Effect of Nb Content on High-Temperature Strength and Precipitates of Nb-Containing Steel
Abstract
:1. Introduction
2. Experimental Scheme
2.1. Smelting Experiment
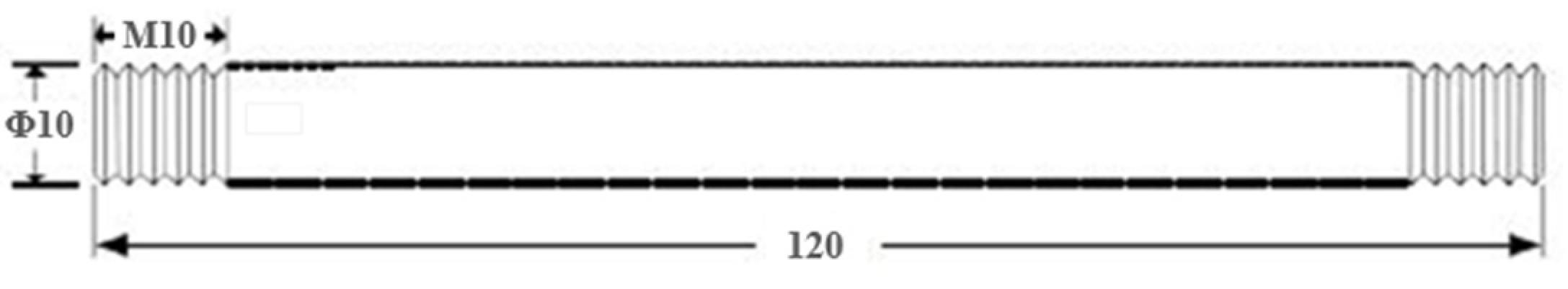
2.2. High-Temperature Strength Performance Experiment
2.3. Precipitate Analysis
