Abstract
Grape pomace (grape skin and seeds) and stems are major by-products of winemaking, of lignocellulosic nature. The aim of this study was to value grape pomace and stems to produce prebiotic oligosaccharides (OS). Grapes from Touriga Nacional and Marselan cultivars (Vitis vinifera L.) were used for conventional red winemaking. The total of extractives, obtained by sequential extraction with dichloromethane, ethanol, and water, was approx. 64.0% (d.w.) for both pomaces, and 46.2% and 59.5% for Marselan and Touriga Nacional stems, respectively. Lignin contents in Marselan stems (26.4%) and pomace (20.4%) were higher than in Touriga Nacional pomace (19.3%) or stems (17.3%). Polysaccharides (hemicelluloses and cellulose) represented 9 and 8.2% of Marselan and Touriga pomaces, and 22.3 and 18.7% of respective stems. After extractives removal, the pomaces and stems were submitted to a hydrothermal treatment (autohydrolysis) to release oligosaccharides from the hemicellulose fraction. Autohydrolysis was carried out following a central composite rotatable design (CCRD) as a function of temperature (142–198 °C) and time (48–132 min). For all materials of both varieties, the production of sugars by autohydrolysis could be described by second-order models. Highest sugar productions were: 81.2 g/kg (d.w.) extracted Marselan pomace; 76.3 g/kg (d.w.) extracted Touriga Nacional pomace; 116.3 g/kg (d.w.) extracted Marselan stems; and 168.4 g/kg (d.w.) extracted Touriga Nacional stems. Yields of 99% OS were obtained by autohydrolysis at 170 °C/90 min.
1. Introduction
Wine production is one of the most important agricultural activities contributing substantially to national economies around the world. In 2021, the global wine production was 260 million hL, with more than 60% of wine being produced by the European Union (EU). In particular, Portugal has a long tradition of wine production, is the 9th country in terms of vineyards (194 thousand hectares) and the 10th largest wine producer in the world. According to the International Organisation of Vine and Wine [1], about 7.3 million hL were produced in Portugal during the 2021 harvest.
There is an increasing interest in the management and valorization of secondary products generated at different stages of the wine production chain, with a significant number of studies being developed on the reuse of grape by-products [2,3].
During the vinification phase of winemaking, from grapes harvest to the conclusion of the alcoholic fermentation (and malolactic fermentation depending on the technological option), large volumes of waste and by-product streams, namely grape stems, grape pomace, and lees, are generated. The yields of these by-products considerably vary depending on grape cultivar, environmental conditions, grape maturation stage, and winemaking technology [3].
Grape stems that build up the lignocellulosic skeleton of grapes are obtained after the destemming process and represent between 3 and 6% (w/w) of the grapes [4]. Grape pomace represents 45–62% (w/w) of total organic wastes resulting from the pressing and fermentation stages. It is a mixture of grape skins, seeds, and pulp. Both stems and pomace contain lignocellulosic compounds with potential to be transformed into value-added products by selective fractionation of the main components (cellulose, hemicelluloses, and lignin) following the biorefinery concept [5,6,7,8,9]. Polysaccharides constitute more than 50% of grape stems (cellulose 15–40%; and hemicelluloses 12–20%) [5] with xylans as the most abundant hemicelluloses [5,6,7,10]. Grape pomace mainly consists of dietary fibers (43–75% of dry pomace) and extractives (e.g., polyphenols and oil) [11,12,13]. Grape pomace contains 25.2–44.5% lignin, 8.04–15% glucan, 4.1–7.05% xylan, and trace amounts of pectinaceous polysaccharides (less than 3% in total) [12,13].
The interest in new prebiotics such as xylo-oligosaccharides (XOS) has increased in recent years. These compounds are considered soluble dietary fibers with recognized prebiotic activity, since they promote health benefits such as bowel functions, immunity, and antimicrobial activity [14]. XOS can be obtained from xylan-rich agricultural residues by xylan hydrolysis. Hydrothermal processing (autohydrolysis) is an effective and selective treatment to carry out the hydrolysis of the hemicellulosic fraction. Autohydrolysis enables a high production of soluble hemicellulosic oligosaccharides and offers environmental advantages over other methods due to the absence of chemicals, and its low economic cost [15]. Autohydrolysis has been carried out by several authors to produce oligosaccharides and sugars from grape stems [16] and from grape pomaces [13]. Other pre-treatments such as ethanol organosolv extraction [16,17], wet oxidation [17], or dilute sulfuric acid hydrolysis [17,18,19] have been used for the fractionation of lignocellulosic material of grape pomace or stalks. The recovery of polysaccharides from pomaces and lees by extraction with aqueous tartaric acid or ammonium oxalate solutions was also reported [20,21]. However, these methods are more pollutant than autohydrolysis.
The aim of this study was (i) to evaluate the effect of time and temperature on autohydrolysis of hemicelluloses from grape stems and pomaces of red grape cultivars Touriga Nacional (Portuguese variety) and Marselan (French variety), (ii) the optimization of autohydrolysis conditions, aimed at the production of bioactive oligosaccharides (OS), (iii) to evaluate which type of by-product (stems or pomace) is the most adequate for OS production, and (iv) the effect of cultivar on sugar yield.
