Study on Influencing Factors of Molecular Sieve Oxygen-Production System
Abstract
:1. Introduction
2. Operating Principle of Molecular Sieve Oxygen Generation
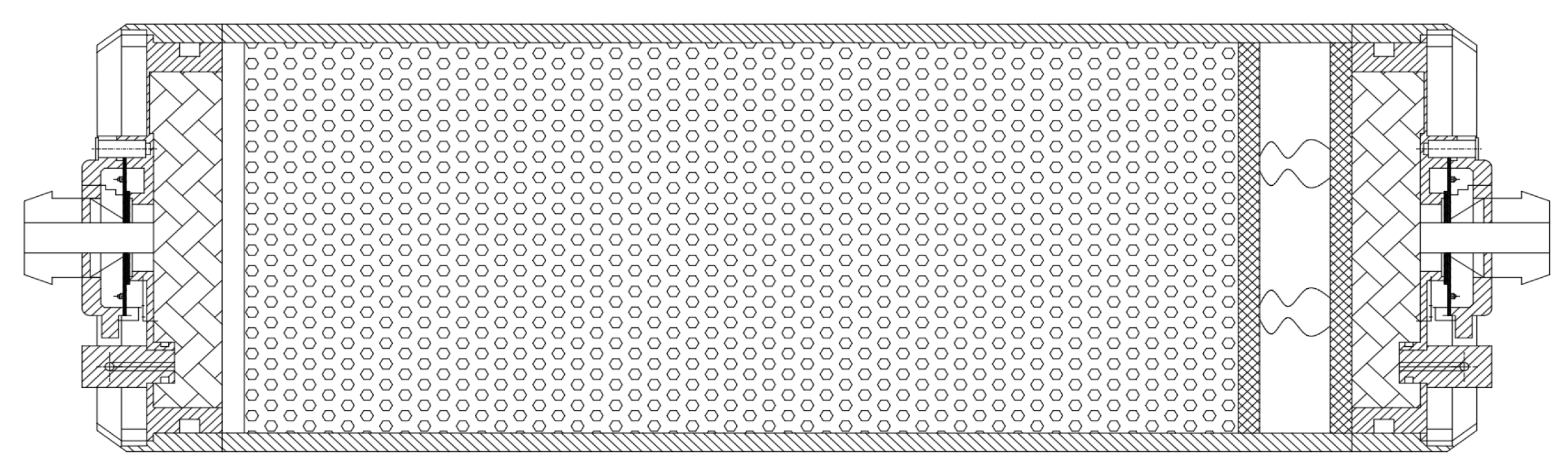
3. Composition Analysis of Molecular Sieve Oxygen-Generation System
3.1. Oxygen System Air-Intake Unit
3.2. Oxygen-Production System Air Compressor
- (1)
- In general, the exhaust pressure of an air compressor in the process of oxygen production should be controlled between 0.5–4 bar. According to the amount of their exhaust pressure, air compressors can be divided into 0.05–2.0 MPa low-pressure air compressors, 20–100 bar medium-pressure air compressors, 100–1000 bar high-pressure air compressors and 1000 bar ultra-high-pressure air compressors. The exhaust pressure of the air compressor used in the molecular sieve oxygen system studied in this paper should be controlled between 0.8–1.7 bar, so the low-pressure air compressor was selected.
- (2)
- Due to the characteristics of air compressors, many of them work with an oil medium as the piston, with oil attached to the gas, so if there is oil gas in the oxygen-production process that goes into the oxygen system, causing the oil gas and a high concentration of oxygen to be mixed with an open fire, it can very easily explode, which is a great safety hazard; in addition, the oil gas will contaminate the oxygen, so that it can not meet the requirements of breathing oxygen. The most serious concern is that the oil gas can contaminate the molecular sieve, which may make the molecular sieve fail. In order to make the gas oxygen concentration high enough, the molecular sieve should be used normally, so it is necessary to avoid the interference of other impurities in the process of oxygen production and choose an oil-free air compressor.
- (3)
- The exhaust volume of the air compressor is generally around 3.5 m3/h. The molecular sieve oxygen system’s theoretical demand for oxygen flow has a limit value of 5 L/min, due to the molecular sieve bed cycle’s reciprocal work; the actual state of the air compressor is neither a continuous working state nor a completely continuous state. From the previous experimental research, it is known that the production gas recovery rate of the general oxygen system is generally about 25%; by taking a recovery rate of 25%, the required exhaust volume can be calculated as shown in Formula (1).