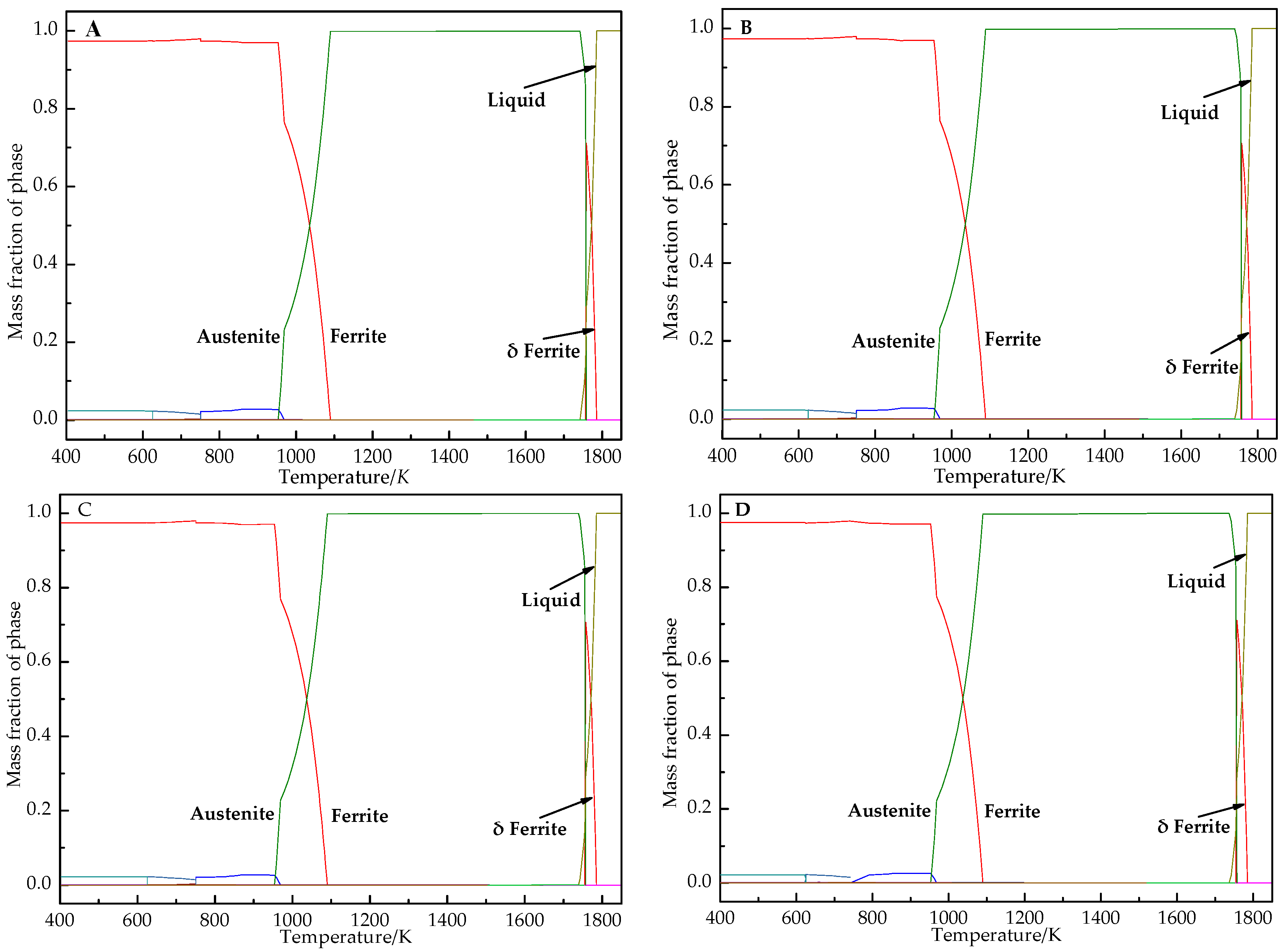
| TS/K | TL/K | |
|---|---|---|
| A | 1731.69 | 1784.40 |
| B | 1732.46 | 1785.45 |
| C | 1731.84 | 1783.97 |
| D | 1732.50 | 1784.53 |
3. Experimental Results and Analysis
3.1. Analysis of High-Temperature Strength Test Results
