A Petal-like Structured NiCuOOH-NF Electrode by a Sonochemical Combined with the Electrochemical Method for Ammonia Oxidation Reaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrode Fabrication
2.3. Physical Characterization
2.4. Electrochemical Measurements
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. The Preparation Principle of Electrode
3.2. Characterization of Electrode
3.3. Electrochemical Activity Analysis
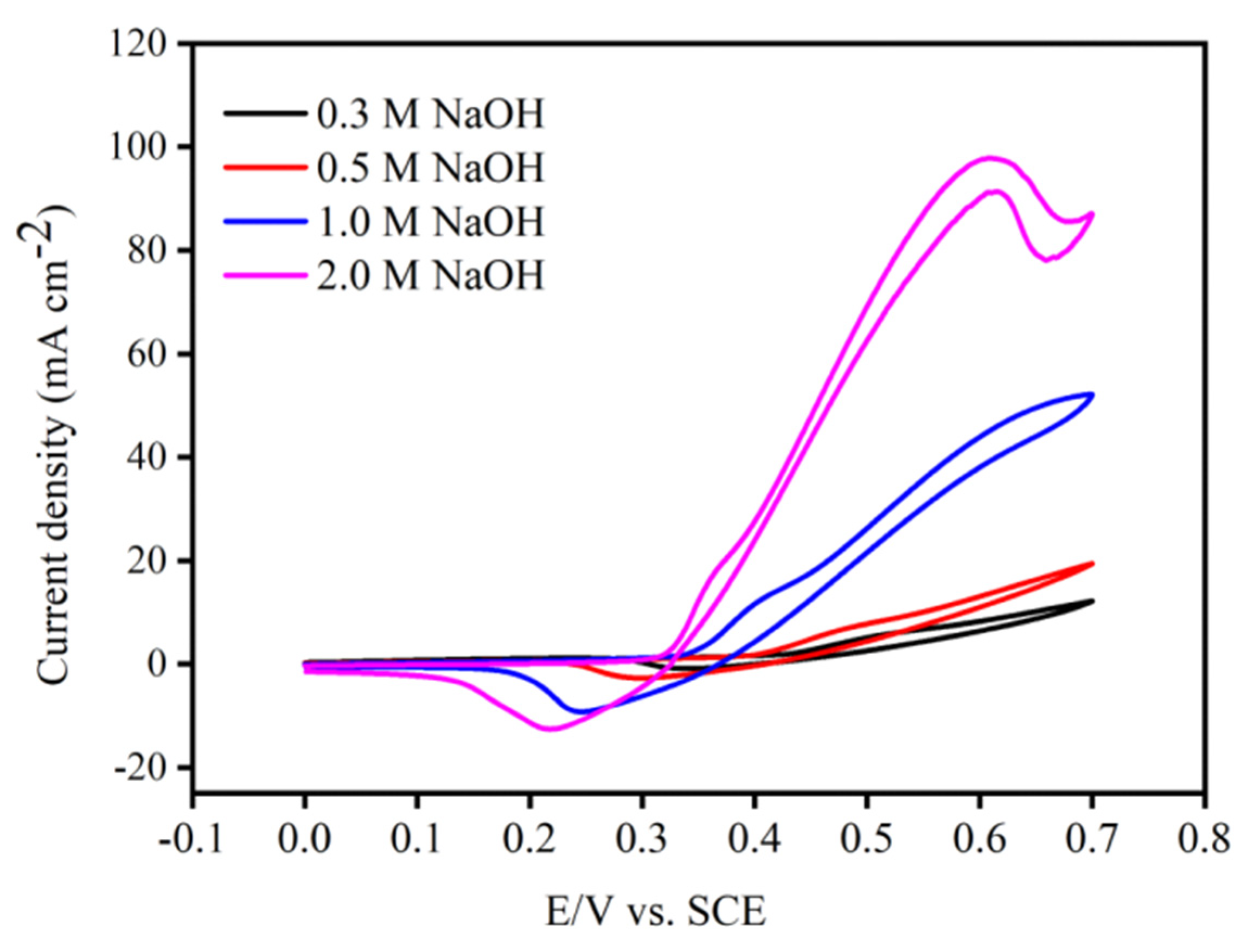
3.4. The Influence Factors on AOR
3.5. Application in Real Wastewater
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bin Shahid, U.; Siddharth, K.; Shao, M. Electrifying the nitrogen cycle: An electrochemical endeavor. Curr. Opin. Electrochem. 2021, 30, 100790. [Google Scholar] [CrossRef]
- Rosca, V.; Duca, M.; de Groot, M.T.; Koper, M.T.M. Nitrogen Cycle Electrocatalysis. Chem. Rev. 2009, 109, 2209–2244. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Gao, M.; Zhang, M.; Feng, L.; Liu, Q.; Zhang, G. Application of encapsulated algae into MBR for high-ammonia nitrogen wastewater treatment and biofouling control. Water Res. 2020, 187, 116430. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Wu, P.; Wang, H.; Deng, L.; Wang, W. Nitrification performance evaluation of activated sludge under high potassium ion stress during high-ammonia nitrogen organic wastewater treatment. J. Environ. Sci. 2021, 111, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.; Lu, M.; Zhu, Y.; Zhang, Z.; Zhou, Z.; Liang, Y.; Vidic, R.D. Consideration of Potential Technologies for Ammonia Removal and Recovery from Produced Water. Environ. Sci. Technol. 2022, 56, 3305–3308. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Zhang, Z.; Luo, W.; Wu, H.; Zhang, L.; Zhang, X.; Zhao, T. Performance and microbial ecology of a novel moving bed biofilm reactor process inoculated with heterotrophic nitrification-aerobic denitrification bacteria for high ammonia nitrogen wastewater treatment. Bioresour. Technol. 2020, 315, 123813. [Google Scholar] [CrossRef]
- Ji, J.; Peng, Y.; Wang, B.; Li, X.; Zhang, Q. Synergistic Partial-Denitrification, Anammox, and in-situ Fermentation (SPDAF) Process for Advanced Nitrogen Removal from Domestic and Nitrate-Containing Wastewater. Environ. Sci. Technol. 2020, 54, 3702–3713. [Google Scholar] [CrossRef]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.J.; Ratnayaka, D.D. Specialized and Advanced Water Treatment Processes. In Twort’s Water Supply; IWA Publishing: London, UK, 2017; pp. 407–473. [Google Scholar] [CrossRef]