- (4)
- Turbulent noise and structural noise control. As the studied molecular sieve oxygen system is mostly used for highland travel or home oxygen health care, the system noise level generally needs to be kept under 55 dB. The main sources of noise are the gas turbulence and structural vibrations in the air compressor, so the noise generated by the air compressor in the process of gas introduction and the working vibrations should be strictly controlled.
3.3. Molecular Sieve of Oxygen-Generation System
3.4. Molecular Sieve Bed of Oxygen-Generation System
4. Experiment on Influence of Cycle Period and Calibrating Hole
4.1. Oxygen Cycle Selection
4.2. Effect of Different Sizing Hole Diameters on Oxygen Concentration
5. Conclusions
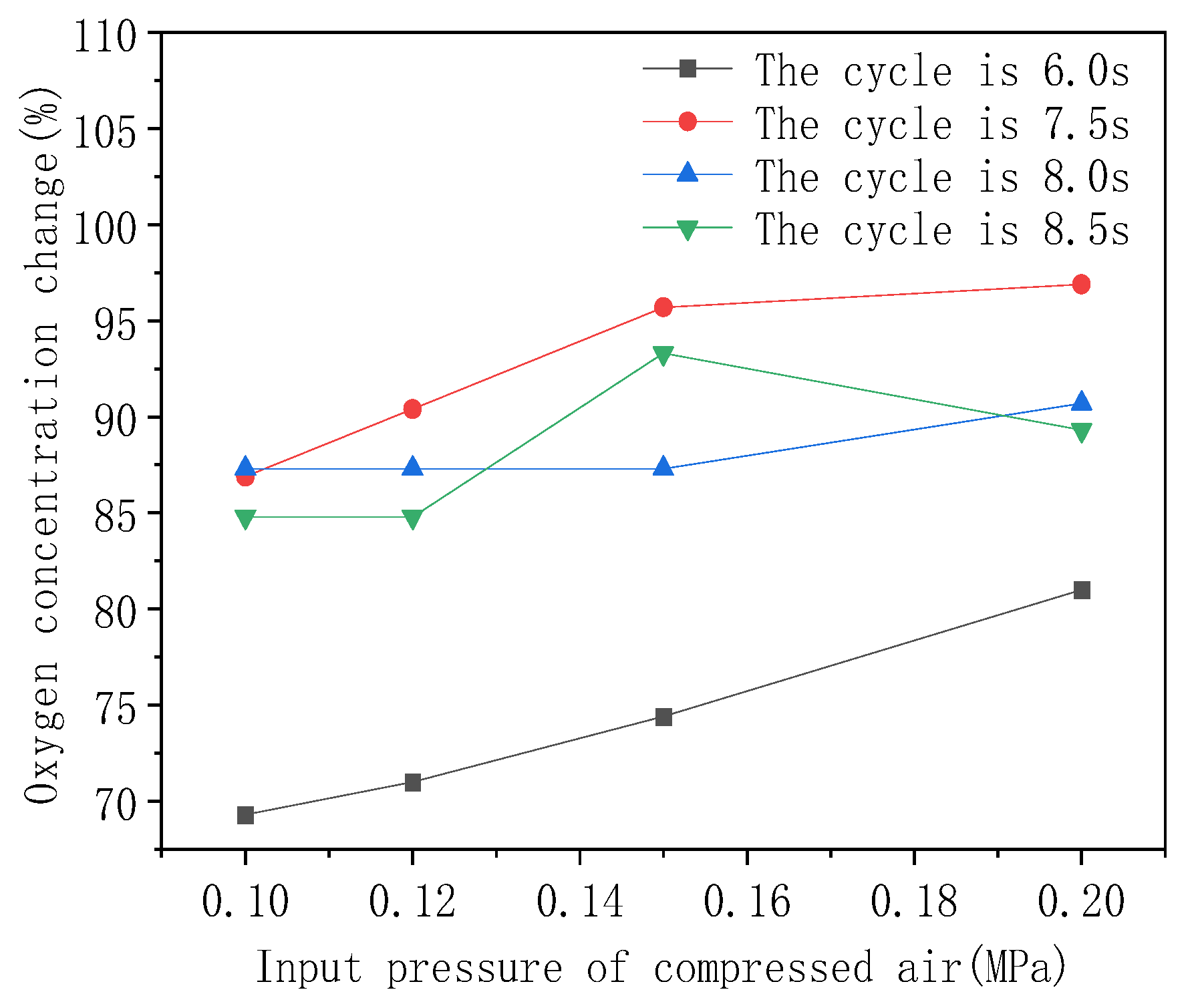
- In this paper, the two-bed type molecular sieve oxygen system is used as the experimental object, and key units of the system are selected. When the cycle is stable, the relationship between the outlet oxygen concentration of the two-bed-type molecular sieve oxygen system and the cycle period and the flushing sizing hole is obtained. With the change in the flushing cycle, the outlet oxygen concentration range changes, and an oxygen cycle of 8.0 s is more significant compared to 7.0 s and 8.5 s.
