The Role of Non-Covalent Bonds in the Deformation Process of Coal: An Experimental Study on Bituminous Coal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
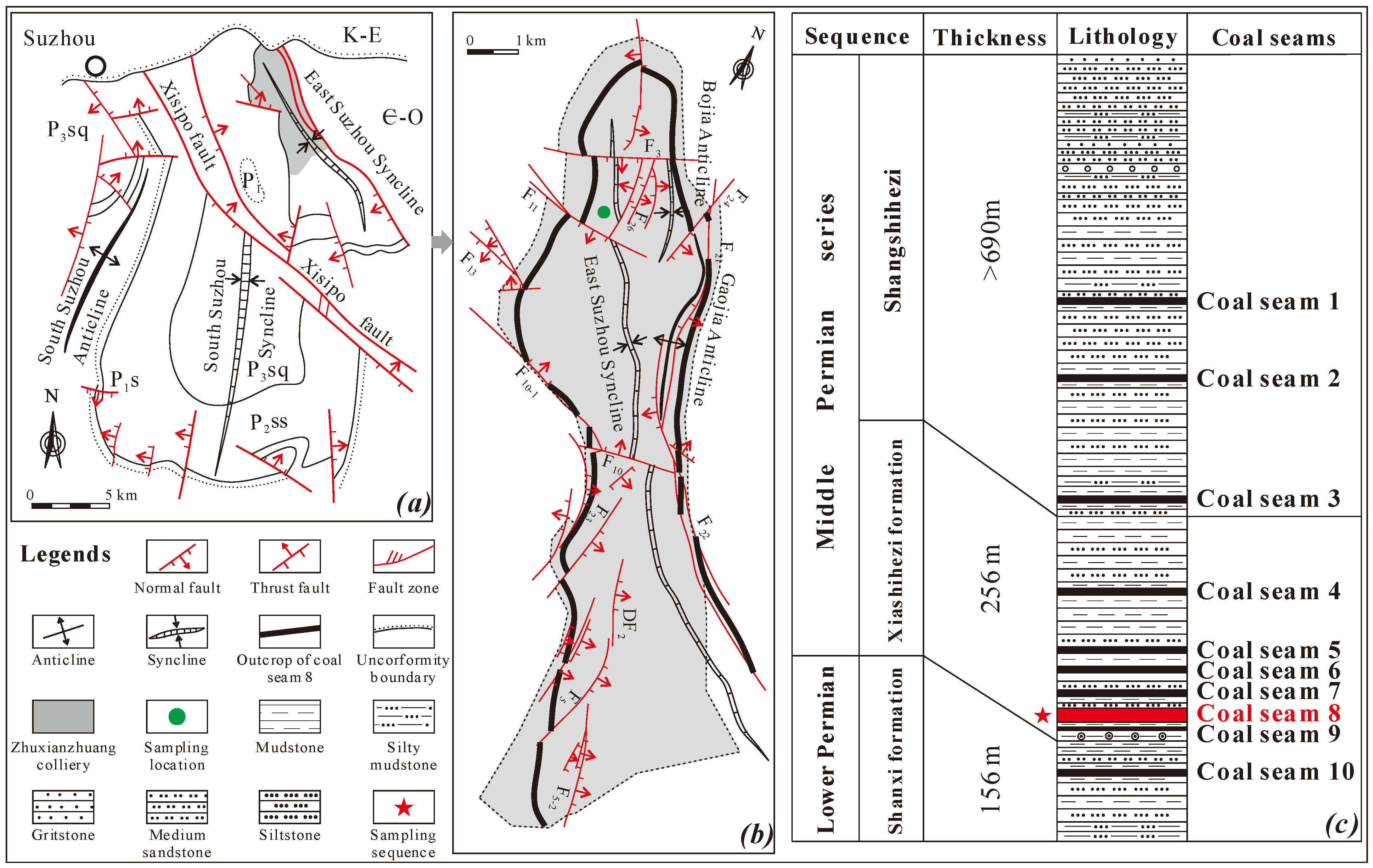
2.1.1. Geological Setting
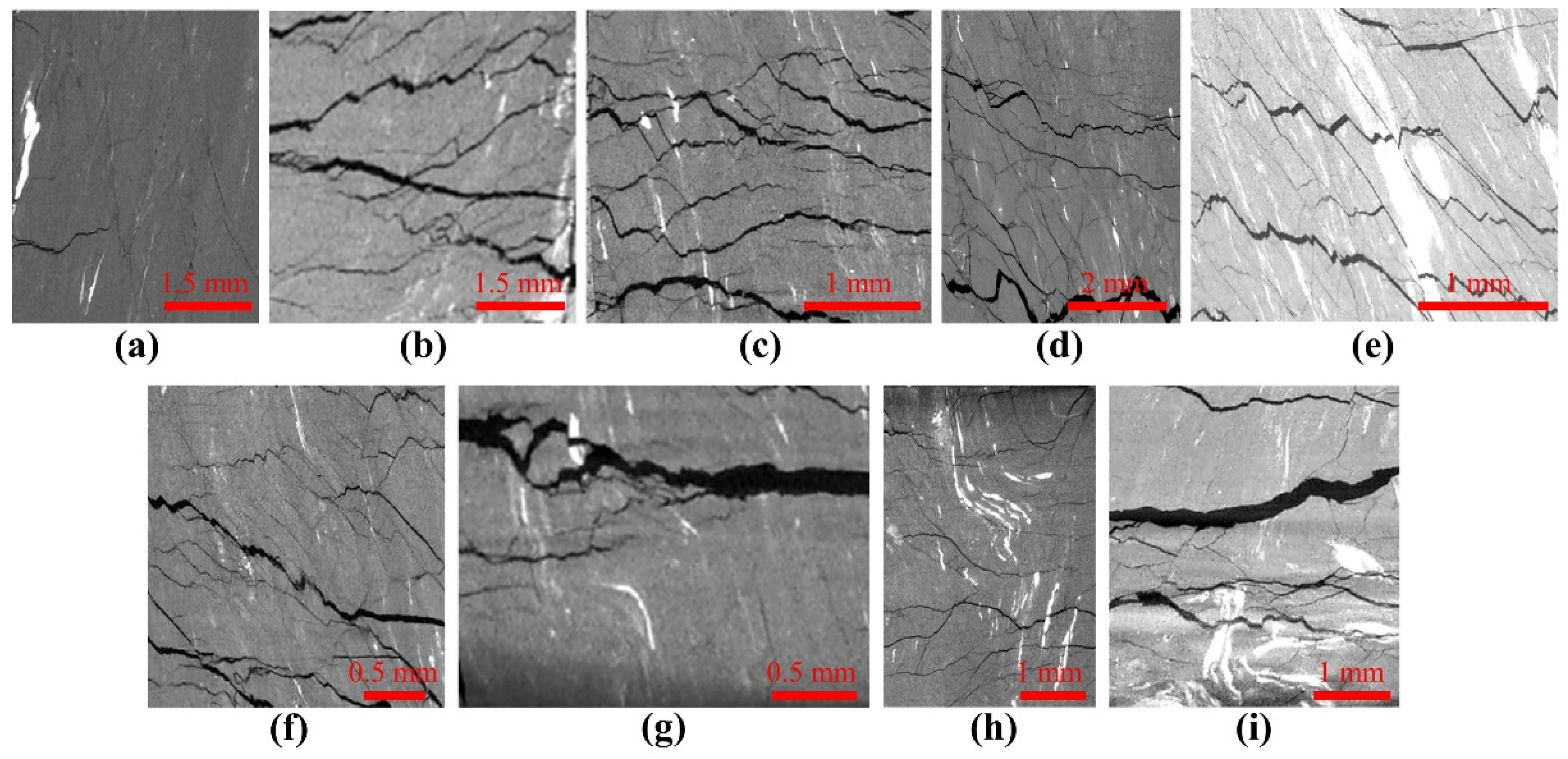
2.1.2. Samples Preparation
2.1.3. Characterization of Coal Properties
2.2. Methods
Simulative Experiment
3. Results and Discussions
3.1. Deformation Behavior of Samples
3.2. Evolution of HBs
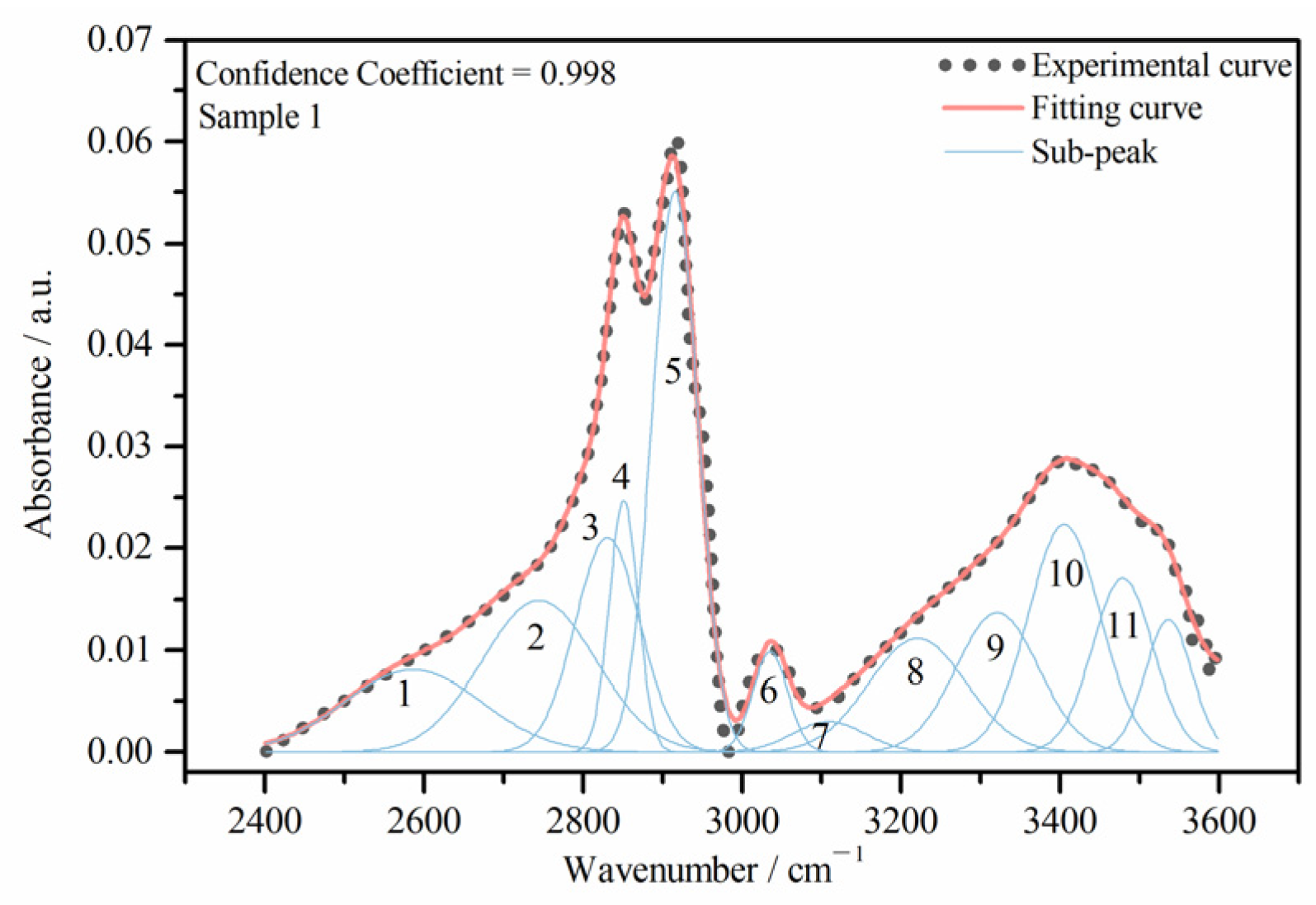
3.2.1. Calculation
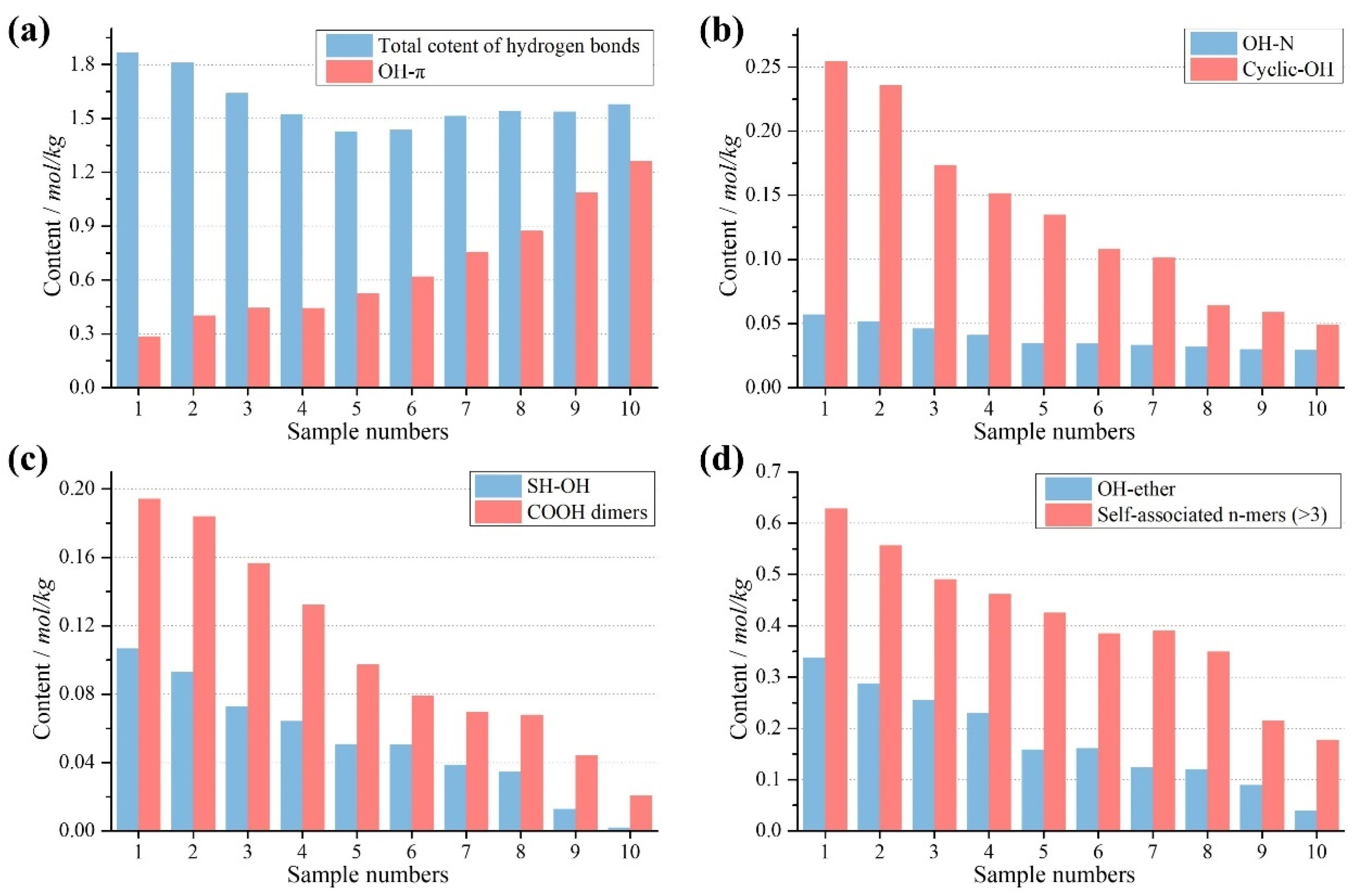
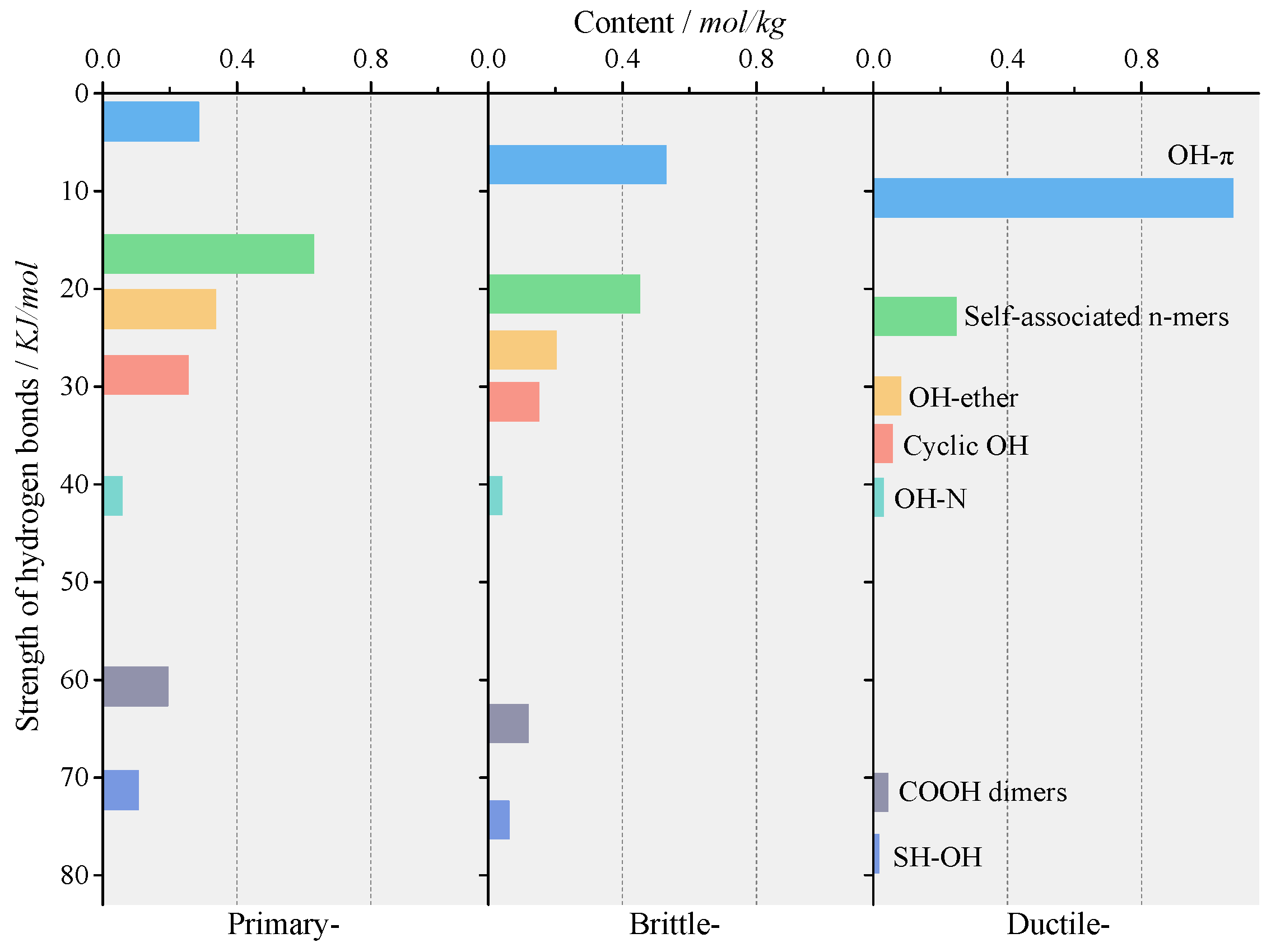
3.2.2. Content of HBs
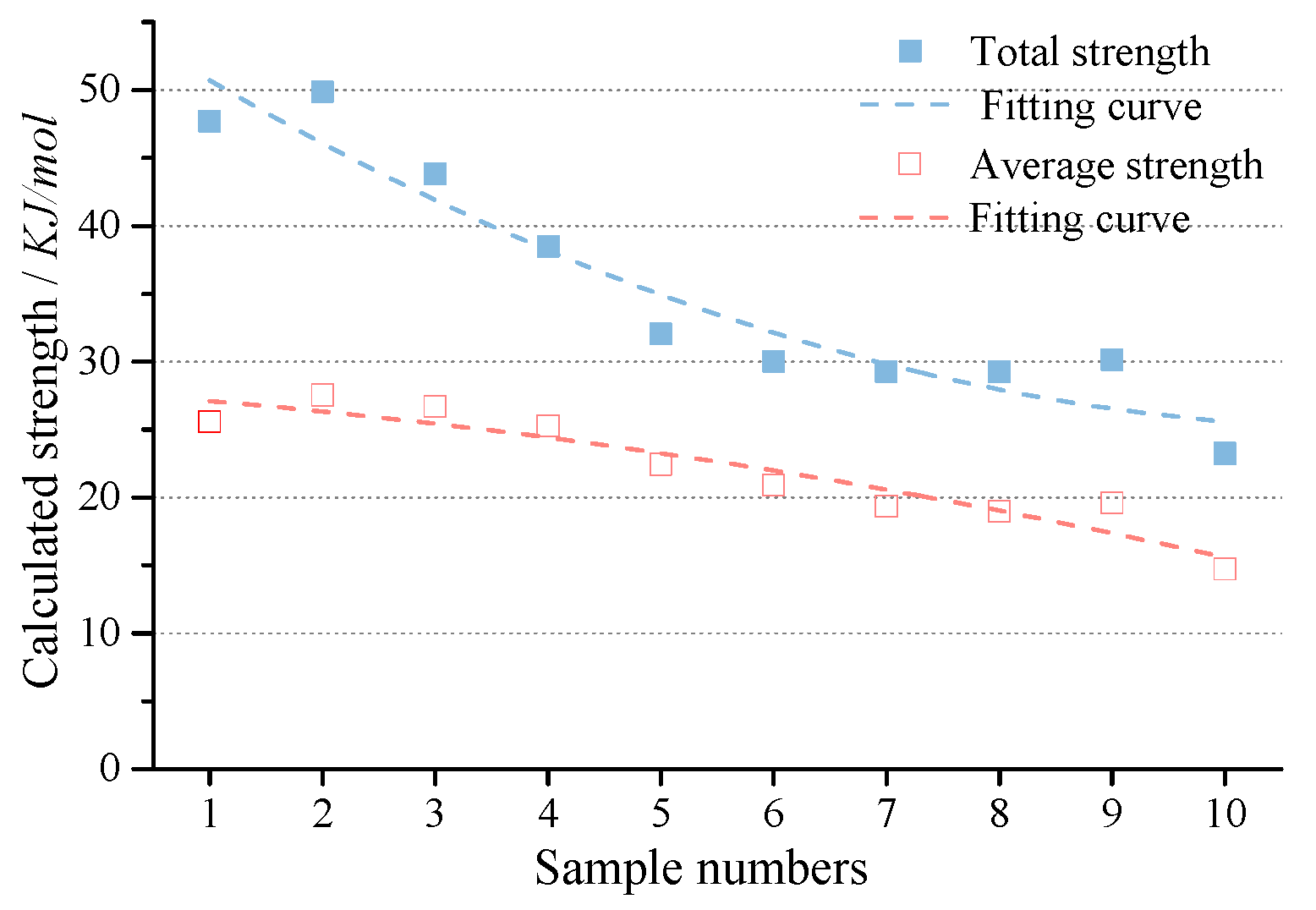
3.2.3. Strength of HBs
Distribution Patterns of HBs
Total and Average Enthalpy of HBs
3.3. Functional Groups and π−π Bonds
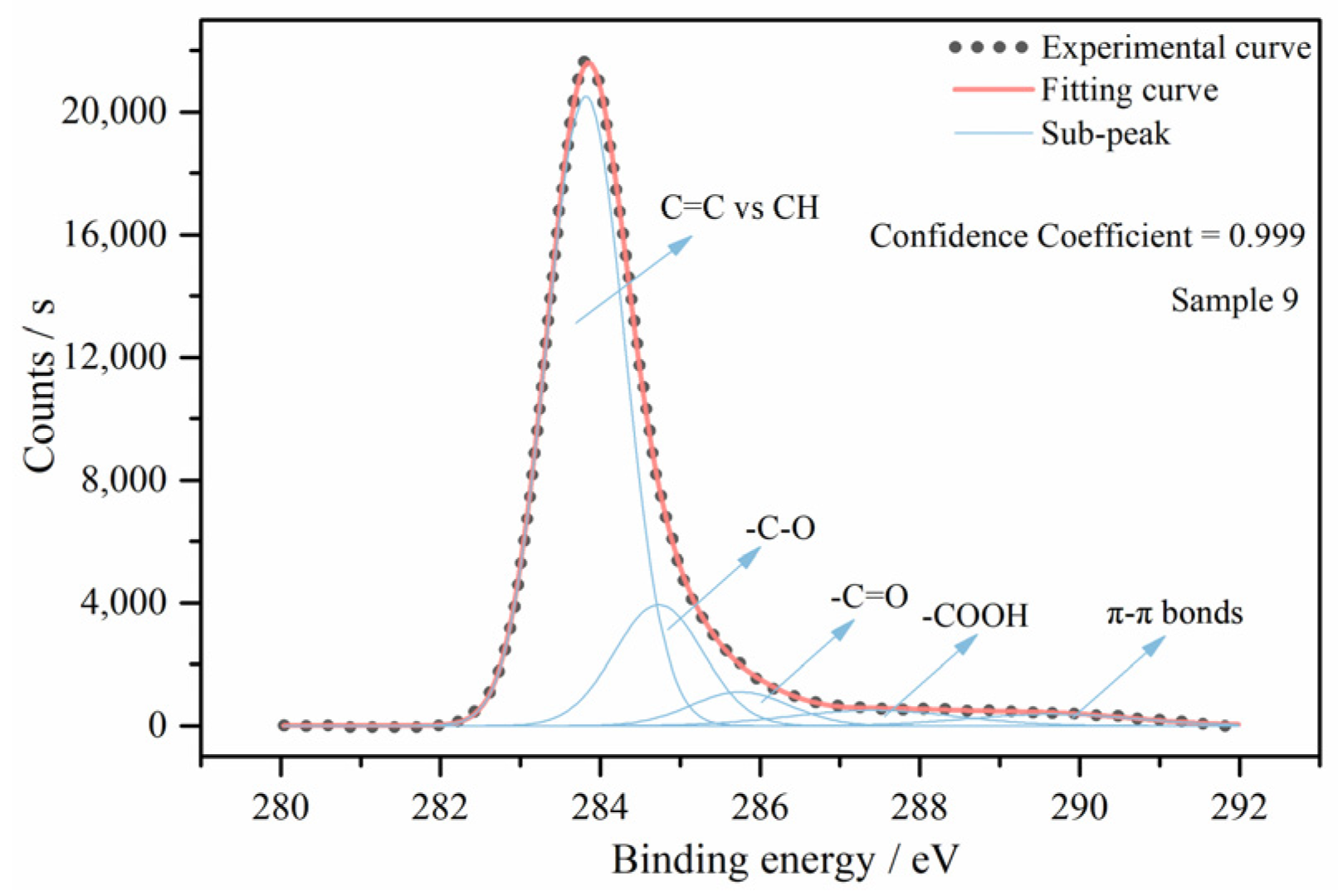
3.3.1. Curve Fitting of XPS Spectrums
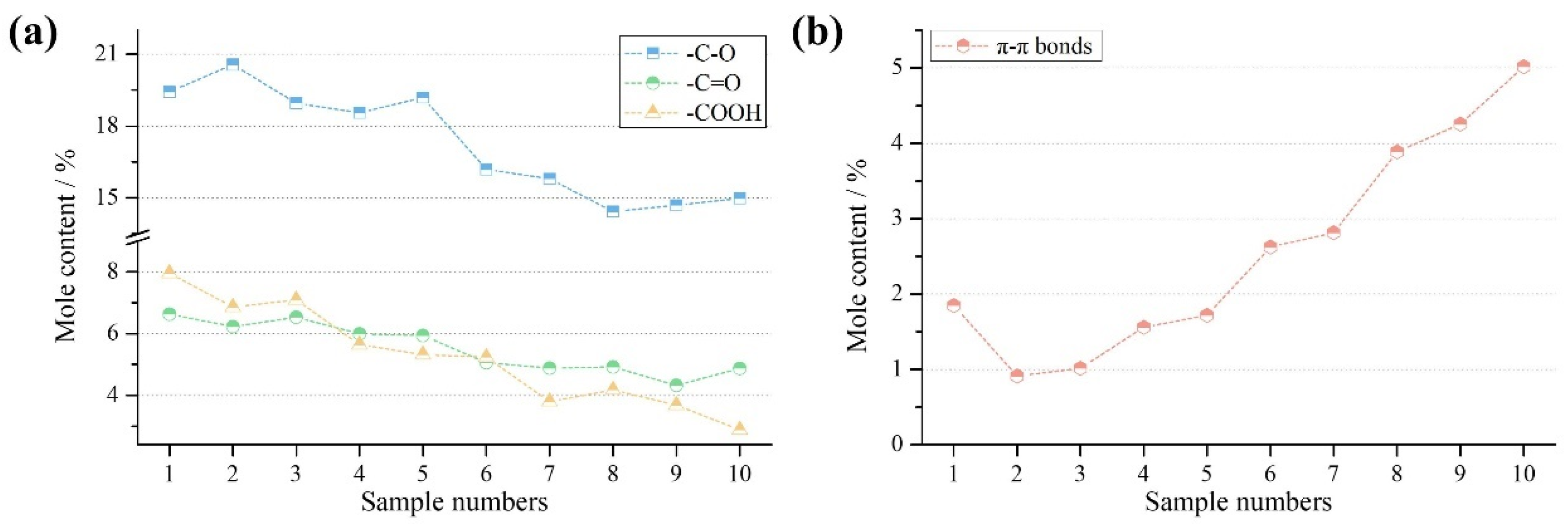
3.3.2. Variation of Functional Groups
3.3.3. Differentiation of π–π Bonds
3.4. Order Degree of Coal Molecular Structures
3.5. Evolutionary Patterns of Coal Network Structures
3.6. Understanding of Coal Deformation Mechanisms from the Nano-Scale Perspective
3.6.1. Brittleness and Plasticity of Coals
3.6.2. Transition Conditions from Brittle to Ductile Deformation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, B.; Qu, Z.; Wang, G.G.; Li, M. Effects of structural deformation on formation of coalbed methane reservoirs in Huaibei coalfield, China. Int. J. Coal Geol. 2010, 82, 175–183. [Google Scholar] [CrossRef]
- Hou, Q.; Li, H.; Fan, J.; Ju, Y.; Wang, T.; Li, X.; Wu, Y. Structure and coalbed methane occurrence in tectonically deformed coals. Sci. China Earth Sci. 2012, 55, 1755–1763. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, X.; Wei, C.; Zhang, P.; Zou, M.; Wang, B.; Quan, F.; Ju, W. Review on the Application of Low-Field Nuclear Magnetic Resonance Technology in Coalbed Methane Production Simulation. ACS Omega 2022, 7, 26298–26307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wei, C.; Ju, W.; Yan, G.; Lu, G.; Hou, X.; Kai, Z. Stress sensitivity characterization and heterogeneous variation of the pore-fracture system in middle-high rank coals reservoir based on NMR experiments. Fuel 2018, 238, 331–344. [Google Scholar] [CrossRef]
- Niu, Q.; Cao, L.; Sang, S.; Zhou, X.; Wang, Z.; Wu, Z. The adsorption-swelling and permeability characteristics of natural and reconstituted anthracite coals. Energy 2017, 141, 2206–2217. [Google Scholar] [CrossRef]
- Niu, Q.; Cao, L.; Sang, S.; Wang, W.; Zhou, X.; Yuan, W.; Ji, Z.; Chang, J.; Li, M. Experimental study on the softening effect and mechanism of anthracite with CO2 injection. Int. J. Rock Mech. Min. Sci. 2021, 138, 104614. [Google Scholar] [CrossRef]