- Verstraete, W.; Philips, S. Nitrification-denitrification processes and technologies in new contexts. Environ. Pollut. 1998, 102, 717–726. [Google Scholar] [CrossRef]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping—Acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Pillai, H.S.; Lattimer, J.; Adli, N.M.; Karakalos, S.G.; Chen, M.; Guo, L.; Xu, H.; Yang, J.; et al. Ternary PtIrNi Catalysts for Efficient Electrochemical Ammonia Oxidation. ACS Catal. 2020, 10, 3945–3957. [Google Scholar] [CrossRef]
- Shih, Y.-J.; Hsu, C.-H. Kinetics and highly selective N2 conversion of direct electrochemical ammonia oxidation in an undivided cell using NiCo oxide nanoparticle as the anode and metallic Cu/Ni foam as the cathode. Chem. Eng. J. 2020, 409, 128024. [Google Scholar] [CrossRef]
- Li, Y.; Pillai, H.S.; Wang, T.; Hwang, S.; Zhao, Y.; Qiao, Z.; Mu, Q.; Karakalos, S.; Chen, M.; Yang, J.; et al. High-performance ammonia oxidation catalysts for anion-exchange membrane direct ammonia fuel cells. Energy Environ. Sci. 2021, 14, 1449–1460. [Google Scholar] [CrossRef]
- Zhong, C.; Hu, W.B.; Cheng, Y.F. Recent advances in electrocatalysts for electro-oxidation of ammonia. J. Mater. Chem. A 2013, 1, 3216–3238. [Google Scholar] [CrossRef]
- He, S.; Somayaji, V.; Wang, M.; Lee, S.-H.; Geng, Z.; Zhu, S.; Novello, P.; Varanasi, C.V.; Liu, J. High entropy spinel oxide for efficient electrochemical oxidation of ammonia. Nano Res. 2021, 15, 4785–4791. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Li, J.; Hua, C.; Xue, S.; Lei, Z. High performance PtxEu alloys as effective electrocatalysts for ammonia electro-oxidation. Int. J. Hydrogen Energy 2017, 42, 18959–18967. [Google Scholar] [CrossRef]
- Chan, Y.T.; Siddharth, K.; Shao, M. Investigation of cubic Pt alloys for ammonia oxidation reaction. Nano Res. 2020, 13, 1920–1927. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Leon, M.N.; Fernandes, C.M.; Antoniassi, R.M.; Alves, O.C.; Ponzio, E.A.; Silva, J.C.M. PtSnO2/C and Pt/C with preferential (100) orientation: High active electrocatalysts for ammonia electro-oxidation reaction. Appl. Catal. B Environ. 2020, 264, 118458. [Google Scholar] [CrossRef]
- Li, Z.-F.; Wang, Y.; Botte, G.G. Revisiting the electrochemical oxidation of ammonia on carbon-supported metal nanoparticle catalysts. Electrochim. Acta 2017, 228, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Du, D.; Lan, R.; Humphreys, J.; Miller, D.N.; Walker, M.; Wu, Z.; Irvine, J.T.; Tao, S. Electrodeposited NiCu bimetal on carbon paper as stable non-noble anode for efficient electrooxidation of ammonia. Appl. Catal. B Environ. 2018, 237, 1101–1109. [Google Scholar] [CrossRef]
- Zhang, H.M.; Wang, Y.F.; Kwok, Y.H.; Wu, Z.C.; Xia, D.H.; Leung, D.Y.C. A Direct Ammonia Microfluidic Fuel Cell using NiCu Nanoparticles Supported on Carbon Nanotubes as an Electrocatalyst. Chemsuschem 2018, 11, 2889–2897. [Google Scholar] [CrossRef]