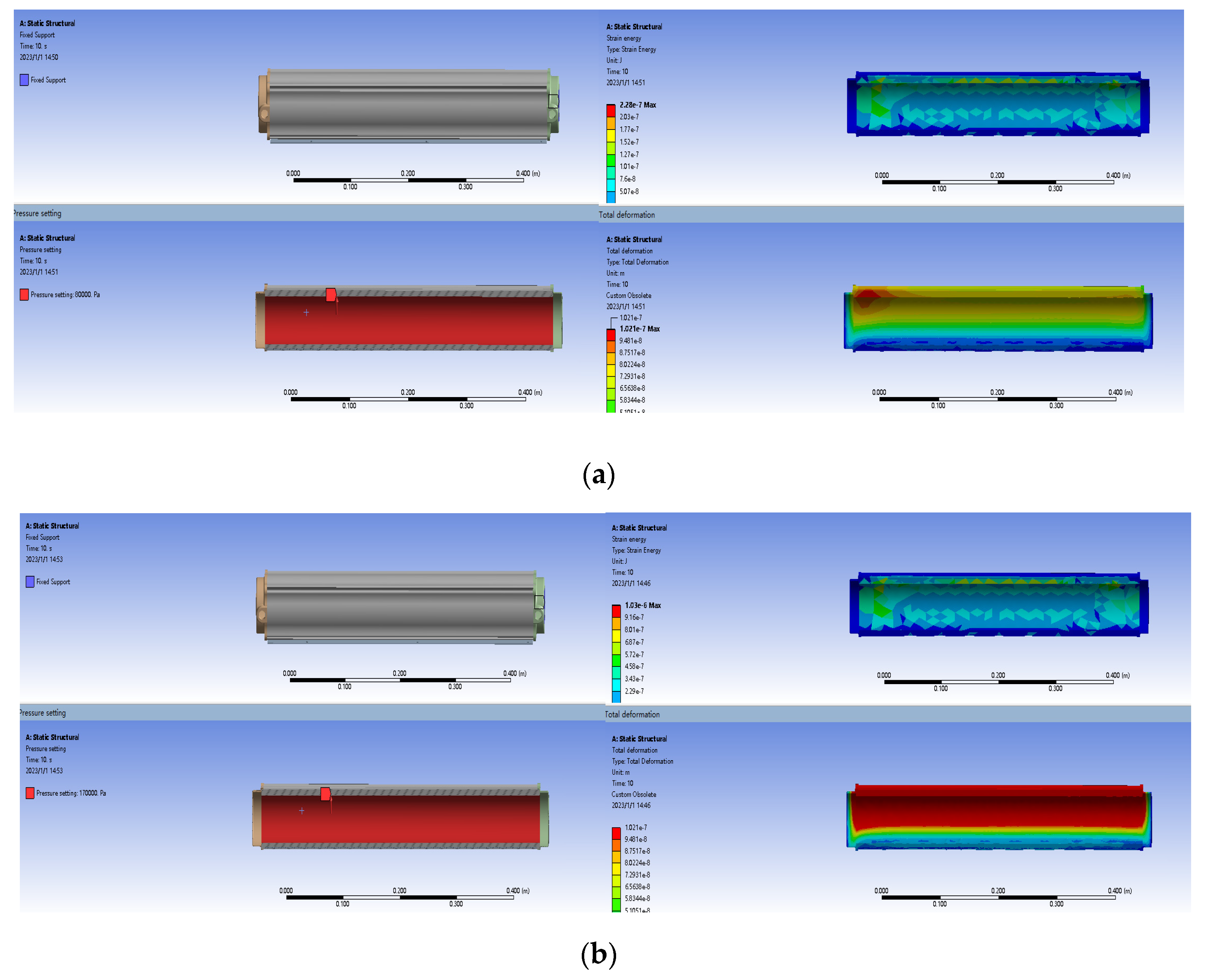
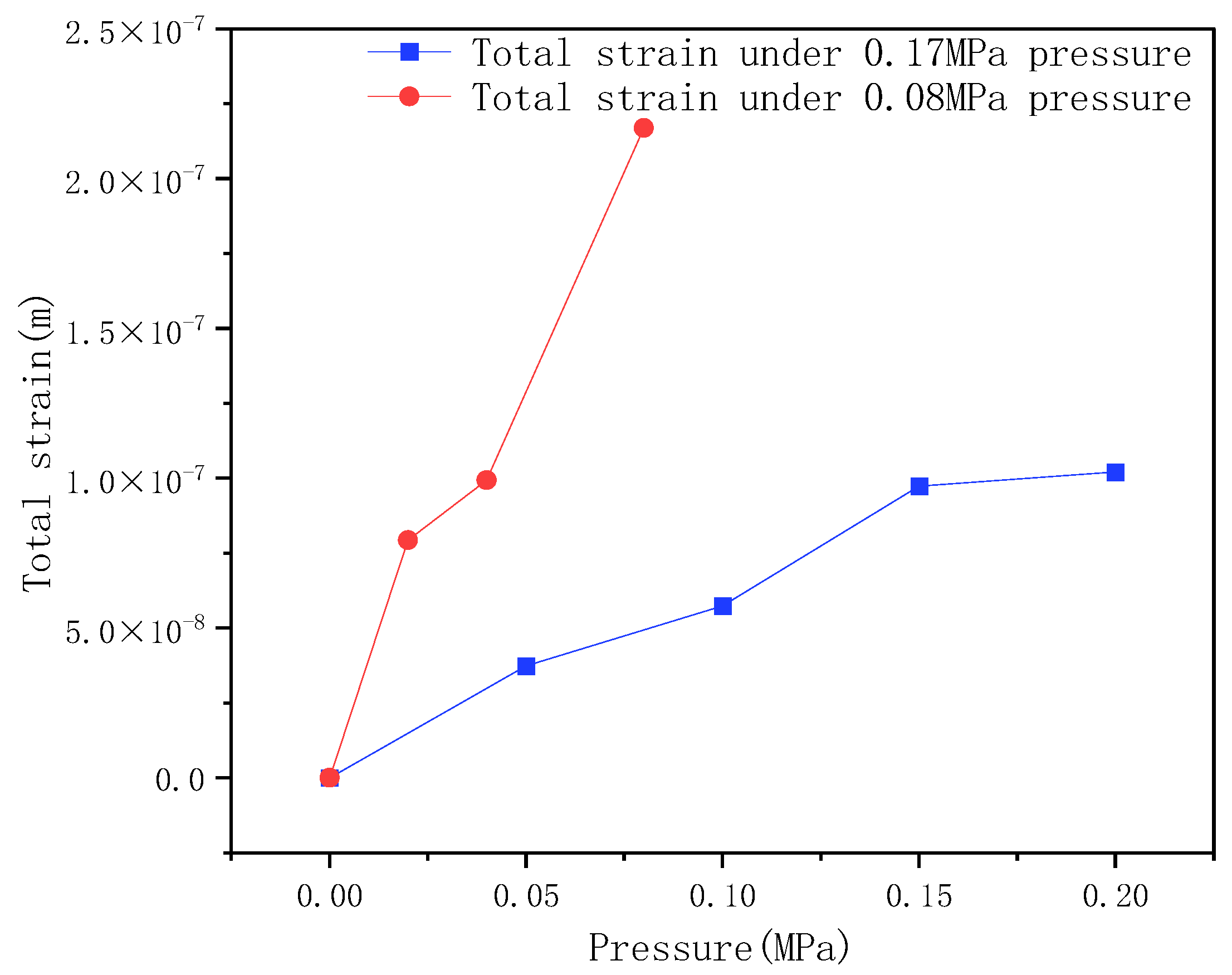
- Since the Li-X type lithium molecular sieve material has good adsorption performance under 0.08–0.17 MPa pressure, it is selected as the molecular sieve in the molecular sieve bed for the molecular sieve oxygen-production system studied in this paper. From the experimental data, it can be obtained that when the system lead gas is in the range of 0.08–0.17 MPa, the larger the inlet pressure is, the larger the oxygen concentration of the gas obtained from the oxygen production system is.