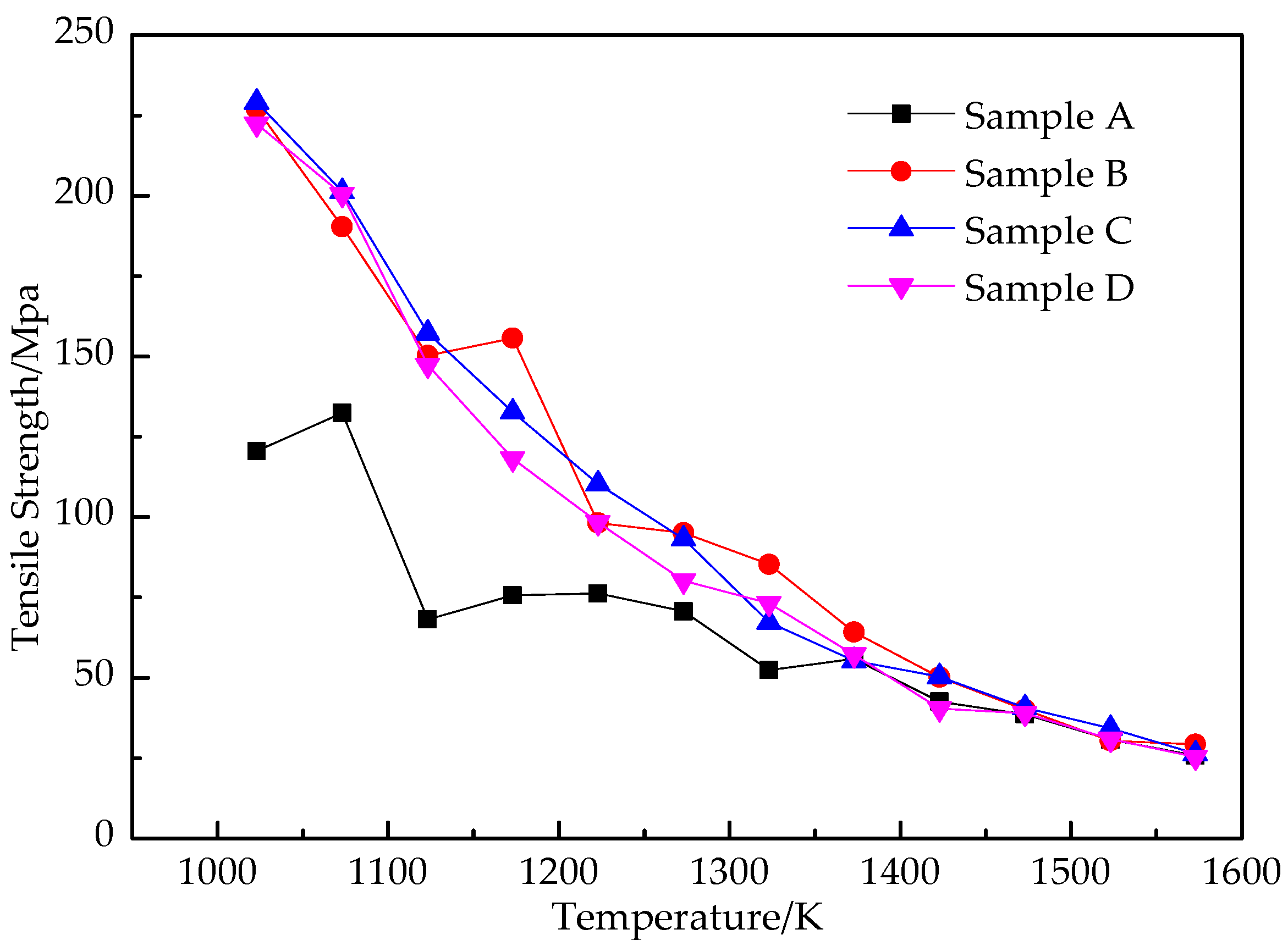
3.1.1. Tensile Strength Curve
3.1.2. Yield Strength Curve
4. Analysis and Discussion
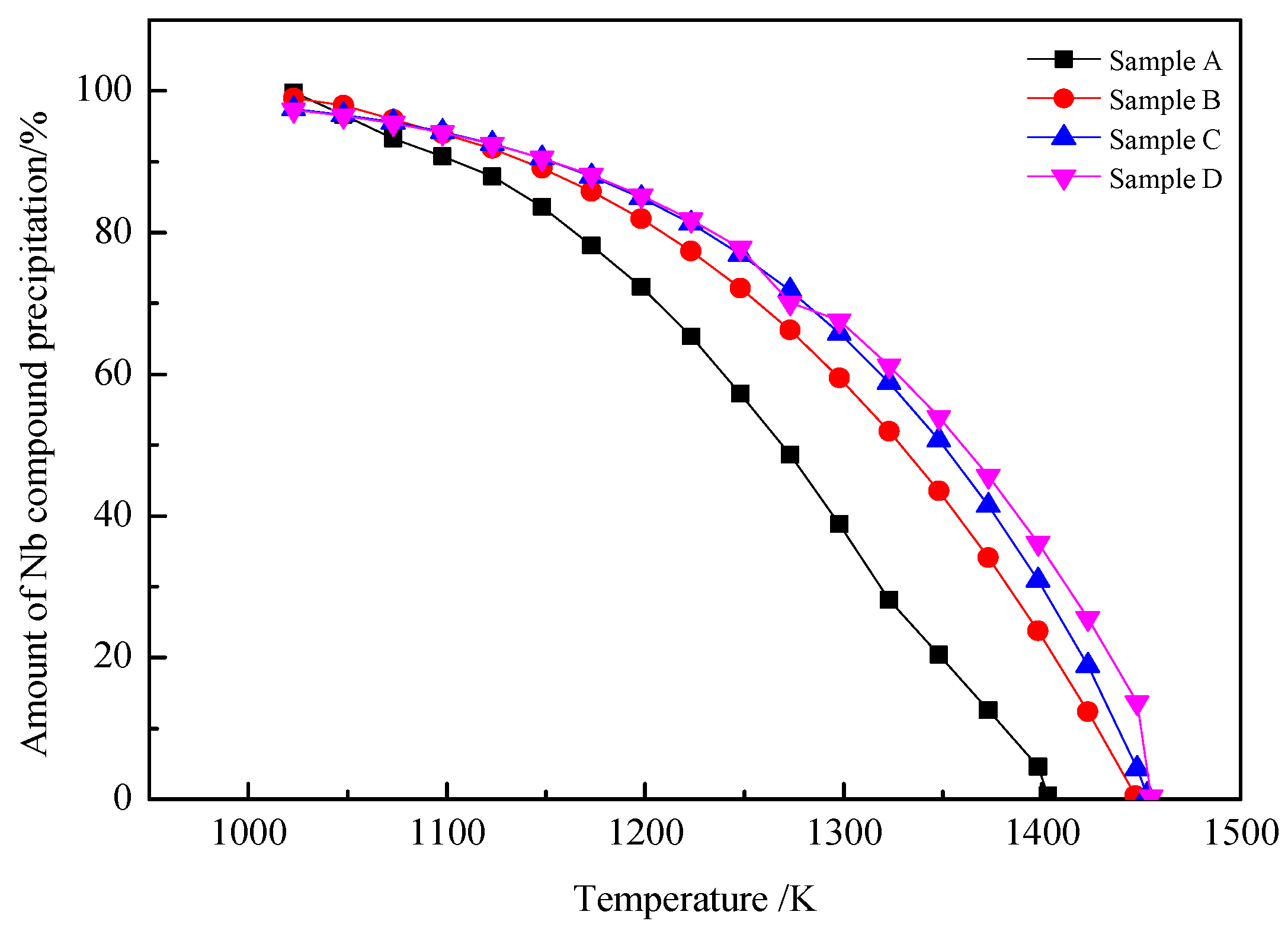
4.1. Thermodynamic Analysis
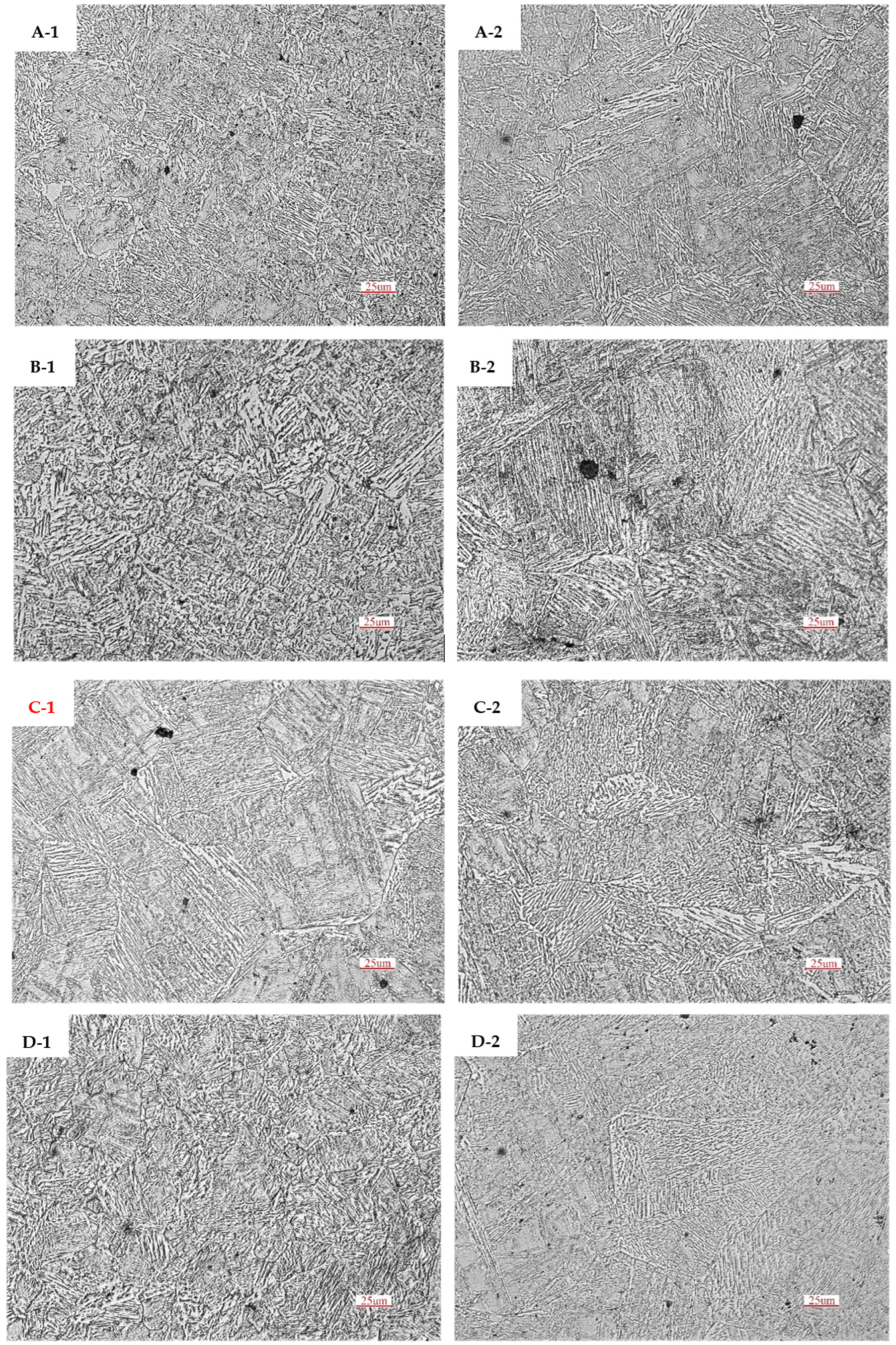
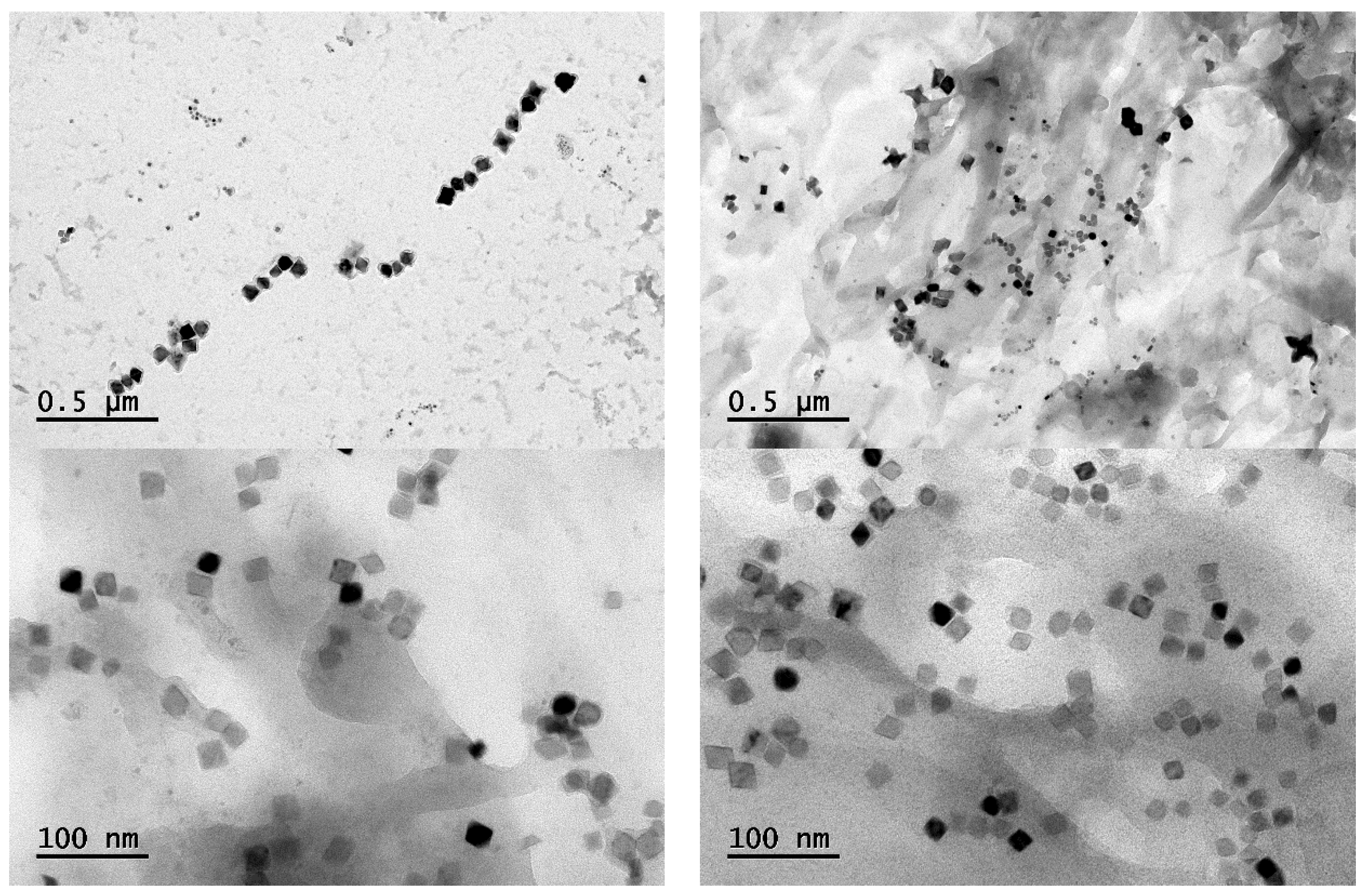