2. Materials and Methods
2.1. Raw Material and Sample Preparation
Grapes from the Portuguese red variety Touriga Nacional and the French red variety Marselan (hybrid between Cabernet Sauvignon and Grenache varieties) harvested in August–September 2020, were provided by the vineyards of Quinta do Pinto and Quinta de Aroeira (Portugal), respectively, both located in the Lisbon wine region (Portugal). These grapes were vinified in the winery pilot plant of the Instituto Superior de Agronomia (ISA), Portugal, using conventional red wine technology with maceration. Before the alcoholic fermentation of the grape must (in the presence of the solid parts of the berries, namely skins and seeds), the grapes were crushed and destemmed, and the stems were collected for this study. The pomace was sampled after running off and pressing of the mash after seven days of maceration.
Grape pomaces and grape stems of both varieties were stored at −18 °C until use. After defrosting, the material was dried using an oven with air circulation at 60 °C for 24 h and triturated in a knife-mill (Retsch SM 2000) to particle sizes below 2 mm and sieved. The 40/60 mesh fractions (0.250–0.450 mm) were used for chemical analysis.
2.2. Chemical Composition of Pomaces and Stems
The chemical analysis included the determination of ash, extractives soluble in solvents with different polarity, Klason and acid-soluble lignin. Ash values were obtained gravimetrically by incinerating 2 g of dried samples in a muffle furnace (Heraeus MR 170 E) at 525 °C [22].
Extractives were obtained after successive extractions with dichloromethane (6 h), ethanol (16 h) and water (16 h) with a ratio of 1:20 (solid:solvent; m/v) in a Soxhlet extractor, using approximately 7 g of dried sample. The mass of extractives solubilized by each solvent was determined by the difference between the initial mass of dry sample and the mass of the solid residue obtained after extractions dried at 105 °C. Acid-insoluble (Klason) lignin and soluble lignin were determined in the extractive-free material according to TAPPI standard methods, [23,24], respectively. In brief, sulfuric acid (72%, 3.0 mL) was added to 0.35 g of extracted sample and the mixture was placed in a water bath at 30 °C for 1 h. After this time, the sample was diluted to a concentration of 3% sulfuric acid and hydrolyzed for 1 h at 120 °C. The solid residue after hydrolysis was recovered by filtration (G-3 porosity glass filter) and dried and weighed to determine the Klason lignin content. The concentration of soluble lignin in the filtrate was determined by measuring the absorbance of the solute at 206 nm with a spectrophotometer (Shimadzu UV-160A, Shimadzu Corporation, Kyoto, Japan), using the value of 110 L/cm.g as the absorptivity of soluble lignin. Total lignin content is the sum of Klason and acid-soluble lignins. The quantity of polysaccharides (cellulose and hemicelluloses) was calculated as the difference from 100 and the sum of ash, total extractives, and total lignin (Klason and acid-soluble) contents.
The analyses were performed in triplicate, and therefore results were expressed as mean values in percentage of the original dry sample.
2.3. Autohydrolysis Experiments and Modelling
After removal of extractives with successive Soxhlet extractions with dichloromethane, ethanol, and water, grape pomaces and stems were submitted to autohydrolysis to release oligosaccharides from the hemicelluloses. The autohydrolysis reactions were carried out in 100 mL stainless steel reactors rotating in an oil bath with temperature control. A load of 3 g (dry weight basis) of each material and 60 mL of water (liquid-to-solid ratio = 20:1, v/m) was used in each reactor. Autohydrolysis experiments were performed at different times and temperatures according to the Central Composite Rotatable Design (CCRD) as in Table 1. Using this experimental design, each factor (variable) will be tested at five different levels and the levels of the tested variables change simultaneously, conversely to the classic approach of “one-variable-at-a-time” (OVAT). Moreover, the same precision of the classical approach is attained with a much lower number of trials. This type of experimental design will allow detecting the factors and interactions between factors with significant effects on the response. In addition, since five factor levels are tested, it is possible to fit second-order polynomial models to the experimental data and, therefore, obtain a response surface describing the obtained results as a function of the tested factors [25,26]. A total of 13 experiments were carried out for each material, consisting of a set of 4 factorial points (runs 1–4), 4 star points (runs 5–8), and 5 replicates of the center point (runs 9–13). The replicate of the central point allows the estimation of the experimental error, which is assumed to be constant for all experimental regions [25,26]. Autohydrolysis temperature varied from 142 to 198 °C and time from 48 to 132 min.

Table 1.
Experimental design (Central Composite Rotatable Design, CCRD) followed for autohydrolysis reaction of extracted grape pomaces or stems, as a function of time and temperature (coded and decoded values for the variables), and respective severity factor (S.F.) values.
At the end of each experiment, the reactors were quickly cooled on ice to stop the reaction. Subsequently, solid and liquid phases were separated by filtration to recover the liquid stream containing the hydrolysates. For each experiment, the severity factor S.F. (logR0) was calculated by Equation (1):
where the temperature (T, °C) is a function of time (t, min), 100 °C is the reference temperature and 14.75 °C is a constant regarding the normal energy of activation, assuming that, in general, the process is hydrolytic and follows first-order kinetics [27]. The experiments of CCRD had a severity factor varying from 3.2 (star point at 142 °C/90 min) to 4.8 (star point at 198 °C/90 min).
2.4. Sugar Analysis for Autohydrolysis Assessment
The efficacy of the autohydrolysis process was assessed by the quantification of the total sugar content released to the liquid medium, using the phenol–sulfuric acid method [28]. In brief, 100 μL of hydrolysate solution reacted with 2.5 mL of concentrated sulfuric acid (96%) and 1 mL of phenol solution (5%, w/v). The solution was homogenized in a vortex, cooled, and the absorbance at 490 nm was read in a spectrophotometer (Shimadzu UV-160A). The results were converted into glucose equivalent using a calibration curve prepared with D-glucose standard solution at 10 concentration levels (10–100 mg/L; R2 = 0.987).
