-REFPROP, version 8.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2007. [Google Scholar]
- GAMS. A User’s Guide; GAMS Development Corporation: Washington, DC, USA, 2008. [Google Scholar]
- Su, W.; Zhao, L.; Deng, S. Developing a performance evaluation model of Organic Rankine Cycle for working fluids based on the group contribution method. Energ. Convers. Manag. 2017, 132, 307–315. [Google Scholar] [CrossRef]

| Properties | Units | Lower Bound | Upper Bound |
|---|---|---|---|
| Pc,i, Pcm | bar | 10 | 90 |
| Tc,i, Tcm | K | 330 | 900 |
| Tb,i | K | 250 | 500 |
| ni,k | / | 2 | 15 |
| TWH,in = 473.15 K, 453.15 K, 433.15 K, 413.15 K | |||
| a = 0.92917, b = 11.27806, σ ≥ 0.8 | |||
| Carbon Groups | Halogen Groups | Oxygen Groups | Nitrogen Groups | Sulfur Groups | Aromatic Groups |
|---|---|---|---|---|---|
| -CH3 | -F | -OH | -CH2NH2 | -CH2SH | ACH |
| -CH2- | -Cl | -CHO | >CHNH2 | CH3S- | AC |
| -CH< | -Br | CH3CO- | CH3NH- | -CH2S- | ACCH3 |
| >C< | -I | -CH2CO- | -CH2NH- | >CHS- | ACCH2 |
| -COO- | >CHNH- | ACCH | |||
| >CH-O- | CH3N< | ACOH | |||
| -CH2O- | -CH2N< | ACNH2 | |||
| CH3O- | -CH2NO2 | ACCl | |||
| -COOH | >CHNO2 | ACF |
| Scenario | TWH,in | Tcopt | Working Fluid | |||
|---|---|---|---|---|---|---|
| S1 | 473.15 | 447.7 | existing | R245ca (CHF2-CF2-CH2F) | ||
| designed | pure | COO(F)-CN(F4) | ||||
| mixture | 0.89154 | -CF2-CF2-CF2-CF(CH3)-(monocyclic) | ||||
| 0.10846 | (CH3)2-C(I)-CHClF | |||||
| S2 | 453.15 | 429.1 | existing | R245fa (CF3-CH2-CHF2) | ||
| designed | pure | CH3N-CH(F)-CH(F)-CH(F2) | ||||
| mixture | 0.20034 | CHNO2(Br)-CF2-CF2-CH2SH | ||||
| 0.79966 | CH3-CH2O-CF2-CF2-CF3 | |||||
| S3 | 433.15 | 410.5 | existing | R600A (CH(CH3)3) | ||
| designed | pure | CO(F3)-C(F2)OF | ||||
| mixture | 0.32451 | CH3-CF2-CHS(CH3)-CH3 | ||||
| 0.67549 | CH3-CH(CH3)-CF2-CF2-CHClF | |||||
| S4 | 413.15 | 392 | existing | RC318 (C4F10) | ||
| designed | pure | CH3-CF2-CF2Cl | ||||
| mixture | 0.89736 | -CH-O(CH3)-CF2-CF2-CF2-(monocyclic) | ||||
| 0.10264 | CHClF-CH2-CH(CHClF)-CH2SH | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Kang, L.; Liu, Y. An Effective Method for Working Fluid Design of Organic Rankine Cycle. Processes 2022, 10, 1857. https://doi.org/10.3390/pr10091857
Tang J, Kang L, Liu Y. An Effective Method for Working Fluid Design of Organic Rankine Cycle. Processes. 2022; 10(9):1857. https://doi.org/10.3390/pr10091857
Chicago/Turabian StyleTang, Jianping, Lixia Kang, and Yongzhong Liu. 2022. "An Effective Method for Working Fluid Design of Organic Rankine Cycle" Processes 10, no. 9: 1857. https://doi.org/10.3390/pr10091857
APA StyleTang, J., Kang, L., & Liu, Y. (2022). An Effective Method for Working Fluid Design of Organic Rankine Cycle. Processes, 10(9), 1857. https://doi.org/10.3390/pr10091857