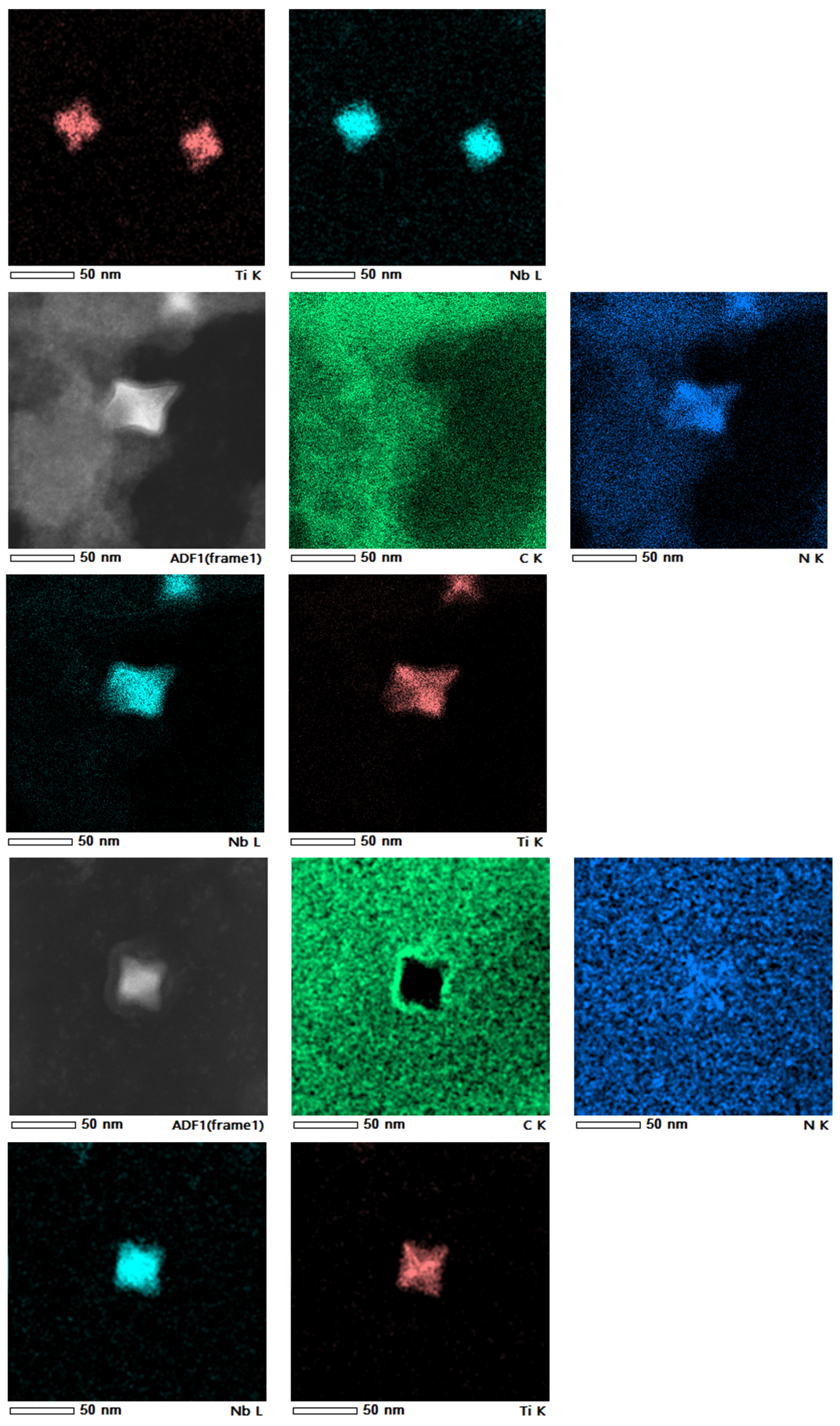
4.2. Phase and Composition of Precipitates
5. Conclusions
- (1)
- In the temperature range of 1373~1023 K, the increase in Nb content significantly improves the high-temperature tensile strength and yield strength of Q355GJ steel, which makes Q355GJ steel have strong resistance to deformation at high temperatures. When Nb content is 0.031%, the high-temperature strength performance of the steel is the best.
- (2)
- According to the thermodynamic calculation results, at 1023 K, almost all Nb compounds in samples A, B, C and D are precipitated. The increase in Nb content increases the initial precipitation temperature of Nb compounds (the initial precipitation temperature of Nb (C, N) in samples A, B, C and D is 1403 K, 1447 K, 1453 K and 1455 K, respectively).