- Niu, Q.; Pan, J.; Cao, L.; Ji, Z.; Wang, H.; Wang, K.; Wang, Z. The evolution and formation mechanisms of closed pores in coal. Fuel 2017, 200, 555–563. [Google Scholar] [CrossRef]
- Ju, Y.; Li, X. New research progress on the ultrastructure of tectonically deformed coals. Prog. Nat. Sci. 2009, 19, 1455–1466. [Google Scholar] [CrossRef]
- Yu, S.; Bo, J.; Fengli, L.; Jiegang, L. Structure and fractal characteristic of micro- and meso-pores in low, middle-rank tectonic deformed coals by CO2 and N2 adsorption. Microporous Mesoporous Mater. 2017, 253, 191–202. [Google Scholar] [CrossRef]
- Cao, Y.; Davis, A.; Liu, R.; Liu, X.; Zhang, Y. The influence of tectonic deformation on some geochemical properties of coals—A possible indicator of outburst potential. Int. J. Coal Geology 2003, 53, 69–79. [Google Scholar] [CrossRef]
- Hochella, M.F. Nanoscience and technology: The next revolution in the Earth sciences. Earth Planet. Sci. Lett. 2002, 203, 593–605. [Google Scholar] [CrossRef]
- Hochella, M.F.; Lower, S.K.; Maurice, P.A.; Penn, R.L.; Sahai, N.; Sparks, D.L.; Twining, B.S. Nanominerals, mineral nanoparticles, and earth systems. Science 2008, 319, 1631–1635. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xu, R.; Hou, Q.; Wang, J.; Pan, J. Deformation mechanisms and macromolecular structure response of anthracite under different stress. Energy Fuels 2016. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, B.; Liu, J.; Song, Y. The evolutionary characteristics and mechanisms of coal chemical structure in micro deformed domains under sub-high temperatures and high pressures. Fuel 2018, 222, 258–268. [Google Scholar] [CrossRef]
- Pan, J.; Wang, S.; Ju, Y.; Hou, Q.; Niu, Q.; Wang, K.; Li, M.; Shi, X. Quantitative study of the macromolecular structures of tectonically deformed coal using high-resolution transmission electron microscopy. J. Nat. Gas Sci. Eng. 2015, 27, 1852–1862. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, Y.; Hou, Q.; Jin, Y. Nanoscale pores in coal related to coal rank and deformation structures. Transp. Porous Media 2015, 107, 543–554. [Google Scholar] [CrossRef]
- Zhang, J.; Wei, C.; Zhao, C.; Zhang, T.; Lu, G.; Zou, M. Effects of nano-pore and macromolecule structure of coal samples on energy parameters variation during methane adsorption under different temperature and pressure. Fuel 2021, 289, 119804. [Google Scholar] [CrossRef]
- Liu, Y.; Zhu, Y.; Li, W.; Zhang, C.; Wang, Y. Ultra micropores in macromolecular structure of subbituminous coal vitrinite. Fuel 2017, 210, 298–306. [Google Scholar] [CrossRef]
- Pan, J.; Zhu, H.; Hou, Q.; Wang, H.; Wang, S. Macromolecular and pore structures of Chinese tectonically deformed coal studied by atomic force microscopy. Fuel 2015, 139, 94–101. [Google Scholar] [CrossRef]