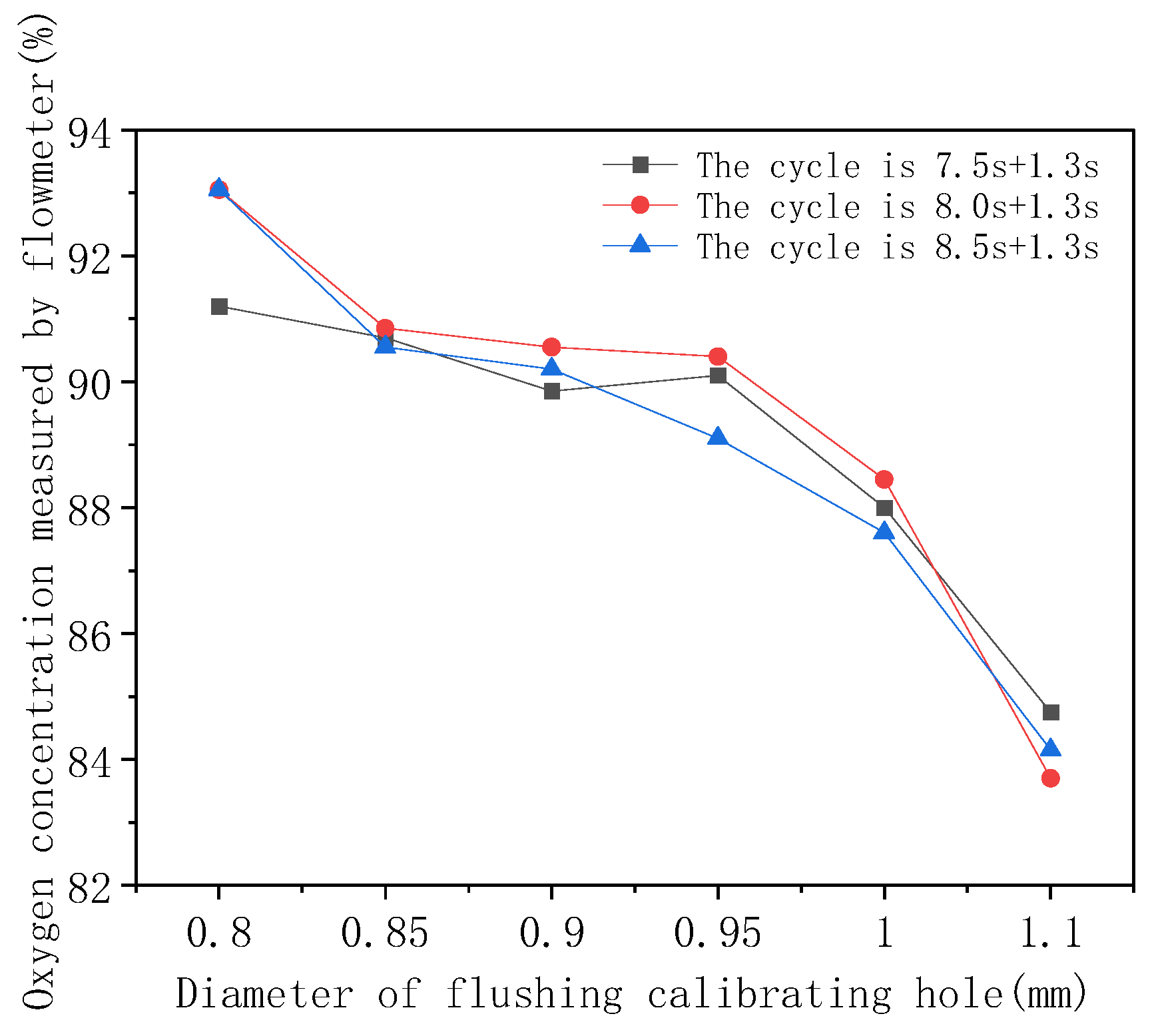
- The size of the flushing sizing pore diameter directly affects the desorption effect of the gas passing through the molecular sieve bed, thus affecting the oxygen concentration at the oxygen outlet of the system. Other conditions are certain, under different cycle configurations, and, according to the data analysis, the larger the oxygen concentration is, the smaller the corresponding optimal sizing hole diameter is; the best flushing sizing hole diameter of the molecular sieve system is 0.8 mm.
