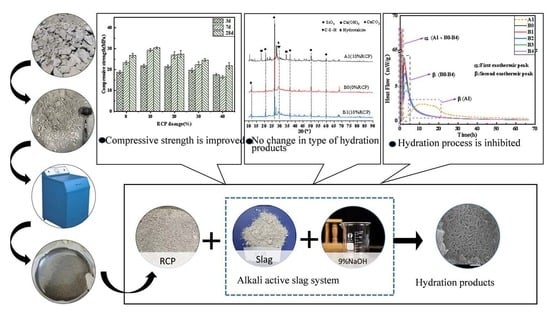
A Study on the Application of Recycled Concrete Powder in an Alkali-Activated Cementitious System
Abstract
:Highlights
Abstract
1. Introduction
2. Experimental
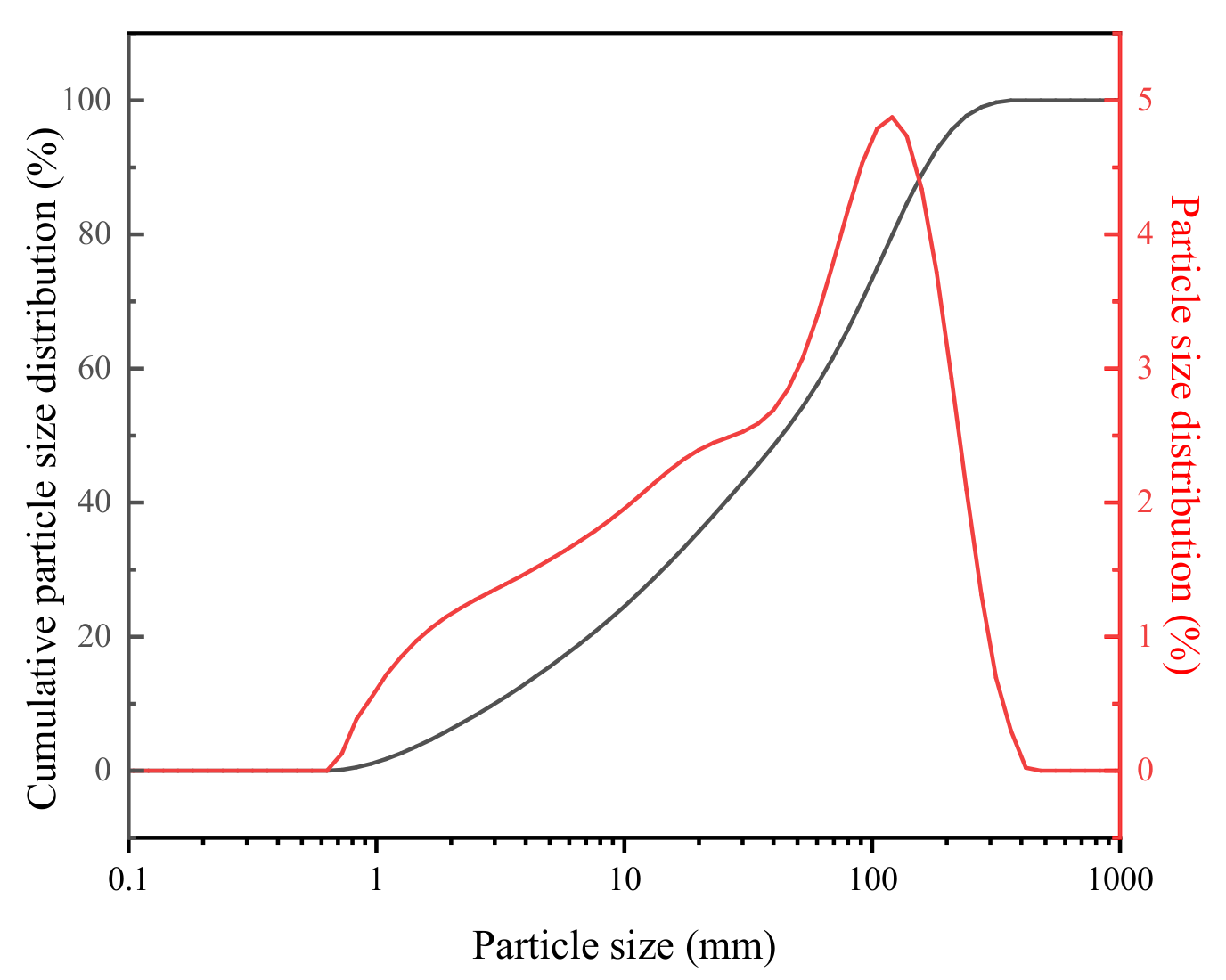
2.1. Materials
2.2. Preparation of Specimens
2.3. Test and Characterization
2.3.1. Fluidity and Mechanical Properties
2.3.2. Rheological Properties
2.3.3. Hydration Heat Analysis
3. Results and Discussion
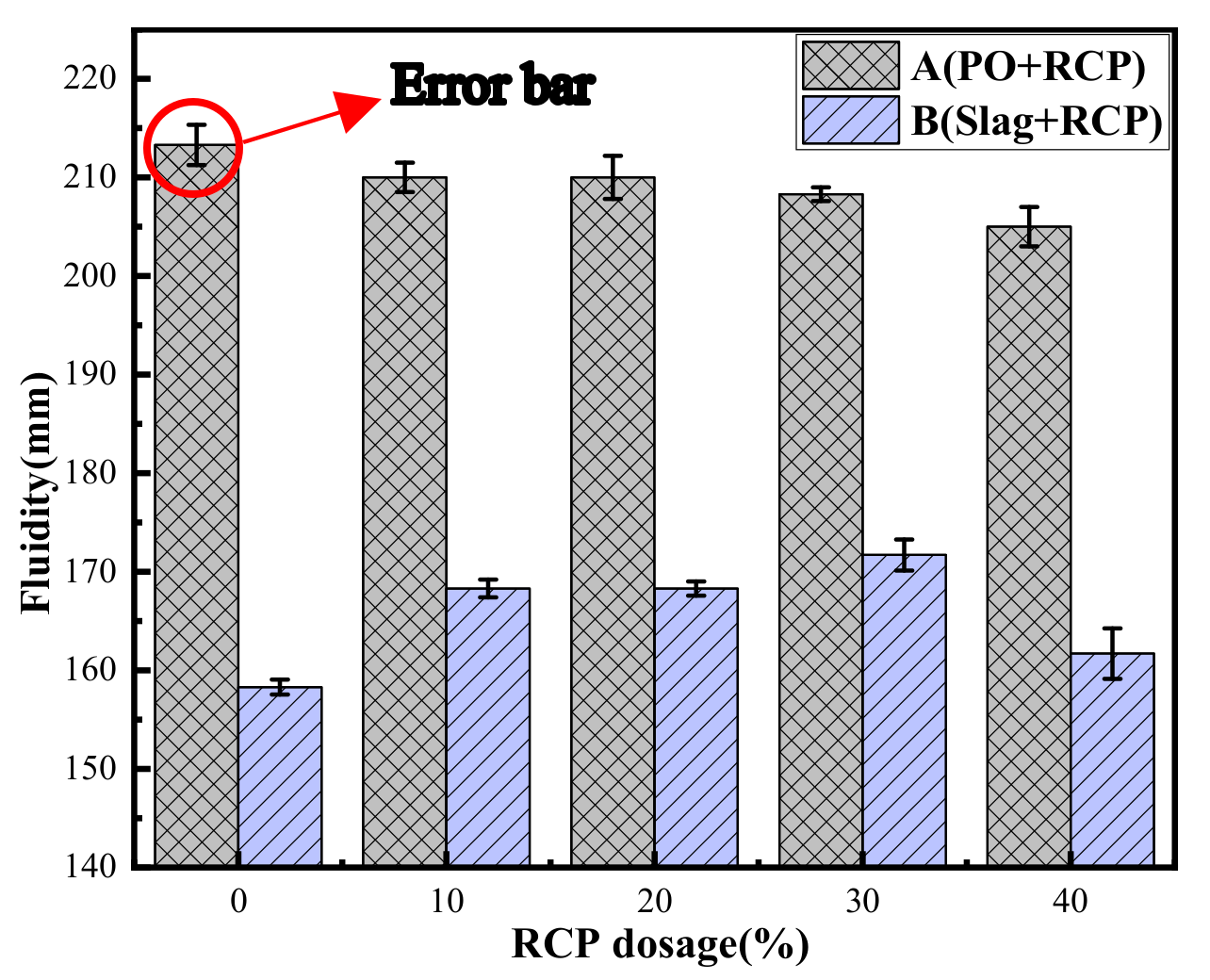
3.1. Fluidity
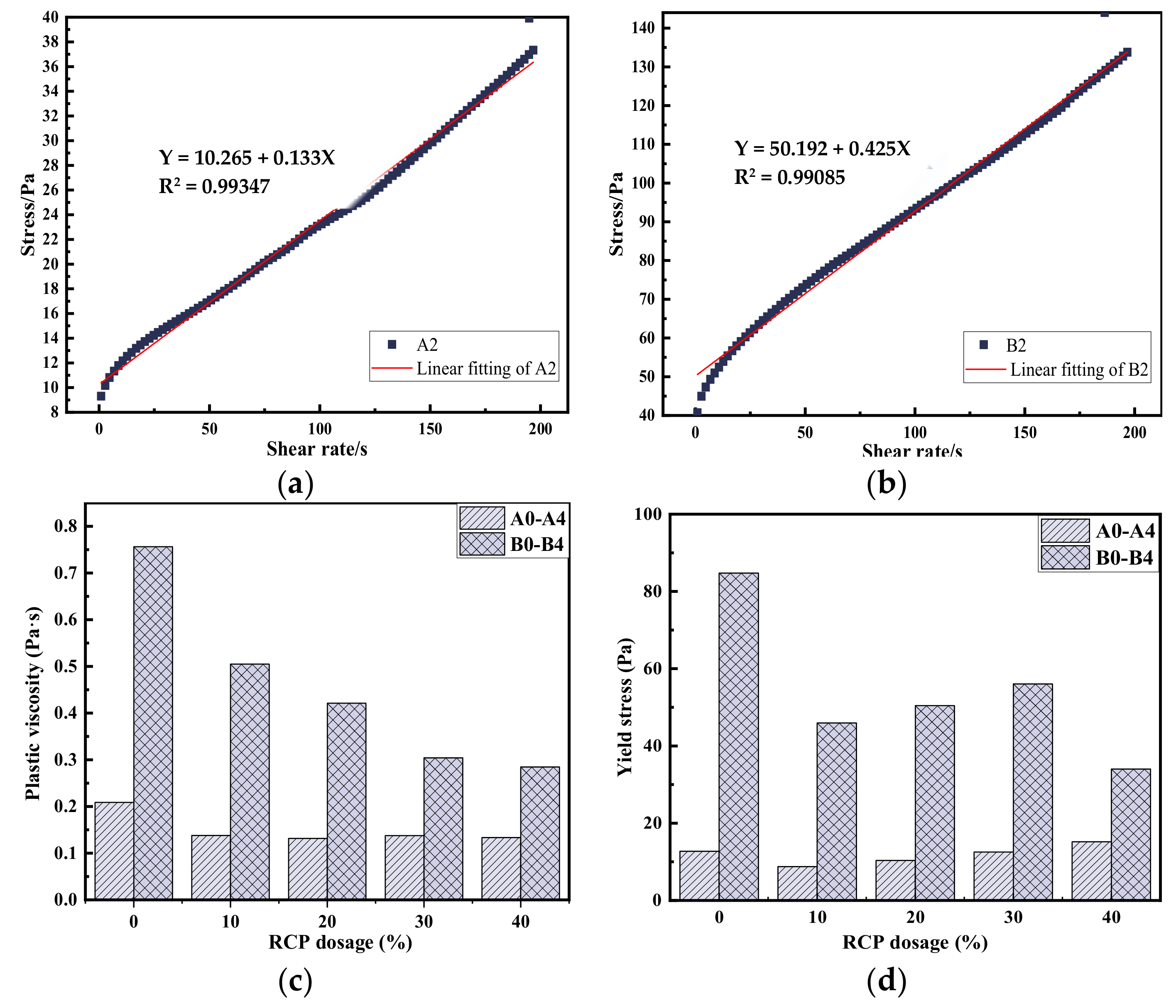
3.2. Rheological Properties
3.3. Mechanical Properties
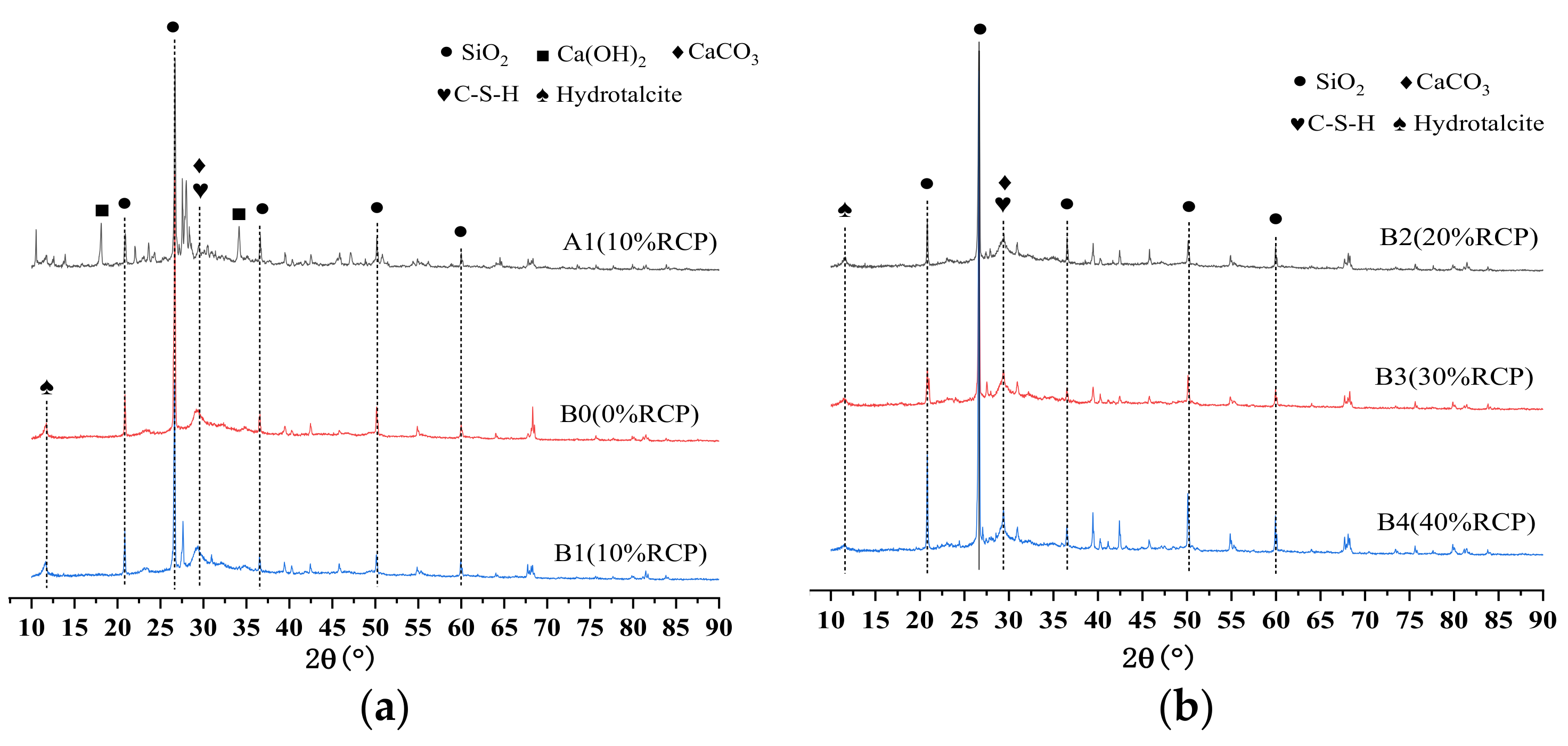
3.4. XRD Analysis
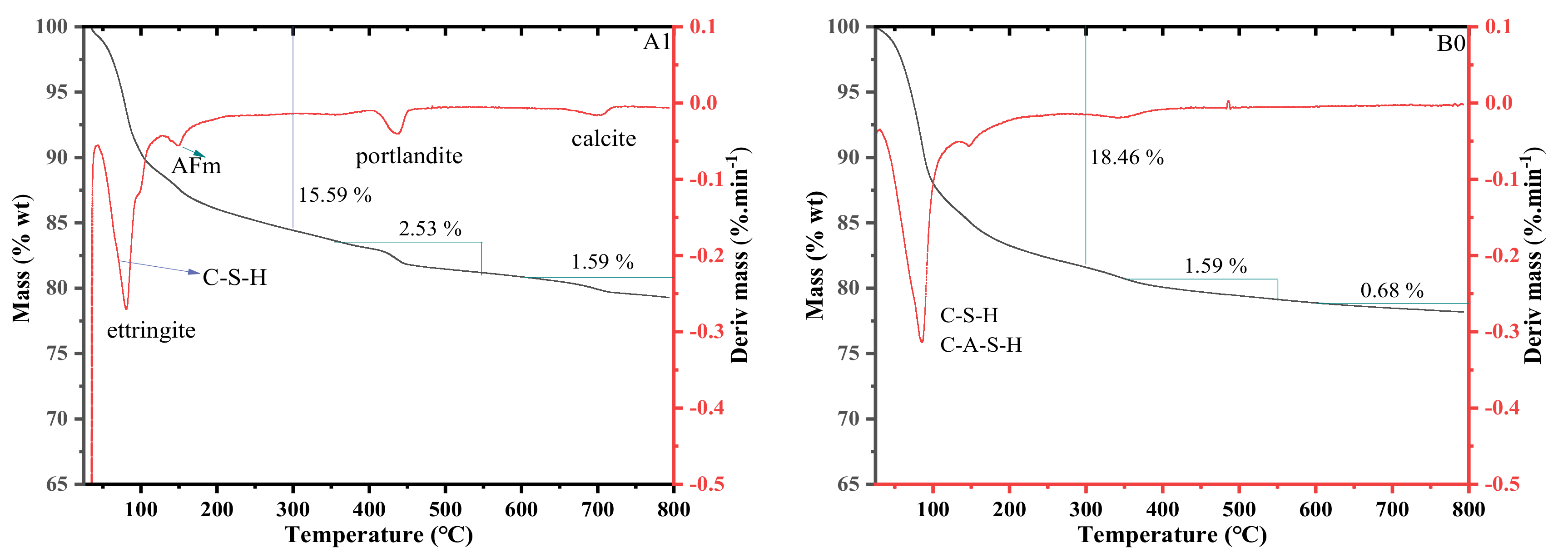
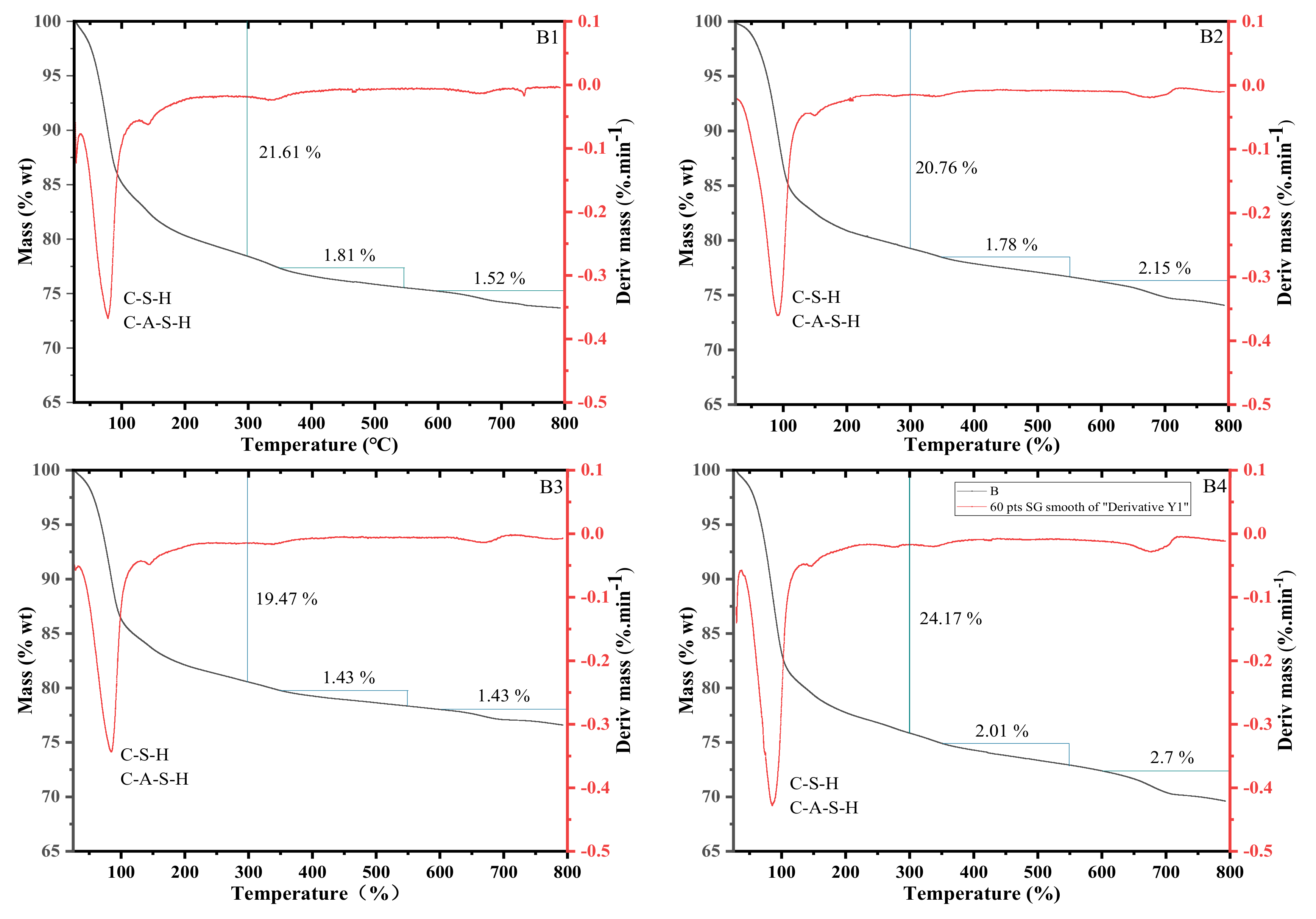
3.5. Thermogravimetric Analysis
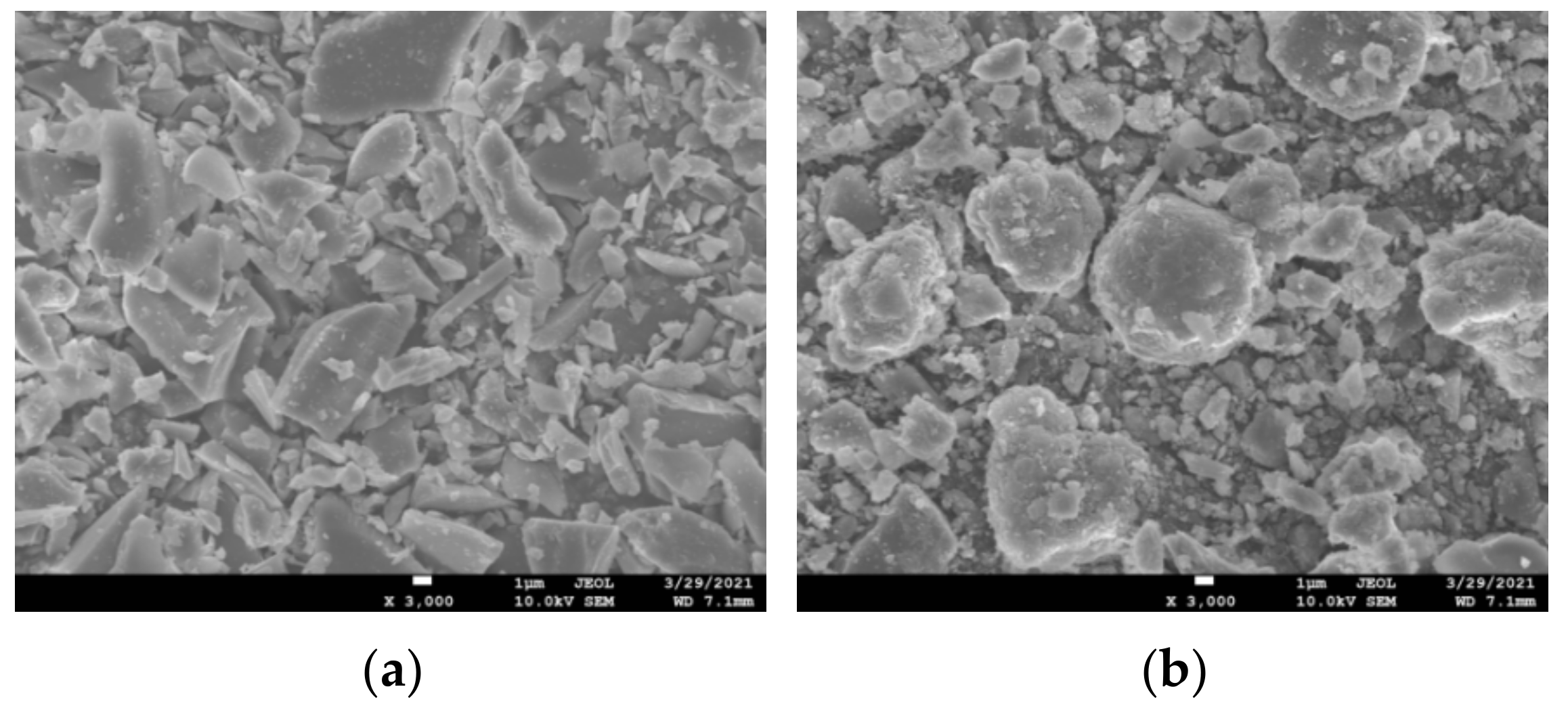
3.6. SEM-EDS Analysis
3.7. Hydration Heat Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yazdani, M.; Kabirifar, K.; Frimpong, B.E.; Shariati, M.; Mirmozaffari, M.; Boskabadi, A. Improving construction and demolition waste collection service in an urban area using a simheuristic approach: A case study in Sydney, Australia. J. Clean. Prod. 2021, 280, 124138. [Google Scholar] [CrossRef]
- Yue, G.; Ma, Z.; Liu, M.; Liang, C.; Ba, G. Damage behavior of the multiple ITZs in recycled aggregate concrete subjected to aggressive ion environment. Constr. Build. Mater. 2020, 245, 118419. [Google Scholar] [CrossRef]
- Wu, H.; Zuo, J.; Zillante, G.; Wang, J.; Yuan, H. Status quo and future directions of construction and demolition waste research: A critical review. J. Clean. Prod. 2019, 240, 118163. [Google Scholar] [CrossRef]