Some liquid samples obtained after the autohydrolysis reaction were assayed by pressure ion-exchange chromatography with a pulsed amperometric detector (HPIC-PAD) for the determination of the monosaccharide profile (e.g., glucose, xylose, arabinose, and rhamnose) and individual quantification. A Dionex ICS-3000 system, with an Aminotrap plus Carbopac PA10 column (250 × 4 mm), was used. The eluent consisted of sodium hydroxide/sodium acetate solution at a flow rate of 1 mL/min at 25 °C. The quantification was performed by external calibration using standard solutions (concentration from 5 ppm to 100 ppm) of the measured compounds (HPLC grade) [29,30]. All the analyses were made in duplicate.
Oligosaccharide content was estimated by the difference between the total sugar content of the liquid phase recovered from the hydrothermal treatment, and the total amount of monosaccharides [30,31].
2.5. Statistical Analysis
One-way analysis of variance (ANOVA) of the results of chemical composition of the various stem and pomace samples, as well as the handling of the results obtained from autohydrolysis performed under the conditions dictated by the CCRD, were performed using the software Statistica, version 7, from Statsoft, Tulsa, OK, USA. In ANOVA, a post-hoc Duncan’s test was carried out to analyse pairwise differences between varieties for each material (pomace or stems), considering a p value of 0.05.
3. Results and Discussion
3.1. Chemical Characterization of the Winery Wastes
The results obtained for the chemical composition of the winery wastes (grape pomace and stems) of varieties Touriga Nacional and Marselan are shown in Table 2.

Table 2.
Chemical composition of grape pomaces and stems from Marselan and Touriga Nacional varieties (% dry basis). For each type of material (pomace or stems) different letters indicate significant differences at p < 0.05 (post-hoc Duncan’s test).
3.1.1. Grape Pomace
The studied pomace samples included grape skin and seeds, that were visible to the naked eye, obtained after the alcoholic fermentation stage. The total content of extractives was high and similar in both pomace samples: 63.87% of the Marselan and 64.40% of the Touriga Nacional pomaces, with no significant differences among them (p = 0.158). A large proportion of the extractives correspond to polar compounds that are soluble in ethanol and water, representing about 60% of the total extractives. Ethanol-soluble extractives obtained from Touriga Nacional pomace were significantly higher than those from Marselan pomace. No significant differences were found between water-soluble extractives in both pomaces. Previous studies in pomaces have shown that the extractives released by polar solvents are mainly ascribed to the presence of non-structural sugars and other compounds, which depend on grape variety, and winemaking process [19]. Corbin et al., 2015 [19] reported for red pomace (Cabernet Sauvignon) and white pomace (Sauvignon Blanc) total extractives of 20.7% and 60.5%, respectively, which included 14.8% and 43.3% of soluble carbohydrates, respectively. These values are lower than those of the present study.
The non-polar compounds extracted by dichloromethane represented about 40% of the total extractives. This value found in pomaces should arise from the presence of grape seeds since grape seeds contain about 8–10% of oil [32]. The ash content of both grape pomaces was around 7.4%. These values are higher than the ash values (1.2–3.0%) reported by Corbin et al., 2015 [19] and González-Centeno et al., 2010 [10] for grape pomaces.
After extraction, an insoluble residue is obtained, which mainly consists of cell wall structural material, predominantly composed of lignin and polysaccharides. Lignin was the main component with concentrations on an extractive-free basis of 55% (20.4% initial pomace) and 53% (19.3% initial pomace) in Marselan and Touriga Nacional pomaces, respectively, without statistically significant differences (p = 0.511). Higher lignin content (34.8% of pomace, previously washed with water) was observed in pomace from the Greek variety Agiorgitiko [33]. Corbin et al., 2015 [19] reported 32.5% and 10.5% of acid-insoluble lignin for alcohol-insoluble pomaces of Cabernet Sauvignon and Sauvignon Blanc grape pomace, respectively. The alcohol-insoluble residue from grape pomaces of ten varieties (six red grape varieties: Cabernet Sauvignon, Callet, Manto Negro, Merlot, Syrah, and Tempranillo, and four white grape varieties: Chardonnay, Macabeu, Parellada and Prensal Blanc) had lignin contents ranging from 16.8 to 24.2% [10]. The differences in relation to the present results may be due to grape cultivar, but also to different proportions of seed and skin in the grape pomace samples, or environmental conditions, grape maturation stage, and winemaking technology [3]. The structural polysaccharides, which mainly consist of glucan and xyloglucans [10,19], represented a small proportion of the total mass, 9.0% of the dry mass (extractive-free basis: 24.6%) and 8.0% (extractive-free basis: 23.0%) for Marselan and Touriga Nacional pomaces, respectively, with no significant difference (p = 0.457). The results are within the range as those determined for extracted pomaces of Cabernet Sauvignon and Sauvignon Blanc grape with 11–17% [19] and the amounts of cell wall polysaccharides obtained by González-Centeno et al., 2010 [10] in ten extracted grape pomaces, ranging from 15.6% to 29.6%.