- Cao, Y.; Mitchell, G.D.; Davis, A.; Wang, D. Deformation metamorphism of bituminous and anthracite coals from China. Int. J. Coal Geol. 2000, 43, 227–242. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Guo, C.; Hou, Q. The mechanisms of gas generation during coal deformation: Preliminary observations. Fuel 2014, 117, 326–330. [Google Scholar] [CrossRef]
- Hou, Q.; Han, Y.; Wang, J.; Dong, Y.; Pan, J. The impacts of stress on the chemical structure of coals: A mini-review based on the recent development of mechanochemistry. Sci. Bull. 2017, 62, 965–970. [Google Scholar] [CrossRef]
- Cao, D.; Li, X.; Zhang, S. Influence of tectonic stress on coalification: Stress degradation mechanism and stress polycondensation mechanism. Sci. China Ser. D Earth Sci. 2007, 50, 43–54. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Han, Y. Macromolecular response to tectonic deformation in low-rank tectonically deformed coals (TDCs). Fuel 2018, 219, 279–287. [Google Scholar] [CrossRef]
- Li, X.; Ju, Y.; Hou, Q.; Lin, H. Spectra response from macromolecular structure evolution of tectonically deformed coal of different deformation mechanisms. Sci. China Earth Sci. 2012, 55, 1269–1279. [Google Scholar] [CrossRef]
- Liu, X.; Song, D.; He, X.; Nie, B.; Wang, L. Insight into the macromolecular structural differences between hard coal and deformed soft coal. Fuel 2019, 245, 188–197. [Google Scholar] [CrossRef]
- Wang, J.; Guo, G.-J.; Han, Y.; Hou, Q.; Geng, M.; Zhang, Z. Mechanolysis mechanisms of the fused aromatic rings of anthracite coal under shear stress. Fuel 2019, 253, 1247–1255. [Google Scholar] [CrossRef]
- Han, Y.; Wang, J.; Dong, Y.; Hou, Q.; Pan, J. The role of structure defects in the deformation of anthracite and their influence on the macromolecular structure. Fuel 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, B.; Qu, M. Macromolecular evolution and structural defects in tectonically deformed coals. Fuel 2019, 236, 1432–1445. [Google Scholar] [CrossRef]
- Ross, J.V.; Bustin, R.M. The role of strain energy in creep graphitization of anthracite. Nature 1990, 343, 58–60. [Google Scholar] [CrossRef]
- Bustin, R.; Rouzaud, J.-N.; Ross, J. Natural graphitization of anthracite: Experimental considerations. Carbon 1995, 33, 679–691. [Google Scholar] [CrossRef]
- Ross, J.; Bustin, R. Vitrinite anisotropy resulting from simple shear experiments at high temperature and high confining pressure. Int. J. Coal Geol. 1997, 33, 153–168. [Google Scholar] [CrossRef]
- Kitamura, M.; Mukoyoshi, H.; Fulton, P.M.; Hirose, T. Coal maturation by frictional heat during rapid fault slip. Geophys. Res. Lett. 2012, 39, 1–5. [Google Scholar] [CrossRef]