- Zheng, L.; Wu, H.; Zhang, H.; Duan, H.; Wang, J.; Jiang, W.; Dong, B.; Liu, G.; Zuo, J.; Song, Q. Characterizing the generation and flows of construction and demolition waste in China. Constr. Build. Mater. 2017, 136, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Hu, M.; Di Maio, F.; Sprecher, B.; Yang, X.; Tukker, A. An overview of the waste hierarchy framework for analyzing the circularity in construction and demolition waste management in Europe. Sci. Total Environ. 2022, 803, 149892. [Google Scholar] [CrossRef] [PubMed]
- GB/T 25177-2010. Recycled Coarse Aggregate for Concrete. China Standards Press: Beijing, China, 2010.
- GB/T 25176-2010. Recycled Fine Aggregate for Concrete and Mortar. China Standards Press: Beijing, China, 2010.
- JGJ/T 240-2011. Technical Specification of Reclaimed Aggregate Application. China Architecture & Building Press: Beijing, China, 2011.
- JG/T 573-2020. Recycled Fine Powder for Concrete and Mortar. China Standards Press: Beijing, China, 2020.
- Li, S.; Gao, J.; Li, Q.; Zhao, X. Investigation of using recycled powder from the preparation of recycled aggregate as a supplementary cementitious material. Constr. Build. Mater. 2021, 267, 120976. [Google Scholar] [CrossRef]
- Oliveira, T.C.F.; Dezen, B.G.S.; Possan, E. Use of concrete fine fraction waste as a replacement of Portland cement. J. Clean. Prod. 2020, 273, 123126. [Google Scholar] [CrossRef]
- Kim, J.; Jang, H. Closed-loop recycling of C & D waste: Mechanical properties of concrete with the repeatedly recycled C & D powder as partial cement replacement. J. Clean. Prod. 2022, 343, 130977. [Google Scholar]
- Zhu, P.; Mao, X.Q.; Qu, W.; Li, Z.; Ma, Z.J. Investigation of using recycled powder from waste of clay bricks and cement solids in reactive powder concrete. Constr. Build. Mater. 2016, 113, 246–254. [Google Scholar] [CrossRef]