- Wu, C.; Zhu, J.; Wang, H.; Wang, G.; Chen, T.; Tan, Y. Porous Ni1−xCuxO Nanowire Arrays as Noble-Metal-Free High-Performance Catalysts for Ammonia-Borane Electrooxidation. ACS Catal. 2019, 10, 721–735. [Google Scholar] [CrossRef]
- Faid, A.Y.; Barnett, A.O.; Seland, F.; Sunde, S. NiCu mixed metal oxide catalyst for alkaline hydrogen evolution in anion exchange membrane water electrolysis. Electrochim. Acta 2021, 371, 137837. [Google Scholar] [CrossRef]
- Wei, C.; Sun, Y.; Scherer, G.G.; Fisher, A.C.; Sherburne, M.P.; Ager, J.W.; Xu, Z.J. Surface Composition Dependent Ligand Effect in Tuning the Activity of Nickel–Copper Bimetallic Electrocatalysts toward Hydrogen Evolution in Alkaline. J. Am. Chem. Soc. 2020, 142, 7765–7775. [Google Scholar] [CrossRef]
- Jiang, X.; Ying, D.; Liu, X.; Liu, M.; Zhou, S.; Guo, C.; Zhao, G.; Wang, Y.; Jia, J. Identification of the role of Cu site in Ni-Cu hydroxide for robust and high selective electrochemical ammonia oxidation to nitrite. Electrochim. Acta 2020, 345, 136157. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Y.; Wu, Z.; Leung, D.Y. An ammonia electrolytic cell with NiCu/C as anode catalyst for hydrogen production. Energy Procedia 2017, 142, 1539–1544. [Google Scholar] [CrossRef]
- Hussain, I.; Mohapatra, D.; Dhakal, G.; Lamiel, C.; Mohamed, S.G.; Sayed, M.S.; Lee, Y.R.; Lee, J.; Lee, M.; Shim, J.-J. Growth of 2D nanoflakes from 1D long leaf arrays: Electrochemical influence of copper and nickel co-substituted cobalt oxide. J. Energy Storage 2020, 32, 101871. [Google Scholar] [CrossRef]
- Sun, M.; Dong, J.; Lv, Y.; Zhao, S.; Meng, C.; Song, Y.; Wang, G.; Li, J.; Fu, Q.; Tian, Z.; et al. Pt@h-BN core–shell fuel cell electrocatalysts with electrocatalysis confined under outer shells. Nano Res. 2018, 11, 3490–3498. [Google Scholar] [CrossRef]
- Yu, Y.; Hu, X.; Wang, S.; Qiao, H.; Liu, Z.; Song, K.; Shen, X. High mass loading Ni4Co1-OH@CuO core-shell nanowire arrays obtained by electrochemical reconstruction for alkaline energy storage. Nano Res. 2021, 15, 685–693. [Google Scholar] [CrossRef]
- Li, L.; Xu, G.; Liu, X.; Huang, S.; Wei, X.; Yang, L. Combined high catalytic activity and polysulfide confinement in hierarchical carbon-encapsulated CoSe hollow core-shell spheres for high-performance lithium–sulfur batteries. J. Power Sour. 2021, 506, 230177. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, W.; Liu, S.; Chen, X.; Ma, T.; Wang, G.; Lu, Z.; Sun, J. Enhanced interface interaction in Cu2S@Ni core-shell nanorod arrays as hydrogen evolution reaction electrode for alkaline seawater electrolysis. J. Power Sour. 2021, 506, 230235. [Google Scholar] [CrossRef]