- Ma, S.; Shimamoto, T.; Yao, L.; Togo, T.; Kitajima, H. A rotary-shear low to high-velocity friction apparatus in Beijing to study rock friction at plate to seismic slip rates. Earthq. Sci. 2014, 27, 469–497. [Google Scholar] [CrossRef]
- Xu, R.; Li, H.; Hou, Q.; Li, X.; Yu, L. The effect of different deformation mechanisms on the chemical structure of anthracite coals. Sci. China Earth Sci. 2015, 58, 502–509. [Google Scholar] [CrossRef]
- Li, W.; Jiang, B.; Moore, T.; Wang, G.; Liu, J.-G.; Song, Y. Characterization of the chemical structure of tectonically deformed coals. Energy Fuels 2017, 31, 6977–6985. [Google Scholar] [CrossRef]
- Shinn, J.H. From coal to single-stage and two-stage products: A reactive model of coal structure. Fuel 1984, 63, 1187–1196. [Google Scholar] [CrossRef]
- Given, P.H.; Marzec, A.; Barton, W.A.; Lynch, L.J.; Gerstein, B.C. The concept of a mobile or molecular phase within the macromolecular network of coals: A debate. Fuel 1986, 65, 155–163. [Google Scholar] [CrossRef]
- Derbyshire, F.; Marzec, A.; Schulten, H.-R.; Wilson, M.A.; Davis, A.; Tekely, P.; Delpuech, J.-J.; Jurkiewicz, A.; Bronnimann, C.E.; Wind, R.A.; et al. Molecular structure of coals: A debate. Fuel 1989, 68, 1091–1106. [Google Scholar] [CrossRef]
- Vasireddy, S.; Morreale, B.; Cugini, A.; Song, C.; Spivey, J.J. Clean liquid fuels from direct coal liquefaction: Chemistry, catalysis, technological status and challenges. Energy Environ. Sci. 2011, 4, 311–345. [Google Scholar] [CrossRef]
- Nishioka, M. The associated molecular nature of bituminous coal. Fuel 1992, 71, 941–948. [Google Scholar] [CrossRef]
- Van Niekerk, D.; Mathews, J.P. Molecular representations of Permian-aged vitrinite-rich and inertinite-rich South African coals. Fuel 2010, 89, 73–82. [Google Scholar] [CrossRef]
- Qin, K.; Guo, S.; Li, S. New concept on coal structure and new consideration for the generation mechanism of oil from coal. Chin. Sci. Bull. 1998, 43, 2025–2035. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Li, B. Hydrogen bonds in coal. The influence of coal rank and the recognition of a new hydrogen bond in coal. Fuel Energy Abstr. 2004, 45, 164. [Google Scholar]
- Van Niekerk, D.; Halleck, P.M.; Mathews, J.P. Solvent swelling behavior of Permian-aged South African vitrinite-rich and inertinite-rich coals. Fuel 2010, 89, 19–25. [Google Scholar] [CrossRef]
- Nishioka, M.; Larsen, J.W. Association of aromatic structures in coals. Energy Fuels 1990, 4, 100–106. [Google Scholar] [CrossRef]
- Marzec, A. Towards an understanding of the coal structure: A review. Fuel Process. Technol. 2002, 77, 25–32. [Google Scholar] [CrossRef]
- Quinga, E.M.Y.; Larsen, J.W. Noncovalent interactions in high-rank coals. Energy Fuels 1987, 1, 300–304. [Google Scholar] [CrossRef]