- Rani, M.U.; Jenifer, J.M. Mechanical Properties of Concrete with Partial replacement of Portland Cement by Clay brick powder. Int. J. Eng. Res. Technol (IJERT) 2016, 5, 63–67, ISSN 2278–0181. [Google Scholar]
- Xiao, J.; Ma, Z.; Sui, T.; Akbarnezhad, A.; Duan, Z. Mechanical properties of concrete mixed with recycled powder produced from construction and demolition waste. J. Clean. Prod. 2018, 188, 720–731. [Google Scholar] [CrossRef]
- Zhao, Y.; Gao, J.; Liu, C.; Chen, X.; Xu, Z. The particle-size effect of waste clay brick powder on its pozzolanic activity and properties of blended cement. J. Clean. Prod. 2020, 242, 118521. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Zhu, Z.; Ma, L. Evaluation of waste concrete recycled powder (WCRP) on the preparation of low-exothermic cement. J. Build. Eng. 2022, 53, 104511. [Google Scholar] [CrossRef]
- He, Z.; Shen, A.; Wu, H.; Wang, W.; Wang, L.; Yao, C.; Wu, J. Research progress on recycled clay brick waste as an alternative to cement for sustainable construction materials. Constr. Build. Mater. 2021, 274, 122113. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, X.; Lu, N.; Hou, S.; He, Z.; Liang, C. Effect of recycled powders on the mechanical properties and durability of fully recycled fiber-reinforced mortar. J. Build. Eng. 2022, 45, 103574. [Google Scholar] [CrossRef]
- Ma, Z.; Li, W.; Wu, H.; Cao, C. Chloride permeability of concrete mixed with activity recycled powder obtained from C & D waste. Constr. Build. Mater. 2019, 199, 652–663. [Google Scholar]
- Mao, X.; Qu, W.; Zhu, P.; Xiao, J. Influence of recycled powder on chloride penetration resistance of green reactive powder concrete. Constr. Build. Mater. 2020, 251, 119049. [Google Scholar] [CrossRef]