3.1.2. Grape Stems
The grape stems of Marselan and Touriga Nacional varieties were characterized by a high content of extractable compounds (46.19% and 59.51%, respectively), mostly polar compounds (soluble in ethanol and water), which represented more than 95% of total extractives (Table 2). Non-polar compounds extracted by dichloromethane, including alkanes and fatty acids, corresponded to a low proportion of the total extractives (less than 2%). The extractives released by water and ethanol are mainly due to the presence of polyphenolic compounds, inorganic salts, non-structural sugars, and other compounds, depending on the destemming stage [34,35]. The differences between stems of both varieties were significant for the total extractives (p < 0.001) and lipophilic extractives. The results are comparable to the total 58.86% of extractives reported for red grape stems (Vinhão variety) [36] and to the ethanol and water 52.25% of extractives for grape stems from Pinot nero grapes [35]. Previously washed red grape stalk wastes presented 11.8–21.6% of water extractives and 4.5–7.1% of ethanol extractives [7]. A similar low concentration of lipophilic compounds (1.0% of dichloromethane extract) was reported for grape stems from red grape pomaces of the Vitis vinifera variety [17] and for grape stem wastes (1.02% and 1.9% of dichloromethane extract) [7].
The ash content of 5% is similar to that previously reported for red grape stems by Ping et al., 2011 [17], 4.9% for red grape stems (Vinhão variety), and 3.12% for white grape stems (Loureiro variety) by Atatoprak et al., 2022 [36], but lower than the values between 6.5 and 9%, reported by Spigno et al., 2013 [34] for grape stems from six varieties (Chardonnay, Moscato, Muller-Thurgau, Barbera, Nebbiolo, and Pinot Noir).
The structural components of Marselan and Touriga Nacional grape stems contain 26.4 and 17.3% of lignin and 22.32 and 18.73% of structural polysaccharides, respectively. The differences between stems of two varieties were significant for the lignin (p < 0.001) and structural polysaccahrides (p < 0.001). These values, when calculated on the extractive-free basis, are 49.1 and 42.7% lignin and 41.5 and 46.3% structural polysaccharides, respectively. The lignin content of the studied grape stems is similar to the 17.4% lignin determined by Prozil et al., 2012 [6] in grape stems of Touriga Nacional and to the 14.4% lignin in grape stem wastes reported by Pujol et al., 2013 [7]. The lignin content, when expressed on the extractive-free basis, was comparable to 48.5% obtained in extracted stems of Spanish grapes of the Vinhão variety [36], 47.3% and 40.9% of lignin in grape stems previously washed with water from Pinot nero grapes [35] and in stems from the French grape variety [17]. Concerning the structural carbohydrates, Atatoprak et al., 2022 [36] reported 18.5% of polysaccharides (10.5% of glucan and 8.01% of hemicellulose rich in xylose) for Spanish grape stems (Vinhão variety), while 27.57% of structural carbohydrates were reported by Pujol et al., 2013 [7] for grape stem wastes. Spigno et al., 2008 [35] reported 39.25% of polysaccharides (25.30% of glucan and 13.95% of hemicelluloses) for grape stems from Pinot nero and total polysaccharides of 37.9% (12.2 cellulose and 25.7 hemicelluloses) for a mixture of grape stems from two Italian red cultivars (Bonarda and Barbera) [16].
3.2. Autohydrolysis Experiments and Modelling
The autohydrolysis experiments were carried out for the grape pomaces and stems of both grape varieties, after extractives removal. The operation conditions were dictated by the experimental matrix, corresponding to different severity factors (S.F.) varying from 3.2 to 4.8 (Table 1). The quantity of soluble sugars released by autohydrolysis was assayed and expressed as g glucose equivalent per kg of dry extracted pomace or stems. The results obtained for each autohydrolysis experiment are presented in Table 3. In general, sugar production obtained from stems was higher than that from pomaces and Touriga Nacional stems had higher sugar yields. This may be explained by the high content of polysaccharides (hemicelluloses and cellulose) in stems, which is about 2.5- and 2.3-fold the value for Touriga Nacional and Marselan pomaces, respectively. In addition, grape pomaces are rich in grape skins, representing about 50% of the weight. The cutin layer present in the skins may hinder the autohydrolysis of hemicelluloses [37]. Sugar production varied as follows: from 19.0 to 81.2 g/kg (d.w.) extracted Marselan pomace; from 15.8 to 76.3 g/kg (d.w.) extracted Touriga Nacional pomace; from 28.0 to 116.3 g/kg (d.w.) extracted Marselan stems; and from 38.2 to 168.4 g/kg (d.w.) extracted Touriga Nacional stems. Moreover, the highest sugar yields were obtained under autohydrolysis conditions with severity factors higher than 3.6, corresponding to experiments, no. 2 (S.F. = 3.6), no. 3 (S.F.= 4.4), no. 4 (S.F. = 4.7), no. 8 (S.F. = 4.2); and the central points (Experiments no. 9–13; S.F. = 4.0). In fact, maximum sugar yields were obtained for Touriga Nacional stems: 168.4 g/kg (d.w.) (Exp. 8), 162.6 g/kg (Exp. 4), 162.3 g/kg (Exp. 3). It seems that applying higher temperature and prolonged times will help to decompose hemicelluloses into soluble sugars, namely oligosaccharides and monomers, to a greater extent. However, either for stems or for pomaces of both varieties, sugar yields decreased when the autohydrolysis was conducted under the highest S.F. tested of 4.8 (Exp. 6).

Table 3.
Sugar production (g glucose equivalent/kg dry extracted pomace and stems) by autohydrolysis of extracted grape pomaces and stems of Marselan and Touriga Nacional varieties, carried out under different experimental conditions (CCRD). The experiments no. 12 and 13 (repetitions of the central point) with Marselan stems were not carried out due to lack of material.