- Nishioka, M. The associative nature of lower rank coal. Fuel 1993, 72, 1725–1731. [Google Scholar] [CrossRef]
- Brenner, D. The macromolecular nature of bituminous coal. Fuel 1985, 64, 167–173. [Google Scholar] [CrossRef]
- Larsen, J.W.; Baskar, A.J. Hydrogen bonds from a subbituminous coal to sorbed solvents. An infrared study. Energy Fuels 1987, 1, 230–232. [Google Scholar] [CrossRef]
- Krzesińska, M. Averaged structural units in bituminous coals studied by means of ultrasonic wave velocity measurements. Energy Fuels 2001, 15, 930–935. [Google Scholar] [CrossRef]
- Krzesińska, M.; Pilawa, B.; Pusz, S. The physical parameters of different rank coals related to their degree of cross-linking and the caking ability. Energy Fuels 2006, 20, 1103–1110. [Google Scholar] [CrossRef]
- Larsen, J.W.; Green, T.K.; Kovac, J. The nature of the macromolecular network structure of bituminous coals. J. Org. Chem. 1985, 50, 4729–4735. [Google Scholar] [CrossRef]
- Nishioka, M. Molecular mobility of high-volatile bituminous coals accompanying drying. Fuel 1994, 73, 57–62. [Google Scholar] [CrossRef]
- Shui, H.; Wang, Z.; Cao, M. Effect of pre-swelling of coal on its solvent extraction and liquefaction properties. Fuel 2008, 87, 2908–2913. [Google Scholar] [CrossRef]
- Hu, H.; Sha, G.; Chen, G. Effect of solvent swelling on liquefaction of Xinglong coal at less severe conditions. Fuel Process. Technol. 2000, 68, 33–43. [Google Scholar] [CrossRef]
- Diaz, M.C.; Duffy, J.J.; Snape, C.; Steel, K.M. Use of high-temperature, high-torque rheometry to study the viscoelastic properties of coal during carbonization. J. Rheol. 2007, 51, 895. [Google Scholar] [CrossRef]
- Nissan, A.H. Three modes of dissociation of H bonds in hydrogen-bond dominated solids. Nature 1976, 263, 759. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, B. Stress response of noncovalent bonds in molecular networks of tectonically deformed coals. Fuel 2019, 255, 115785. [Google Scholar] [CrossRef]
- Li, C.-W.; Wang, J.-G.; Xie, B.-J.; Dong, L.-H.; Sun, Y.-F.; Cao, X. FTIR study of hydrogen bonds in coal under drop weight impact testing. Spectrosc. Spectr. Anal. 2014, 34, 2961–2967. [Google Scholar]
- Engelund, E.T.; Salmén, L. Tensile creep and recovery of Norway spruce influenced by temperature and moisture. Holzforschung 2012, 66, 959–965. [Google Scholar] [CrossRef]
- Chen, M.; Runge, T.; Wang, L.; Li, R.; Feng, J.; Shu, X.-L.; Shi, Q.-S. Hydrogen bonding impact on chitosan plasticization. Carbohydr. Polym. 2018, 200, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-L. Sulfur in coals: A review of geochemistry and origins. Int. J. Coal Geol. 2012, 100, 1–13. [Google Scholar] [CrossRef]