- Ding, R.; He, Y.; Li, X. Research status of alkali-activated cementitious materials. China Concr. Cem. Prod. 2021, 7, 7–11. (In Chinese) [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; Mejía-Arcila, J.M.; de Gutiérrez, R.M. Eco-efficient alkali-activated cement based on red clay brick wastes suitable for the manufacturing of building materials. J. Clean. Prod. 2017, 166, 242–252. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M. Comparison of different activators for alkaline activation of construction and demolition wastes. Constr. Build. Mater. 2021, 281, 122599. [Google Scholar] [CrossRef]
- Ulugöl, H.; Kul, A.; Yıldırım, G.; Şahmaran, M.; Aldemir, A.; Figueira, D.; Ashour, A. Mechanical and microstructural characterization of geopolymers from assorted construction and demolition waste-based masonry and glass. J. Clean. Prod. 2021, 280, 124358. [Google Scholar] [CrossRef]
- Meng, T.; Hong, Y.; Ying, K.; Wang, Z. Comparison of technical properties of cement pastes with different activated recycled powder from construction and demolition waste. Cem. Concr. Compos. 2021, 120, 104065. [Google Scholar] [CrossRef]
- Robayo, R.A.; Mulford, A.; Munera, J.; de Gutiérrez, R.M. Alternative cements based on alkali-activated red clay brick waste. Constr. Build. Mater. 2016, 128, 163–169. [Google Scholar] [CrossRef]
- Vásquez, A.; Cárdenas, V.; Robayo, R.A.; de Gutiérrez, R.M. Geopolymer based on concrete demolition waste. Adv. Powder Technol. 2016, 27, 1173–1179. [Google Scholar] [CrossRef]
- Fang, Q. Rheological Properties and Application of Polymer Cement Slurry. Doctoral Thesis, Guangzhou University, Guangzhou, China, 2018. (In Chinese). [Google Scholar]