To model autohydrolysis and optimize reaction conditions, in terms of temperature and time, the results from Table 3 were used. The linear and quadratic effects of each variable (factors) as well as their interaction (time x temperature) on sugar production by autohydrolysis were calculated (data not shown). The calculation of these effects and respective significance levels are important to select the factors to be included in the polynomial models describing the response surface fitted to the experimental results. The factors with significant effects (p ≤ 0.05) or those with p value higher than 0.05 that, when removed, originate a lack of fit of the model, must be considered in the polynomial second-order models fitted to the experimental data points [25].
For both pomaces, sugar production by autohydrolysis depended on the time and temperature used but the interaction effect of time with temperature showed to be not significant. For both pomaces, temperature had a positive linear effect on sugar production, which means that an increase in temperature will promote autohydrolysis. The negative quadratic effect of temperature in the autohydrolysis of both pomaces and the negative quadratic effect of time in Touriga Nacional pomace thermal treatment indicate that sugar hydrolysis can be described by convex response surfaces as a function of temperature (both pomaces) and time (Touriga pomace).
Concerning Touriga Nacional stems, the temperature used in the autohydrolysis also showed a significant positive effect on sugar release. For Marselan stems, the linear effect of temperature could be ignored. In addition, for both stems, the quadratic negative effect of temperature had to be considered in the models fitted to the experimental data, showing a convex-shaped surface as a function of this factor. The effect of time was positive and linear for both stems, indicating that the quantity of soluble sugars released by autohydrolysis increased with the operation time. The quadratic effect of time was considered only for Marselan stems and was negative, indicating a convex response surface as a function of reaction time. The interaction (temperature x time) was not significant for the autohydrolysis of Touriga Nacional stems, while it had to be considered for the autohydrolysis of Marselan stems.
Table 4 shows the second-order polynomial equations fitted to sugar production by autohydrolysis, expressed in glucose (g/kg pomace), and respective coefficients of determination (R2) and the adjusted coefficients of determination (R2adj). These models correspond to the response surfaces presented in Figure 1 and Figure 2, for pomaces and stems, respectively. According to Haaland, 1989 [25], for practical purposes, determination coefficients should be at least 0.75, and are considered very good above 0.90. Following this criterion, all the models, except the one for Marselan stems, have a good fit to the experimental results. In fact, the models for Touriga Nacional pomace and stems and for Marselan pomace explain 88.2, 75.1, and 82.7% of the experimental results (R2 × 100), respectively, while the model for Marselan stems only explains 54.5% of the data variability.

Table 4.
Polynomial equations of the models fitted to sugar production, expressed as [Glu] (g glucose equivalent/kg of extracted pomace, dry weight), by autohydrolysis of extracted pomaces and stems as a function of temperature (T, °C) and time (t, min), and respective R2 and R2adj.

Figure 1.
Soluble sugars, expressed as g glucose equivalent per kg of dried extracted material, obtained by autohydrolysis of Touriga Nacional or Marselan pomaces, as a function of reaction temperature and time (response surfaces and contour plots). White dots represent the experimental data points.
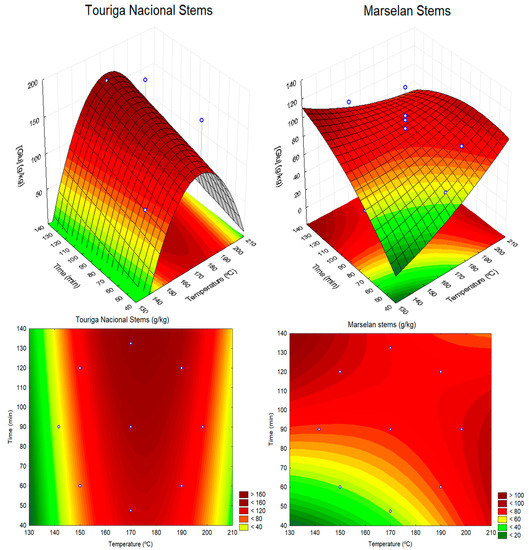
Figure 2.
Soluble sugars, expressed as g glucose equivalent per kg of dried extracted material, obtained by autohydrolysis of Touriga Nacional or Marselan stems, as a function of reaction temperature and time (response surfaces and contour plots). White dots represent the experimental data points.
Both response surfaces for pomaces (Figure 1) have a convex shape. However, the response surface fitted to Touriga Nacional results has an optimum point inside the experimental region, while the optimal conditions are not attained for Marselan pomaces in this region. The best temperature to attain a higher sugar yield appears to be around 170–180 °C for Touriga Nacional and between 170–190 °C for Marselan pomace. Regarding the optimal time, it is between 90 and 135 min for Touriga Nacional and higher than 125 min for Marselan pomace.
The response surfaces describing sugar production by autohydrolysis of stems show a convex surface for Touriga Nacional stems (Figure 2). The optimal temperature to attain the maximum sugar production is outside the experimental region used. However, the visualization of both the surface and its projection (contour plot) shows that higher sugar yields are attained for autohydrolysis temperatures around 160–190 °C and a reaction time higher than 80 min (severity factor > 3.6). For the Marselan stems, a saddle-like surface was fitted, which means that high sugar yields can be obtained under several combinations of temperature and time (Figure 2). Higher sugar yields were observed for treatments with (i) higher temperature (>190 °C) and time between 50–100 min (severity factor > 4.3), or (ii) with lower temperatures (~150 °C) and longer times (>120 min) (severity factor > 3.6).