- (3)
- After heat treatment at 1373 K and 1473 K, the precipitation amount of the two-phase particles in samples B, C and D is obviously more than that in sample A, and the precipitation amount of the two-phase particles is obviously increased as the heat treatment temperature increases. The second-phase particles are mainly square- and star-shaped (Ti, Nb) (C, N) composite precipitates. The precipitated two-phase particles in sample B have a smaller size and more uniform distribution; those smaller than 80 nm account for about 84% of the total number, which is more effective for improving the overall performance of Q355GJ steel.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, C.; Jia, B.; Wang, J. Influence of artificial cooling methods on post-fire mechanical properties of Q355 structural steel. Constr. Build. Mater. 2020, 252, 119092. [Google Scholar] [CrossRef]
- Outinen, J.; Mäkeläinen, P. Mechanical properties of structural steel at elevated temperatures and after cooling down. Fire Mater. 2004, 28, 237–251. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R. Influence of stress concentration and cooling methods on post-fire mechanical behavior of ASTM A36 steels. Constr. Build. Mater. 2018, 186, 920–945. [Google Scholar] [CrossRef]
- Sajid, H.U.; Kiran, R. Post-fire mechanical behavior of ASTM A572 steels subjected to high stress triaxialities. Eng. Struct. 2019, 191, 323–342. [Google Scholar] [CrossRef]
- Deardo, A.J. Niobium in modern steels. Int. Mater. Rev. 2003, 48, 371–402. [Google Scholar] [CrossRef]
- Pradhan, N.; Banerjee, N.; Reddy, B.B.; Sahay, S.K.; Viswanathan, C.S.; Bhor, P.K.; Basu, D.S.; Mazumdar, S. Control of transverse cracking in special quality slabs. Ironmak. Steelmak. 2001, 28, 305–311. [Google Scholar] [CrossRef]
- Abushosha, R.; Ayyad, S.; Mintz, B. Influence of cooling rate on hot ductility of C-Mn-AI and C-Mn-Nb-AL steels. Mater. Sci. Technol. 1998, 14, 346–351. [Google Scholar] [CrossRef]
- Banks, K.; Koursaris, A.; Verdoorn, F.; Tuling, A. Precipitation and hot ductility of low C-V and low C-V-Nb microalloyed steels during thin slab casting. Mater. Sci. Technol. 2001, 17, 1596–1604. [Google Scholar] [CrossRef]
- Mintz, B.; Abushosha, R. Influence of Vanadium on Hot Ductility of Steel. Ironmak. Steelmak. 1993, 20, 445–452. [Google Scholar]
- Abushosha, R.; Comineli, O.; Mintz, B. Influence of Ti on hot ductility of C-Mn-Al steels. Mater. Sci. Technol. 1999, 15, 278–286. [Google Scholar] [CrossRef]
- Comineli, O.; Abushosha, R.; Mintz, B. Influence of titanium and nitrogen on hot ductility of C–Mn–Nb–Al steels. Mater. Sci. Technol. 1999, 15, 1058–1068. [Google Scholar] [CrossRef]
- Spradbery, C.; Mintz, B. Influence of undercooling thermal cycle on hot ductility of C–Mn–Al–Ti and C–Mn–Al–Nb–Ti steels. Ironmak. Steelmak. 2005, 32, 319–324. [Google Scholar] [CrossRef]
- Maubane, D.R.N.; Mostert, R.J.; Banks, K.M. Re-Austenitisation of Thin Ferrite Films in C–Mn Steels during Thermal Rebound at Continuously Cast Slab Corner Surfaces. Metals 2022, 12, 2155. [Google Scholar] [CrossRef]
- Jie, F.; Li, G.; Yu, Y.; Mao, X.; Fang, K. Comprehensive strengthening mechanism of steel based on nano-scale cementite precipitates. Eng. Sci. 2011, 13, 31–42. [Google Scholar]
- Hong, S.G.; Jun, H.J.; Kang, K.B.; Park, C.G. Evolution of precipitates in the Nb–Ti–V microalloyed HSLA steels during reheating. Scr. Mater. 2003, 48, 1201–1206. [Google Scholar] [CrossRef]
- Hong, S.G.; Kang, K.B.; Park, C.G. Strain-induced precipitation of NbC in Nb and Nb–Ti microalloyed HSLA steels. Scr. Mater. 2002, 46, 163–168. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, C.-H.; Heo, Y.-U.; Suh, D.-W. Stability of (Ti, M)C (M=Nb, V, Mo and W) carbide in steels using first-principles calculations. Acta Mater. 2012, 60, 208–217. [Google Scholar] [CrossRef]
- Yen, H.-W.; Chen, P.-Y.; Huang, C.-Y.; Yang, J.-R. Interphase precipitation of nanometer-sized carbides in a titanium–molybdenum-bearing low-carbon steel. Acta Mater. 2011, 59, 6264–6274. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, C.Y.; Wang, T.Y.; Huang, C.Y.; Yang, J.R. Orientation relationship transition of nanometre sized interphase precipitated TiC carbides in Ti bearing steel. Mater. Sci. Technol. 2010, 26, 421–430. [Google Scholar] [CrossRef]