- Yip, C.K.; Van Deventer, J.S.J. Microanalysis of calcium silicate hydrate gel formed within a geopolymeric binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Duan, Z.; Hou, S.; Xiao, J.; Singh, A. Rheological properties of mortar containing recycled powders from construction and demolition wastes. Constr. Build. Mater. 2020, 237, 117622. [Google Scholar] [CrossRef]
- Kim, Y.J.; Choi, Y.W. Utilization of waste concrete powder as a substitution material for cement. Constr. Build. Mater. 2012, 30, 500–504. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, T.; Liu, S.; Yang, D.; Yu, Q. Investigation of using hybrid recycled powder from demolished concrete solids and clay bricks as a pozzolanic supplement for cement. Constr. Build. Mater. 2014, 73, 754–763. [Google Scholar] [CrossRef]
- Dai, J.; Shi, X.; Wang, Q.; Zhang, H.; Luan, C.; Zhang, K.; Yang, F. Effect of Multi-factor on the Compressive Strength of Construction and Demolition Waste Based Geopolymer Concrete. Mater. Rep. 2021, 35, 9077–9082. (In Chinese) [Google Scholar]
- Ahmari, S.; Ren, X.; Toufigh, V.; Zhang, L. Production of geopolymeric binder from blended waste concrete powder and fly ash. Constr. Build. Mater. 2012, 35, 718–729. [Google Scholar] [CrossRef]
- Liu, C. Research on Preparation and Application of Alkali Activated Recycled Concrete Powder Cementitious Material. Master’s Thesis, Yangzhou University, Yangzhou, China, 2021. (In Chinese). [Google Scholar]
- Yang, L. Study on Preparation of Recycled Cementitious Materials by Recycled Concrete Powder. Master’s Thesis, Southeast University, Nanjing, China, 2016. (In Chinese). [Google Scholar]