From the autohydrolysis of a mixture of grape stems from Bonarda and Barbera Italian red cultivars, carried out at 180 °C for 30 min (SF = 3.8), Amendola et al., 2012 [16] obtained about 150 g of soluble sugars (glucose, xylose, and arabinose) per kg (d.w.) extracted stems. This value is in the range of the values obtained in the present study.
Freitas et al., 2022 [30] performed the optimization of autohydrolysis conditions (time and temperature) of olive pomaces from Galega Vulgar or Cobrançosa cultivars, following the same design used in the present study. Higher sugar yields (180 g glucose equivalent/kg extracted pomace) were obtained under conditions corresponding to severity factors of 4.7 (190 °C/120 min) or 4.9 (198 °C/90 min). Even using a different raw material, the autohydrolysis conditions that maximize sugar production from grape pomaces or stems are not very different from those observed for olive pomace.
3.3. Monomeric and Oligosaccharides Quantification
Table 5 shows the identification and quantification of neutral monomeric sugars, mainly derived from hemicelluloses, present in the liquid phase resulting from hydrothermal treatment of grape pomace and stem samples of Marselan and Touriga Nacional grape varieties at 170 °C/90 min (central point; S.F. = 4.0) and at 198 °C/90min (star point corresponding to the most severe conditions tested; S.F. = 4.8). These results, together with the quantification of total soluble sugars, were used to estimate oligosaccharide (OS) yields (Table 5). The hydrolysates obtained from grape pomaces and stems presented a mixture of carbohydrates in oligomeric and monomeric forms. The oligosaccharide content especially consisted of the xylooligosaccharide form (XOS).

Table 5.
Sugar composition (monomeric sugars and oligosaccharides, OS) (g/kg of extracted raw material) of the liquid phase resulting from autohydrolysis treatments of extracted grape pomaces and stems under different conditions of temperature (T) and time (t) (Rha: rhamnose; Ara: arabinose; Gal: galactose; Glu: glucose; Xyl: xylose).
In grape pomace hydrolysates, sugars were predominantly in oligomeric form. The highest OS yields were observed at 170 °C/90 min (S.F. = 4.0), being 98.8 and 98.9% of total sugars for Touriga Nacional and Marselan pomaces, respectively. These yields correspond to 68.9 g and 56.6 g oligomers released/kg, respectively. When autohydrolysis was conducted under more severe conditions (severity factor of 4.8), the quantity of monomeric sugars (mostly xylose) increased to 2.92 and 2.14 g/kg for Touriga Nacional or Marselan pomaces, respectively, and the concentration of OS decreased to 34.9 and 25.6 g/kg, corresponding to OS yields of 80.1 and 82.1%, respectively.
A similar behavior was observed in autohydrolysis of stems. Concentrations of 137.0 g oligomers/kg grape stems in Touriga Nacional liquor and 101.2 g oligomers/kg grape stems in Marselan liquor were achieved when the autohydrolysis was performed at 170 °C/90 min. These OS concentrations represent 99.2% and 98.8% yield, respectively. The concentration of monomeric sugars in the liquors, including xylose, glucose, arabinose, galactose, and rhamnose, were at a very low levels (1.16 and 1.24 g total sugars released/kg of Touriga Nacional and Marselan stems, respectively). After autohydrolysis at 198 °C for 90 min, the yield of oligosaccharides in the liquid phase decreased considerably, reaching 41.89 g and 12.25 g oligomers/kg of Touriga Nacional and Marselan grape stems, respectively. This decrease was accompanied by an increase in monomeric sugars up to 8.9 and 23.6 g/kg of Touriga Nacional or Marselan stems, respectively. This may indicate that a depolymerisation or hydrolysis of the oligosaccharides and the degradation of solubilized sugars occurred, which is commonly associated with more severe autohydrolysis conditions [38]. The optimization of operating conditions is extremely important to achieve high oligosaccharide yield.
Autohydrolyis has also been used for OS production from other lignocellulosic materials. For example, the maximum contents of oligosaccharides were obtained at 200 °C (severity of 4.01) from vine shoots (22.8 g/L) [39] and at 180 °C (severity of 4.13) from vine pruning (13.19 g/L) [40]. Cara et al., 2012 [31] reported high concentrations of oligosaccharides from olive tree pruning (37.5 g/L total OS) under milder autohydrolysis conditions (180 °C/10 min isothermal process). Freitas et al., 2022 [30] reported 146.6 to 162 g oligosaccharides/kg extracted olive pomace from Galega Vulgar or Cobrançosa cultivars, corresponding to OS yields of 98%, by autohydrolysis at 170 °C/90 min (S.F. = 4.0). Chestnut shells were also submitted to autohydrolysis and the highest sugar yields (18.3 g/L) were reached at 180 °C (S.F. = 3.08) [41]. In all these studies, the best autohydrolysis conditions to maximize OS yields were similar to those found for grape pomace and stems.