- The optimal oxygen production and flushing cycle configuration exist with each condition of the system being constant. From Figure 7, it can be seen that the oxygen concentration at the system outlet is optimal under a cycle time configuration of 8.0 s + 1.3 s, so 8.0 s + 1.3 s is the optimal oxygen production and pressure-equalization cycle configuration.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, R. Gas Separation by Adsorption Processes; Butterwonh: London, UK, 1987. [Google Scholar]
- Sircar, S. Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2002, 41, 1389–1392. [Google Scholar] [CrossRef]
- Ruthven, D.M. Principles of Adsorption and Adsorption Process; John Wiley & Sons: New York, NY, USA, 1984; pp. 29–85. [Google Scholar]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Rouquerol, F.; Rouquerol, J.; Sing, K. Adsorption by Powders and Porous Solids; Academic Press: Cambridge, MA, USA, 2014; pp. 1–23. [Google Scholar]
- Li, P.; Handan Tezel, F. Adsorption separation of N2, O2, CO2 and CH4 gases by β-zeolite. Microporous Mesoporous Mater. 2007, 98, 94–101. [Google Scholar] [CrossRef]
- Kim, M.-B.; Jee, J.-G.; Bae, Y.-S.; Lee, C.H. Parametric Study of Pressure Swing Adsorption Process to Purify Oxygen Using Carbon Molecular Sieve. Ind. Eng. Chem. Res. 2005, 44, 7208–7217. [Google Scholar] [CrossRef]
- Skarstrom, C.W. Method and Apparatus for Fractionating Gaseous Mixtures by Adsorption. U.S. 2,944,627, 12 July 1960. [Google Scholar]
- Guerin de Montgareuil, P.; Domine, D. Process for Separating a Binary Gaseous Mixture by Adsorption. U.S. 3,155,468, 3 November 1964. [Google Scholar]
- Mofarahi, M.; Towfighi, J.; Fathi, L. Oxygen Separation from Air by Four-Bed Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2009, 48, 5439–5444. [Google Scholar] [CrossRef]
- Beh, C.C.K.; Webley, P.A. A Practical Method for the Dynamic Determination of the Product Oxygen Concentration in Pressure-Swing Adsorption Systems. Ind. Eng. Chem. Res. 2003, 42, 5287–5292. [Google Scholar] [CrossRef]
- Chao, J.; Ying, Z.; Wei, G.; Cui, L. Anoinic emulsion-mediated synthesis of zeolite beta. Int. J. Mod. Phys. B 2010, 24, 3236–3241. [Google Scholar]
- Sirawich, P.; Chantaraporn, P. Aspen adsorption simulation for the effects of purge flow rate and vacuum pressure in vacuum pressure swing adsorption. Mater. Today Proc. 2022, 52, 2517–2522. [Google Scholar]
- Deng, M.; Zhang, Q.; Huang, Y.; Zhang, X. Integration and optimization for a PEMFC and PSA oxygen production combined system. Energy Convers. Manag. 2021, 236, 114062. [Google Scholar] [CrossRef]
- ExxonMobil Research and Engineering Company. Patent Issued for Pressure Swing Adsorption for Oxygen Production. USPTO 10,259,711, 2019. Energy Weekly News. [Google Scholar]
- Pressure Swing Adsorption for Oxygen Production. USPTO 20,170,305,744, 2017. Journal of Engineering.