- Zawrah, M.F.; Gado, R.A.; Feltin, N.; Ducourtieux, S.; Devoille, L. Recycling and utilization assessment of waste fired clay bricks (Grog) with granulated blast-furnace slag for geopolymer production. Process Saf. Environ. Prot. 2016, 103, 237–251. [Google Scholar] [CrossRef]
- Reig, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Cheeseman, C.R.; Payá, J. Properties and microstructure of alkali-activated red clay brick waste. Constr. Build. Mater. 2013, 43, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.M.; Zeng, Y.H.; Ren, J.; Guo, S.Y.; Lu, Y.; Zhao, T.J.; Zhang, L. Synthesis, application and unique effects of RGOEP on properties of alkali-activated slag binders. Constr. Build. Mater. 2021, 313, 125555. [Google Scholar] [CrossRef]
- Rodriguez, E.T.; Garbev, K.; Merz, D.; Black, L.; Richardson, I.G. Thermal stability of CSH phases and applicability of Richardson and Groves’ and Richardson C-(A)-SH (I) models to synthetic CSH. Cem. Concr. Res. 2017, 93, 45–56. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.L.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Kim, M.S.; Jun, Y.; Lee, C.; Oh, J.E. Use of CaO as an activator for producing a price-competitive non-cement structural binder using ground granulated blast furnace slag. Cem. Concr. Res. 2013, 54, 208–214. [Google Scholar] [CrossRef]
- Bassani, M.; Tefa, L.; Coppola, B.; Palmero, P. Alkali-activation of aggregate fines from construction and demolition waste: Valorisation in view of road pavement subbase applications. J. Clean. Prod. 2019, 234, 71–84. [Google Scholar] [CrossRef]
- Wu, H.; Yang, D.; Ma, Z. Micro-structure, mechanical and transport properties of cementitious materials with high-volume waste concrete powder and thermal modification. Constr. Build. Mater. 2021, 313, 125477. [Google Scholar] [CrossRef]