4. Conclusions
Autohydrolysis of grapevine stems and pomaces showed to be an efficient operation for oligosaccharide production from hemicelluloses. Using Response Surface Methodology, it was possible to model autohydrolysis conditions (time and temperature) and maximize hemicellulose hydrolysis of extracted pomaces and stems from Marselan or Touriga Nacional red grapevine cultivars. Sugar production from stems was always higher than from pomaces under the same autohydrolysis conditions. Sugar quantities obtained from stems varied from 1.9- to 6.6-fold the values obtained from pomace in Touriga Nacional cultivar and from 1.2- to 3.2-fold for Marselan cultivar. The highest sugar productions obtained from stems were 81.2 g/kg (d.w.) and 76.3 g/kg (d.w.) extracted Marselan and Touriga Nacional pomaces, respectively. Extracted Marselan and Touriga Nacional stems had a maximum of 116.3 g and 168.4 g sugars per kg (d.w.), respectively. The response surfaces fitted to each set of autohydrolysis experiments showed that, near to the optimal temperature and time that maximize sugar production, it was possible to identify a region where sugar yields are near to the maximum predicted by the model. These yields were obtained under autohydrolysis conditions with severity factors higher than 3.6 and around 4.0. The oligosaccharides obtained were mainly XOS and represented 99% of soluble sugars released by autohydrolysis at 170 ℃/90min (S.F. = 4.0) of Touriga Nacional and Marselan pomaces, and 99.2% and 98.8% yield from Touriga Nacional and Marselan stems. In conclusion, higher sugar and OS yields were obtained from stems using a green technology such as autohydrolysis than from pomaces. Touriga Nacional stems had higher yields than the Marselan cultivar, indicating that the results are cultivar-dependent.
Author Contributions
Conceptualization, S.F.-D., I.M., S.C. and J.R.-d.-S.; methodology, S.F.-D., I.M., H.P., S.C. and J.R.-d.-S.; validation, S.F.-D., I.M. and J.R.-d.-S.; formal analysis, R.M. and R.S.; investigation, R.M. and R.S.; data curation, R.M., R.S., S.F.-D. and I.M.; writing—original draft preparation, R.M.; writing—review and editing, S.F.-D., I.M., H.P., S.C. and J.R.-d.-S.; visualization, S.F.-D., I.M., H.P., S.C. and J.R.-d.-S.; supervision, S.F.-D., I.M. and J.R.-d.-S.; project administration, S.F.-D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by FCT—Fundação para a Ciência e a Tecnologia, I.P., through the project LEAF—Linking Landscape, Environment, Agriculture and Food Research Centre (UIDB/04129/2020).
Data Availability Statement
Data available upon request.
Acknowledgments
This work was developed under the FCT—Fundação para a Ciência e a Tecnologia, I.P., research units Linking Landscape, Environment, Agriculture and Food Research Centre, LEAF (UIDP/04129/2020; UIDB/04129/2020) and Forest Research Centre (UIDB/00239/2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- OIV. State of the World Vine and Wine Sector 2021. 2022. Available online: https://www.oiv.int/public/medias/8778/eng-state-of-the-world-vine-and-wine-sector-april-2022-v6.pdf (accessed on 3 June 2022).
- OIV. OIV Collective Expertise: Managing by-Products of Vitivinicultural Origin. International Organisation of Vine and Wine. Paris, France. 2018. Available online: https://www.oiv.int/public/medias/6267/managing-viticulture-by-products-web.pdf (accessed on 3 June 2022).
- Teissedre, P.-L.; Catarino, S.; Comuzzo, P. Wine quality production and sustainability. In Improving Sustainable Viticulture and Winemaking Practices; Costa, J.M., Catarino, S., Escalona, J.M., Comuzzo, P., Eds.; Academic Press: Cambridge, MA, USA; Elsevier: Amsterdam, The Netherlands, 2022; pp. 187–196. [Google Scholar]
- Garcia-Perez, J.V.; Blasco, M.; Carcel, J.A.; Clemente, G.; Mulet, A. Drying Kinetics of Grape Stalk. In Defect and Diffusion Forum; Trans Tech Publications Ltd.: Baech, Switzerland, 2006; Volumes 258–260, pp. 225–230. [Google Scholar]
- Prozil, S.O.; Costa, E.V.; Evtuguin, D.V.; Cruz Lopes, L.P.; Domingues, M.R.M. Structural characterization of polysaccharides isolated from grape stems of Vitis vinifera L. Carbohydr. Res. 2012, 356, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Prozil, S.O.; Evtuguin, D.V.; Cruz Lopes, L.P. Chemical composition of grape stems of Vitis vinifera L. from red grape pomaces. Ind. Crops Prod. 2012, 35, 178–184. [Google Scholar] [CrossRef]
- Pujol, D.; Liu, C.; Fiol, N.; Olivella, M.A.; Gominho, J.; Villaescusa, I.; Pereira, H. Chemical characterization of different granulometric fractions of grape stems waste. Ind. Crops Prod. 2013, 50, 494–500. [Google Scholar] [CrossRef]
- Oliveira, M.; Duarte, E. Integrated approach to winery waste: Waste generation and data consolidation. Front. Environ. Sci. Eng. 2016, 10, 168–176. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Sustainability of wine production. Sustainability 2020, 12, 559. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-chemical properties of cell wall materials obtained from ten grape varieties and their byproducts: Grape pomaces and stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Bordiga, M.; Travaglia, F.; Locatelli, M. Valorisation of grape pomace: An approach that is increasingly reaching its maturity–A review. Int. J. Food Sci. Technol. 2019, 54, 933–942. [Google Scholar] [CrossRef]