- Miura, K.; Mae, K.; Li, W.; Kusakawa, T.; Morozumi, A.F.; Kumano, A. Estimation of hydrogen bond distribution in coal through the analysis of oh stretching bands in diffuse reflectance infrared spectrum measured by in-Situ technique. Energy Fuels 2001, 15, 599–610. [Google Scholar] [CrossRef]
- Chen, C.; Gao, J.; Yan, Y. Observation of the type of hydrogen bonds in coal by ftir. Energy Fuels 1998, 12, 446–449. [Google Scholar] [CrossRef]
- Solomon, P.R.; Carangelo, R.M. FTIR analaysis of coal. 1. techniques and determination of hydroxyl concentrations. Fuel 1982, 61, 663–669. [Google Scholar] [CrossRef]
- Perry, D.L.; Grint, A. Application of XPS to coal characterization. Fuel 1983, 62, 1024–1033. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B.; Liang, H.; Gao, Y.; Bi, Q.; Qu, B. Effects of igneous intrusions on the structure and spontaneous combustion propensity of coal: A case study of bituminous coal in Daxing Mine, China. Fuel 2018, 216, 181–189. [Google Scholar] [CrossRef]
- Pan, J.; Lv, M.; Bai, H.; Hou, Q.; Li, M.; Wang, Z. Effects of metamorphism and deformation on the coal macromolecular structure by laser raman spectroscopy. Energy Fuels 2017, 31, 1136–1146. [Google Scholar] [CrossRef]
- Zhang, Z.; Kang, Q.; Wei, S.; Yun, T.; Yan, G.; Yan, K. Large scale molecular model construction of xishan bituminous coal. Energy Fuels 2017, 31, 1310–1317. [Google Scholar] [CrossRef]
- Hou, C.; Jiang, B.; Li, M.; Song, Y.; Cheng, G. Micro-deformation and fracture evolution of in-situ coal affected by temperature, confining pressure, and differential stress. J. Nat. Gas Sci. Eng. 2022, 100, 104455. [Google Scholar] [CrossRef]
- Liu, J.; Guang, Y.; Rui, M. Macro- and microscopic mechanical behaviour of flow of coal samples experimentally deformed at high temperatures and pressures. Chin. Sci. Bull. 2005, 50, 59–66. [Google Scholar] [CrossRef]
- Jiang, B.; Ju, Y. Tectonic coal structure and its petro-physical features. Nat. Gas Ind. 2004, 24, 27–29, (In Chinese with English Abstract). [Google Scholar]
- Yu, S.; Bo, J.; Jie-Gang, L. Nanopore structural characteristics and their impact on methane adsorption and diffusion in low to medium tectonically deformed coals: Case study in the huaibei coal field. Energy Fuels 2017, 31, 6711–6723. [Google Scholar] [CrossRef]
- Li, H. Major and minor structural features of a bedding shear zone along a coal seam and related gas outburst, Pingdingshan coalfield, northern China. Int. J. Coal Geol. 2001, 47, 101–113. [Google Scholar] [CrossRef]
- Li, H.; Ogawa, Y.; Shimada, S. Mechanism of methane flow through sheared coals and its role on methane recovery. Fuel 2003, 82, 1271–1279. [Google Scholar] [CrossRef]