- Xu, X.; Tang, S.; He, Z. Effect of Ca/Si Ratio on Structure and Mechanical Properties of Calcium Silicate Hydrate via Molecular Dynamics Simulations. Bull. Chin. Ceram. Soc. 2021, 40, 3903–3909. (In Chinese) [Google Scholar]
- Guo, Y.; Wu, S.; Lyu, Z.; Shen, A.; Yin, L.; Xue, C. Pore structure characteristics and performance of construction waste composite powder-modified concrete. Constr. Build. Mater. 2021, 269, 121262. [Google Scholar] [CrossRef]
- Jansen, D.; Goetz-Neunhoeffer, F.; Lothenbach, B.; Neubauer, J. The early hydration of Ordinary Portland Cement (OPC): An approach comparing measured heat flow with calculated heat flow from QXRD. Cem. Concr. Res. 2012, 42, 134–138. [Google Scholar] [CrossRef]







| CaO | SiO2 | Al2O3 | MgO | TiO2 | Fe2O3 | Na2O | K2O | SO3 | P2O5 | Others | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 63.27 | 17.05 | 4.58 | 3.89 | 0.51 | 5.57 | 0.00 | 0.69 | 2.80 | 0.45 | 1.19 |
| Slag | 44.26 | 30.17 | 15.56 | 7.39 | 0.92 | 0.35 | 0.00 | 0.41 | 0.00 | 0.11 | 0.83 |
| RCP | 32.64 | 36.85 | 12.66 | 3.67 | 0.93 | 6.47 | 0.09 | 1.80 | 4.13 | 0.00 | 0.76 |
| Code | Cement/g | Slag/g | RCP/g | Water/g | Activator/g | Sand/g |
|---|---|---|---|---|---|---|
| A0 | 450 | - | 0 | 225 | - | 1350 |
| A1 | 405 | - | 45 | 225 | - | |
| A2 | 360 | - | 90 | 225 | - | |
| A3 | 315 | - | 135 | 225 | - | |
| A4 | 270 | - | 180 | 225 | - | |
| B0 | - | 450 | 0 | - | 225 | |
| B1 | - | 405 | 45 | - | 225 | |
| B2 | - | 360 | 90 | - | 225 | |
| B3 | - | 315 | 135 | - | 225 | |
| B4 | - | 270 | 180 | - | 225 |
| Code | Composition (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Ca | Si | Al | O | Na | C | Mg | Ca/Si | |