- Cheng, M.; Verma, P.; Yang, Z.; Axelbaum, R.L. Single-column cryogenic air separation: Enabling efficient oxygen production with rapid startup and low capital costs—Application to low-carbon fossil-fuel plants. Energy Convers. Manag. 2021, 248, 114773. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, W.; Qin, W.; Gui, W.; Xu, S.; Yang, C.; Zhang, Z.; Wang, Y.; Zheng, J.; Liu, X. Analysis of Carbon Footprint Reduction for Three Novel Air Separation Columns. Sep. Purif. Technol. 2021, 262, 118318. [Google Scholar] [CrossRef]
- Cai, R.; Dou, J.; Krzystowczyk, E.; Richard, A.; Li, F. Chemical looping air separation with Sr0.8Ca0.2Fe0.9Co0.1O3-δ perovskite sorbent: Packed bed modeling, verification, and optimization. Chem. Eng. J. 2022, 429, 132370. [Google Scholar] [CrossRef]
- Diego, K.; Øivind, W.; Signe, K. Minimum entropy production in a distillation column for air separation described by a continuous non-equilibrium model. Chem. Eng. Sci. 2020, 218, 115539. [Google Scholar]
- Klein, H.; Fritsch, P.; Haider, P.; Kender, R.; Rößler, F.; Rehfeldt, S.; Freko, P.; Hoffmann, R.; Thomas, I.; Wunderlich, B. Flexible Operation of Air Separation Units. Chem. Bio. Eng. Rev. 2021, 8, 357–374. [Google Scholar] [CrossRef]
- Chuah, C.Y.; Goh, K.; Bae, T.-H. Enhanced Performance of Carbon Molecular Sieve Membranes Incorporating Zeolite Nanocrystals for Air Separation. Membranes 2021, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Rong, Y.; Fang, S.; Wang, K.; Zhi, X.; Qiu, L.; Chi, X. Thermodynamic analysis of an organic Rankine–vapor compression cycle (ORVC) assisted air compression system for cryogenic air separation units. Appl. Therm. Eng. 2021, 189, 116678. [Google Scholar] [CrossRef]
- Qadir, S.; Li, D.; Gu, Y.; Yuan, Z.; Zhao, Y.; Wang, S.; Wang, S. Experimental and Numerical Analysis on the Enhanced Separation Performance of a Medical Oxygen Concentrator through two-bed rapid pressure swing adsorption. Ind. Eng. Chem. Res. 2021, 60, 5903–5913. [Google Scholar] [CrossRef]
- Liang, D.; Hu, Y.; Bao, Q.; Zhang, J.; Feng, J.; Sun, P.; Ma, Y.; Zhang, H. A suitable zeolite Rho for separating CO2/CH4 in pressure swing adsorption (PSA) process. Inorg. Chem. Commun. 2021, 127, 108547. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, Y.; Yang, R.T. Effects of operating temperature on the performance of small scale rapid cycle pressure swing adsorption air separation process. Adsorption 2020, 27, 205–212. [Google Scholar] [CrossRef]
- Hao, P.; Liu, Z.; Shi, Y.; Li, S.; Cai, N. Characteristics of activated carbon in elevated-temperature pressure swing adsorption desulfurization. Adsorption 2019, 25, 1219–1226. [Google Scholar] [CrossRef]


| Pneumatic Components | Air Medium Quality Grade | ||
|---|---|---|---|
| Particulate Matter | Steam | Oil | |
| Air compressor | ≤4 | ≤3 | ≤5 |
| Pneumatic reversing valve | 3—2 | 3—2 | 4—3 |
| Molecular Sieve Code | Particle Size/mm | Compressive Strength | Density of Bulk/(kg·L−1) | N2 Adsorption Capacity (1 bar/25 °C)/(NL·kg−1) | Oxygen Nitrogen Selection Coefficient (1 bar/25 °C) | Rate of Oxygen Production/(NL·kg−1·h−1) |
|---|---|---|---|---|---|---|
| 1 | 1.6–2.5 | >30 N | 0.62 | >8.3 | >3.5 | 60 |
| 2 | 1.6 ± 0.1 | >25 N | 0.63 | >19.5 | >6.2 | 75 |
| 3 | 0.63 ± 0.07 | >1.7 kg | 0.61 | >8.0 | 3.1 ± 0.2 | 120 |
| 4 | 0.4–0.6 | >2.8 MPa | 0.62 | >19 | >6 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, D.; Li, H. Study on Influencing Factors of Molecular Sieve Oxygen-Production System. Processes 2023, 11, 124. https://doi.org/10.3390/pr11010124
Jiang D, Li H. Study on Influencing Factors of Molecular Sieve Oxygen-Production System. Processes. 2023; 11(1):124. https://doi.org/10.3390/pr11010124
Chicago/Turabian StyleJiang, Dongsheng, and Hui Li. 2023. "Study on Influencing Factors of Molecular Sieve Oxygen-Production System" Processes 11, no. 1: 124. https://doi.org/10.3390/pr11010124