- Painter, P.; Sobkowiak, M.; Youtcheff, J. FT-i.r. Study of hydrogen bonding in coal. Fuel 1987, 66, 973–978. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.-Q.; Bai, J.; Guo, Z.-X. Decomposition kinetics of hydrogen bonds in coal by a new method of in-situ diffuse reflectance FT-IR. J. Fuel Chem. Technol. 2011, 39, 321–327. [Google Scholar] [CrossRef]
- Buckingham, A.; Del Bene, J.; McDowell, S. The hydrogen bond. Chem. Phys. Lett. 2008, 463, 1–10. [Google Scholar] [CrossRef]
- Li, D.; Li, W.; Chen, H.; Li, B. The adjustment of hydrogen bonds and its effect on pyrolysis property of coal. Fuel Process. Technol. 2004, 85, 815–825. [Google Scholar] [CrossRef]
- Miura, K.; Mae, K.; Sakurada, K.; Hashimoto, K. Flash pyrolysis of coal following thermal pretreatment at low temperature. Energy Fuels 1992, 6, 16–21. [Google Scholar] [CrossRef]
- Scheiner, S. Forty years of progress in the study of the hydrogen bond. Struct. Chem. 2019, 30, 1119–1128. [Google Scholar] [CrossRef]
- Kelemen, S.R.; Afeworki, M.; Gorbaty, M.L.; Cohen, A.D. Characterization of organically bound oxygen forms in lignites, peats, and pyrolyzed peats by x-ray photoelectron spectroscopy (XPS) and Solid-State 13C NMR methods. Energy Fuels 2002, 16, 1450–1462. [Google Scholar] [CrossRef]
- Lin, H.; Ju, Y.; Hou, Q.; Li, X.; Wu, Y.; Tan, J.; Fan, J. Raman spectrum characteristics andstructural component response of brittle and ductile deformed coal. Prog. Nat. Sci. 2009, 19, 1117–1125. (In Chinese) [Google Scholar]
- Matthews, M.J.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Endo, M. Origin of dispersive effects of the Raman D band in carbon materials. Phys. Rev. B 1999, 59, R6585–R6588. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martínez-Alonso, A.; Tascón, J. Raman Microprobe Studies on Carbon Materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Vomero, M.; Oliveira, A.; Ashouri, D.; Eickenscheidt, M.; Stieglitz, T. Graphitic carbon electrodes on flexible substrate for neural applications entirely fabricated using infrared nanosecond laser technology. Sci. Rep. 2018, 8, 14749. [Google Scholar] [CrossRef]
- Pan, J.; Meng, Z.; Hou, Q.; Ju, Y.; Cao, Y. Coal strength and Young’s modulus related to coal rank, compressional velocity and maceral composition. J. Struct. Geol. 2013, 54, 129–135. [Google Scholar] [CrossRef]
- Strezov, V.; Lucas, J.; Wall, T.F. Effect of pressure on the swelling of density separated coal particles. Fuel 2005, 84, 1238–1245. [Google Scholar] [CrossRef]
- Zubkova, V.; Czaplicka, M. Changes in the structure of plasticized coals caused by extraction with dichloromethane. Fuel 2012, 96, 298–305. [Google Scholar] [CrossRef]