| A1 Point1 | 35.2 | 7.6 | 3.0 | 50.3 | - | 3.5 | 0.3 | 4.63 |
| Point2 | 12.9 | 11.9 | 5.4 | 59.8 | 0.4 | 5.4 | 4.0 | 1.08 |
| Point3 | 19.9 | 1.2 | 0.6 | 72.5 | - | 5.3 | - | - |
| Point4 | 31.9 | 10.9 | 4.8 | 45.6 | - | 3.9 | 2.9 | 2.93 |
| B0 Point1 | 15.9 | 2.9 | 8.1 | 41.6 | - | 31.6 | - | 5.48 |
| B1 Point1 | - | 30.2 | - | - | - | 68.9 | - | - |
| Point2 | - | 34.7 | - | - | - | 65.3 | - | - |
| Point3 | 1.9 | 12.1 | 16.6 | - | 3.4 | 66.0 | - | 0.16 |
| B2 Point1 | 16.6 | 12.5 | 6.1 | 53.9 | 1.8 | 7.9 | 1.2 | 1.33 |
| Point2 | 1.2 | 33.8 | - | 55.4 | 0.7 | 8.9 | - | 0.04 |
| Point3 | 11.4 | 10.6 | 5.9 | 61.8 | 1.0 | 4.8 | 4.5 | 1.08 |
| B3 Point1 | 4.0 | 19.5 | 2.2 | 64.9 | 2.7 | 5.2 | 1.5 | 0.21 |
| Point2 | 4.4 | 15.8 | 4.9 | 59.2 | 3.6 | 8.2 | 1.1 | 0.28 |
| B4 Point1 | 15.0 | 4.2 | 1.6 | 57.4 | - | 21.8 | - | 3.75 |
| Point2 | 16.1 | 4.3 | 1.6 | 61.0 | 2.1 | 15.0 | - | 3.74 |
| Point3 | 11.2 | 4.7 | 1.4 | 56.3 | 1.4 | 24.5 | 0.5 | 2.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Li, H.; Che, X.; Xu, P.; Li, C.; Yu, Q. A Study on the Application of Recycled Concrete Powder in an Alkali-Activated Cementitious System. Processes 2023, 11, 203. https://doi.org/10.3390/pr11010203
Wan X, Li H, Che X, Xu P, Li C, Yu Q. A Study on the Application of Recycled Concrete Powder in an Alkali-Activated Cementitious System. Processes. 2023; 11(1):203. https://doi.org/10.3390/pr11010203
Chicago/Turabian StyleWan, Xiaomei, Hui Li, Xueping Che, Peizhen Xu, Changjiang Li, and Qi Yu. 2023. "A Study on the Application of Recycled Concrete Powder in an Alkali-Activated Cementitious System" Processes 11, no. 1: 203. https://doi.org/10.3390/pr11010203