- Ning, A.G.; Mao, W.W.; Guo, H.J.; Chen, X.C. Precipitation Behaviors and Strengthening of Carbides in H13 Steel during Quenching. Chin. J. Process Eng. 2014, 14, 1041–1046. [Google Scholar]
- Li, X.; Wang, Z. Interphase Precipitation Behaviors Of Nanometer-Sized Carbides In A Nb-Ti-Bearing Low-Carbon Microalloyed Steel. Acta Metall. Sin. 2015, 51, 417–424. [Google Scholar]
- Pereloma, E.V.; Crawford, B.R.; Hodgson, P.D. Strain-induced precipitation behaviour in hot rolled strip steel. Mater. Sci. Eng. A 2001, 299, 27–37. [Google Scholar] [CrossRef]
- Choudhary, S.K.; Ghosh, A. Mathematical Model for Prediction of Composition of Inclusions Formed during Solidification of Liquid Steel. ISIJ Int. 2009, 49, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Hao, Z.; Yuntao, L.; Jinshan, W. Present situation and development tendency of the high strength hull structure steel. Steel Constr. 2004, 19, 38–40+43. [Google Scholar]
- Petch, N.J. The ductile-brittle transition in the fracture of α-iron: I. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1958, 3, 1089–1097. [Google Scholar] [CrossRef]
- Heslop, J.; Petch, N.J. The ductile-brittle transition in the fracture of α-iron: II. Philos. Mag. A J. Theor. Exp. Appl. Phys. 1958, 3, 1128–1136. [Google Scholar] [CrossRef]
- Petch, N.J. The Cleavage Strength of Polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Gong, P.; Palmiere, E.J.; Rainforth, W.M. Dissolution and precipitation behaviour in steels microalloyed with niobium during thermomechanical processing. Acta Mater. 2015, 97, 392–403. [Google Scholar] [CrossRef]
- Campos, S.S.; Kestenbach, H.J.; Morales, E.V. On strengthening mechanisms in commercial Nb-Ti hot strip steels. Metall. Mater. Trans. A 2001, 32, 1245–1248. [Google Scholar] [CrossRef]
- Sharma, R.C.; Lakshmanan, V.K.; Kirkaldy, J.S. Solubility of niobium carbide and niobium carbonitride in alloyed austenite and ferrite. Metall. Trans. A 1984, 15, 545–553. [Google Scholar] [CrossRef]
- Tang, Q.; Niu, G.; Wu, H.; Xu, L.; Yuan, R. Effects of Induction Heating in the ESP Line on Microstructural Evolution and Nb Dissolution Behavior of Nb-Ti Micro-Alloyed Steel. Metals 2021, 11, 251. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Kirkaldy, J.S. Thermodynamics of Fe-Ti-C and Fe-Nb-C Austenites and Nonstoichiometric Titanium and Niobium Carbides. In Advances in Phase Transitions; Embury, J.D., Purdy, G.R., Eds.; Pergamon: Oxford, UK, 1988; pp. 37–51. [Google Scholar]
- Lou, Y.-Z.; Liu, D.-L.; Ni, X.-Q. Precipitates in steels with Ti additive produced by CSP process. J. Iron Steel Res. Int. 2009, 16, 60–66. [Google Scholar] [CrossRef]






| C | Si | Mn | P | S | Al | Ti |
|---|---|---|---|---|---|---|
| ≤0.003 | ≤0.03 | 0.1~0.2 | ≤0.006 | ≤0.007 | 0.02~0.05 | 0.04~0.08 |
| C | Si | Mn | P | S | Nb | Ti | Al | N | |
|---|---|---|---|---|---|---|---|---|---|
| A | 0.174 | 0.206 | 1.567 | 0.014 | 0.009 | 0.005 | 0.029 | 0.021 | 0.0060 |
| B | 0.173 | 0.202 | 1.555 | 0.014 | 0.008 | 0.030 | 0.026 | 0.013 | 0.0055 |
| C | 0.175 | 0.210 | 1.546 | 0.014 | 0.008 | 0.051 | 0.027 | 0.015 | 0.0056 |
| D | 0.173 | 0.208 | 1.557 | 0.014 | 0.008 | 0.064 | 0.026 | 0.013 | 0.0058 |
| A | B | C | D | |
|---|---|---|---|---|
| Total number of particles/piece | 346 | 585 | 477 | 509 |
| Proportion of particle size >80 nm/% | 24.57 | 16.07 | 19.92 | 20.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhu, L.; Wang, B.; Sun, L.; Xiao, P. Effect of Nb Content on High-Temperature Strength and Precipitates of Nb-Containing Steel. Processes 2023, 11, 209. https://doi.org/10.3390/pr11010209
Zhou J, Zhu L, Wang B, Sun L, Xiao P. Effect of Nb Content on High-Temperature Strength and Precipitates of Nb-Containing Steel. Processes. 2023; 11(1):209. https://doi.org/10.3390/pr11010209
Chicago/Turabian StyleZhou, Jingyi, Liguang Zhu, Bo Wang, Ligen Sun, and Pengcheng Xiao. 2023. "Effect of Nb Content on High-Temperature Strength and Precipitates of Nb-Containing Steel" Processes 11, no. 1: 209. https://doi.org/10.3390/pr11010209