- Norinaga, K.; Kuniya, M.; Iino, M. Effect of associative interaction on the dynamic viscoelastic property of coal concentrated solution. Energy Fuels 2002, 16, 62–68. [Google Scholar] [CrossRef]
- Ju, Y.; Luxbacher, K.; Li, X.; Wang, G.; Yan, Z.; Wei, M.; Yu, L. Micro-structural evolution and their effects on physical properties in different types of tectonically deformed coals. Int. J. Coal Sci. Technol. 2014, 1, 364–375. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y.; Chen, S.; Zhou, Y. Research on the structural characteristics of vitrinite in different coal ranks. Fuel 2013, 107, 647–652. [Google Scholar] [CrossRef]

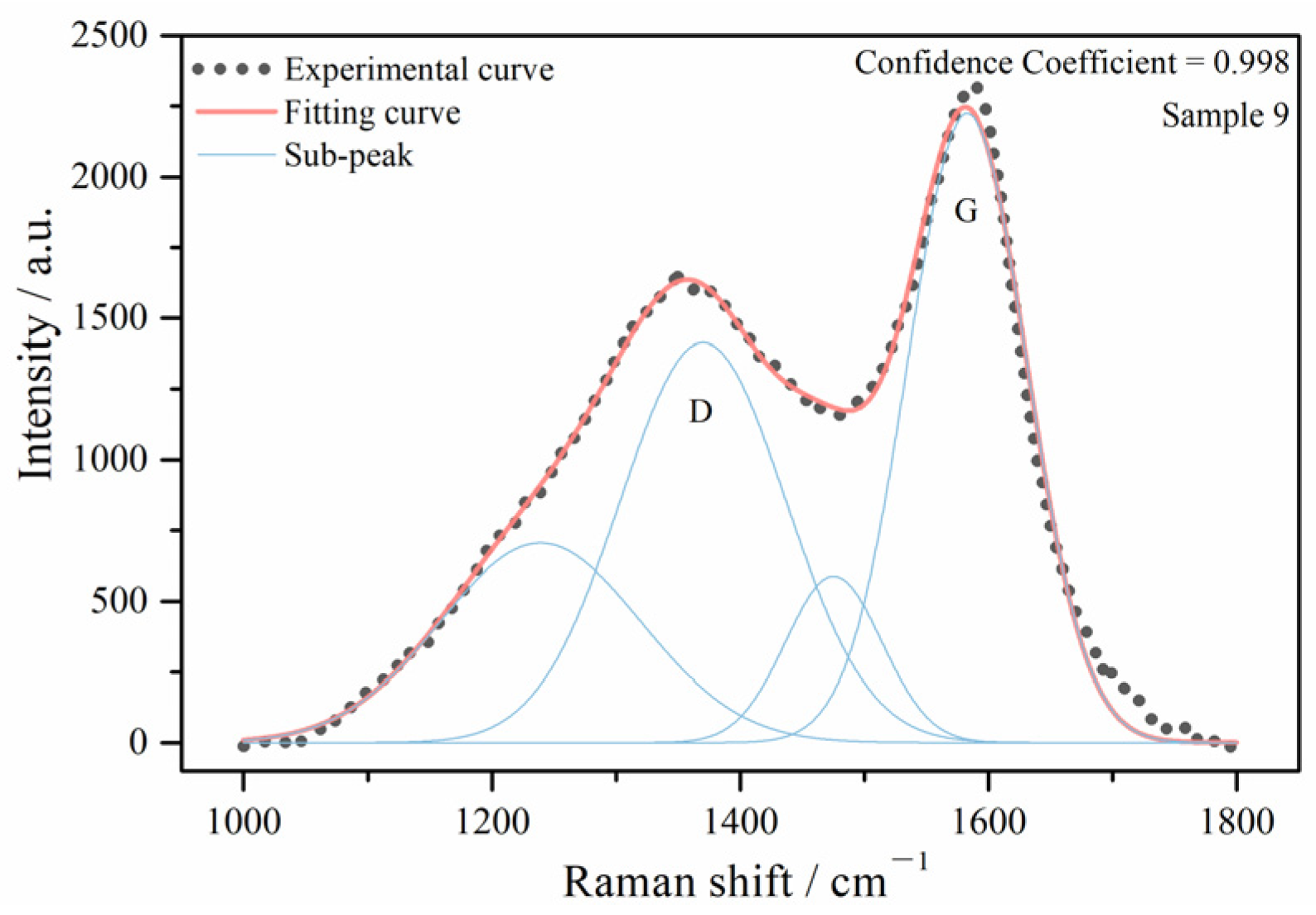
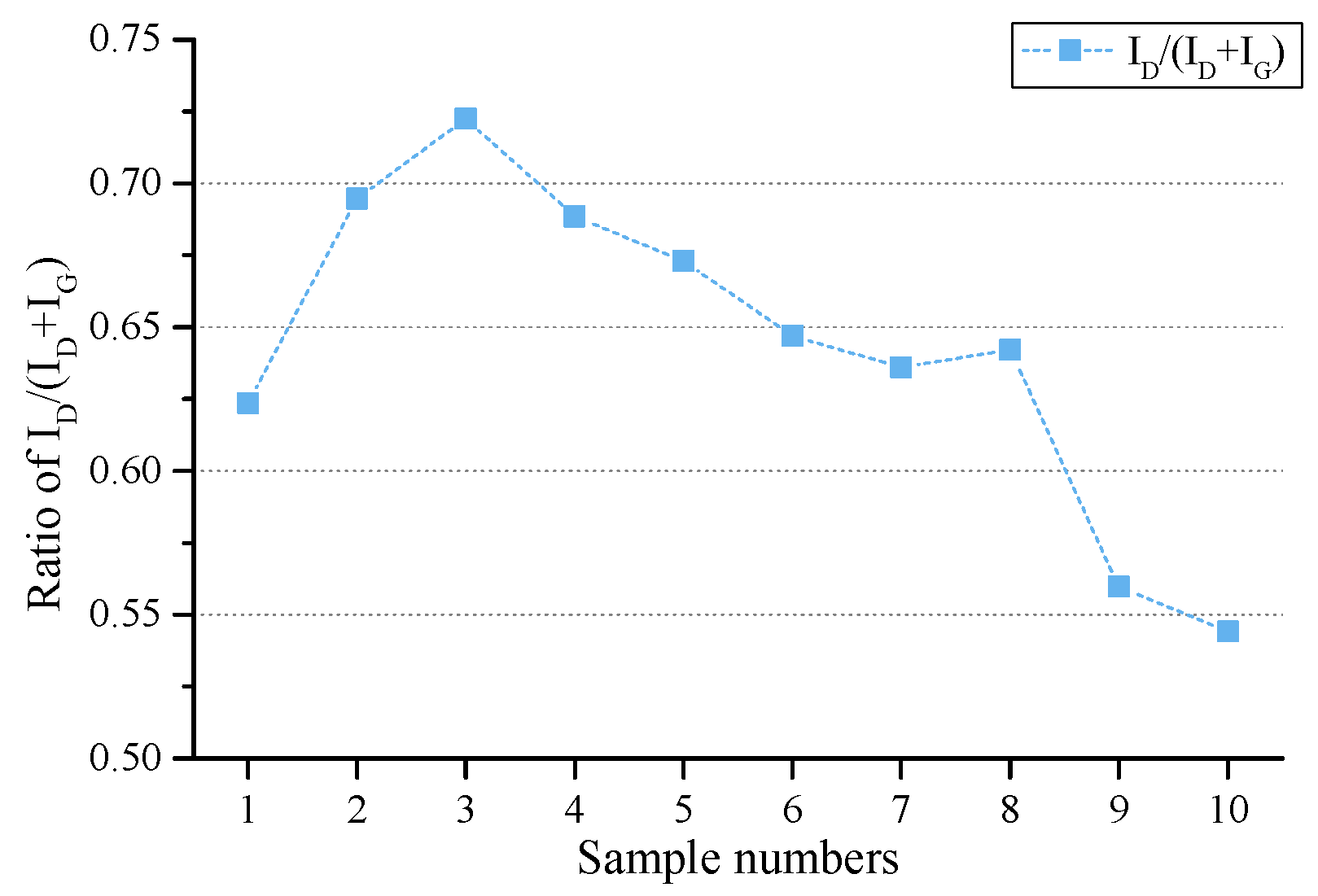
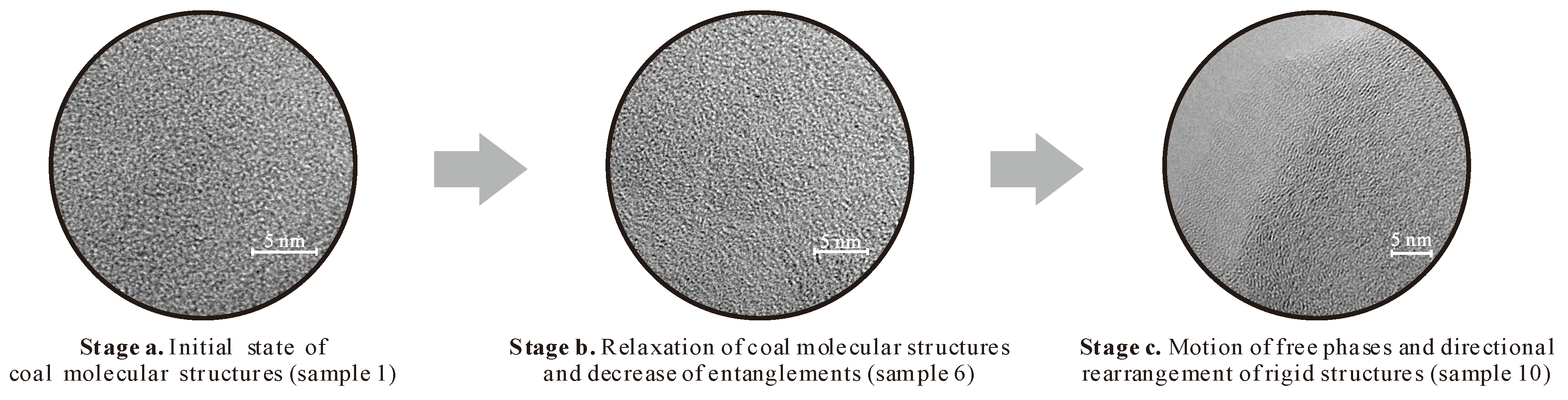
| Ro max | Proximate analysis (%) | Ultimate Analysis (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mad | Ad | Vdaf | FCd | St.d | Odaf | Cdaf | Hdaf | Ndaf | |
| 0.9 | 0.9 | 4.5 | 37.6 | 59.6 | 0.3 | 8.3 | 84.4 | 5.5 | 1.5 |
| Sample Number | Confine Pressure (MPa) | Axial Pressure (MPa) | Temperature (°C) | Creep Experiment Time (h) | Deformation Types |
|---|---|---|---|---|---|
| 1 | 0.0 | 0.0 | 0.0 | 0.0 | Blank sample |
| 2 | 100.0 | 200.0 | 100.0 | 8.0 | Brittle- |
| 3 | 75.0 | 175.0 | 100.0 | 8.0 | Brittle- |
| 4 | 50.0 | 150.0 | 100.0 | 4.0 | Brittle- |
| 5 | 50.0 | 100.0 | 100.0 | 8.0 | Brittle- |
| 6 | 50.0 | 150.0 | 100.0 | 8.0 | Brittle- |
| 7 | 50.0 | 150.0 | 100.0 | 12.0 | Brittle- |
| 8 | 50.0 | 200.0 | 100.0 | 8.0 | Ductile- |
| 9 | 50.0 | 100.0 | 200.0 | 8.0 | Ductile- |
| 10 | 50.0 | 100.0 | 300.0 | 8.0 | Ductile- |
| Peak Number | Types of HBs | Band Positions (cm−1) |
|---|---|---|
| 1 | SH–OH | ~2508.0 |
| 2 | COOH dimers | ~2739.0 |
| 3–5 | CH of aliphatic structures | — |
| 6 | OH–N | ~3006.0 |
| 7 | CH of aromatic structures | ~3050.0 |
| 8 | Cyclic OH | ~3200.0 |
| 9 | OH–ether | ~3300.0 |
| 10 | Self-associated n-mers (n > 3) | ~3406.0 |
| 11 | OH–π | ~3506.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Hou, C. The Role of Non-Covalent Bonds in the Deformation Process of Coal: An Experimental Study on Bituminous Coal. Processes 2022, 10, 1875. https://doi.org/10.3390/pr10091875
Liu H, Hou C. The Role of Non-Covalent Bonds in the Deformation Process of Coal: An Experimental Study on Bituminous Coal. Processes. 2022; 10(9):1875. https://doi.org/10.3390/pr10091875
Chicago/Turabian StyleLiu, Hewu, and Chenliang Hou. 2022. "The Role of Non-Covalent Bonds in the Deformation Process of Coal: An Experimental Study on Bituminous Coal" Processes 10, no. 9: 1875. https://doi.org/10.3390/pr10091875
APA StyleLiu, H., & Hou, C. (2022). The Role of Non-Covalent Bonds in the Deformation Process of Coal: An Experimental Study on Bituminous Coal. Processes, 10(9), 1875. https://doi.org/10.3390/pr10091875