- Jin, Q.; O’Hair, J.; Stewart, A.C.; O’Keefe, S.F.; Neilson, A.P.; Kim, Y.-T.; McGuire, M.; Lee, A.; Wilder, G.; Huang, H. Compositional characterization of different industrial white and red grape pomaces in Virginia and the potential valorization of the major components. Foods 2019, 8, 667. [Google Scholar] [CrossRef]
- Pedras, B.M.; Regalin, G.; Sá-Nogueira, I.; Simões, P.; Pavia, A.; Barreiros, S. Fractionation of red wine grape pomace by subcritical water extraction/hydrolysis. J. Supercrit. Fluids 2020, 160, 104793. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; Neto, P.O.; Fernades da Silva, D.; Pastore, C.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J. Mild autohydrolysis: An environmentally friendly technology for xylooligosaccharide production from wood. J. Chem. Technol. Biotechnol. 1999, 74, 1101–1109. [Google Scholar] [CrossRef]
- Amendola, D.; De Faveri, D.; Egües, I.; Serrano, L.; Labidi, J.; Spigno, G. Autohydrolysis and organosolv process for recovery of hemicelluloses, phenolic compounds and lignin from grape stalks. Bioresour. Technol. 2012, 107, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Evaluation of grape stalks as a bioresource. Ind. Crops Prod. 2011, 33, 200–204. [Google Scholar] [CrossRef]
- Spigno, G.; Moncalvo, A.; de Faveri, D.M.; Silva, A. Valorisation of stalks from different grape cultivars for sugars recovery. Chem. Eng. Trans. 2014, 37, 745–750. [Google Scholar]
- Corbin, K.R.; Hsieh, Y.S.Y.; Betts, N.S.; Byrt, C.S.; Henderson, M.; Stork, J.; DeBolt, S.; Fincher, G.B.; Burton, R.A. Grape marc as a source of carbohydrates for bioethanol. Bioresour. Technol. 2015, 193, 76–83. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S. Optimization of a method to extract polysaccharides from white grape pomace by-products. Food Chem. 2021, 365, 130445. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S.; Doco, T. Characterization of polysaccharide extracts recovered from different grape and winemaking products. Food Res. Int. 2022, 157, 111480. [Google Scholar] [CrossRef]
- TAPPI 211 om-93. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 °C; TAPPI Press: Atlanta, GA, USA, 1993. [Google Scholar]
- TAPPI 222 om-02. Acid-Insoluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 2002. [Google Scholar]
- TAPPI UM 250. Acid-Soluble Lignin in Wood and Pulp; TAPPI Press: Atlanta, GA, USA, 1991. [Google Scholar]
- Haaland, P.D. Experimental Design in Biotechnology; Marcel Dekker Inc.: New York, NY, USA, 1989. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 3rd ed.; John Wiley & Sons: New York, NY, USA, 2017. [Google Scholar]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Philos. Trans. R. Soc. 1987, A321, 523–536. [Google Scholar]
- Zhang, Y.H.P.; Hong, J.; Ye, X. Cellulase assays. Methods Mol. Biol. 2009, 581, 213–231. [Google Scholar]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- Freitas, L.; Simões, R.; Miranda, I.; Peres, F.; Ferreira-Dias, S. Optimization of autohydrolysis of olive pomaces to obtain bioactive oligosaccharides: The effect of cultivar and fruit ripening. Catalysts 2022, 12, 788. [Google Scholar] [CrossRef]
- Cara, C.; Ruiz, E.; Carvalheiro, F.; Moura, P.; Ballesteros, I.; Castro, E.; Gírio, F. Production, purification and characterisation of oligosaccharides from olive tree pruning autohydrolysis. Ind. Crops Prod. 2012, 40, 225–231. [Google Scholar] [CrossRef]
- Antonić, B.; Jančíková, S.; Dordevíć, D.; Tremlová, B. Grape Pomace Valorization: A Systematic Review and Meta-Analysis. Foods 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stems and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crops Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Spigno, G.; Maggi, L.; Amendola, D.; Dragoni, M.; de Faveri, D.M. Influence of cultivar on the lignocellulosic fractionation of grape stems. Ind. Crops Prod. 2013, 46, 283–289. [Google Scholar] [CrossRef]
- Spigno, G.; Pizzorno, T.; de Faveri, D.M. Cellulose and hemicelluloses recovery from grape stems. Bioresour. Technol. 2008, 99, 4329–4337. [Google Scholar] [CrossRef] [PubMed]
- Atatoprak, T.; Amorim, M.M.; Ribeiro, T.; Pintado, M.; Madureira, A.R. Grape Stalk Valorization for Fermentation Purposes. Food Chem. Mol. Sci. 2022, 4, 100067. [Google Scholar] [CrossRef]
- Mendes, J.A.S.; Prozil, S.O.; Evtuguin, D.V.; Cruz Lopes, L.P. Towards comprehensive utilization of winemaking residues: Characterization of grape skins from red grape pomaces of variety Touriga Nacional. Ind. Crops Prod. 2013, 43, 25–32. [Google Scholar] [CrossRef]
- Garrote, G.; Domínguez, H.; Parajó, J.C. Manufacture of xylose-based fermentation media from corncobs by posthydrolysis of autohydrolysis liquors Appl. Biochem. Biotechnol. 2001, 95, 195–207. [Google Scholar] [CrossRef]
- Dávila, I.; Gordobil, O.; Labidi, J.; Gullón, P. Assessment of suitability of vine shoots for hemicellulosic oligosaccharides production through aqueous processing. Bioresour. Technol. 2016, 211, 636–644. [Google Scholar] [CrossRef]
- Jesus, M.S.; Romaní, A.; Genisheva, Z.; Teixeira, J.A.; Domingues, L. Integral valorization of vine pruning residue by sequential autohydrolysis stages. J. Clean. Prod. 2017, 168, 74–86. [Google Scholar] [CrossRef]
- Gullón, B.; Gemma, E.; Dávila, I.; Moreira, M.T.; Labidi, J.; Gullón, P. Hydrothermal treatment of chestnut shells (Castanea sativa) to produce oligosaccharides and antioxidant compounds. Carbohydr. Polym. 2018, 192, 75–83. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).