- Chen, P.; Tong, Y.; Wu, C.; Xie, Y. Surface/Interfacial Engineering of Inorganic Low-Dimensional Electrode Materials for Electrocatalysis. Acc. Chem. Res. 2018, 51, 2857–2866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Jiang, K.; Wang, H.; Hu, S. Fluoride-Induced Dynamic Surface Self-Reconstruction Produces Unexpectedly Efficient Oxygen-Evolution Catalyst. Nano Lett. 2018, 19, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Jiang, H.; Duan, X.; Jiang, Z.; Hu, Y.; Boettcher, S.W.; Zhang, W.; Guo, S.; Li, C. Fluorination-enabled Reconstruction of NiFe Electrocatalysts for Efficient Water Oxidation. Nano Lett. 2020, 21, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; He, Q.; Zhang, Y.; Song, L. Structural Self-Reconstruction of Catalysts in Electrocatalysis. Acc. Chem. Res. 2018, 51, 2968–2977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Y.; Wu, Z.; Leung, D.Y. A direct urea microfluidic fuel cell with flow-through Ni-supported-carbon- nanotube-coated sponge as porous electrode. J. Power Sour. 2017, 363, 61–69. [Google Scholar] [CrossRef]
- Li, X.; Han, G.Q.; Liu, Y.R.; Dong, B.; Hu, W.H.; Shang, X.; Chai, Y.M.; Liu, C.G. NiSe@NiOOH Core-Shell Hyacinth-like Nanostructures on Nickel Foam Synthesized by in Situ Electrochemical Oxidation as an Efficient Electrocatalyst for the Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2016, 8, 20057–20066. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Chen, F. A facile electrochemical fabrication of hierarchically structured nickel–copper composite electrodes on nickel foam for hydrogen evolution reaction. J. Power Sour. 2014, 265, 273–281. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, G.; Shan, M.; Wang, D.; Bai, Z.; Zhou, X.; Lv, Y.; Bai, J. Treatment of high-ammonia-nitrogen landfill leachate nanofiltration concentrate using an Fe-loaded Ni-foam-based electro-Fenton cathode. J. Environ. Chem. Eng. 2020, 8, 104243. [Google Scholar] [CrossRef]
- Tsai, M.-H.; Chen, T.-C.; Juang, Y.; Hua, L.-C.; Huang, C. High catalytic performance of CuCo/nickel foam electrode for ammonia electrooxidation. Electrochem. Commun. 2020, 121, 106875. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, Y.; Xi, S.; Diao, C.; Yu, Z.; Lee, W.S.V.; Xue, J. Deciphering NH3 Adsorption Kinetics in Ternary Ni-Cu-Fe Oxyhydroxide toward Efficient Ammonia Oxidation Reaction. Small 2021, 17, e2005616. [Google Scholar] [CrossRef]
- Silva, J.C.M.; Ntais, S.; Teixeira-Neto, É.; Spinacé, E.V.; Cui, X.; Neto, A.O.; Baranova, E.A. Evaluation of carbon supported platinum–ruthenium nanoparticles for ammonia electro-oxidation: Combined fuel cell and electrochemical approach. Int. J. Hydrogen Energy 2017, 42, 193–201. [Google Scholar] [CrossRef]

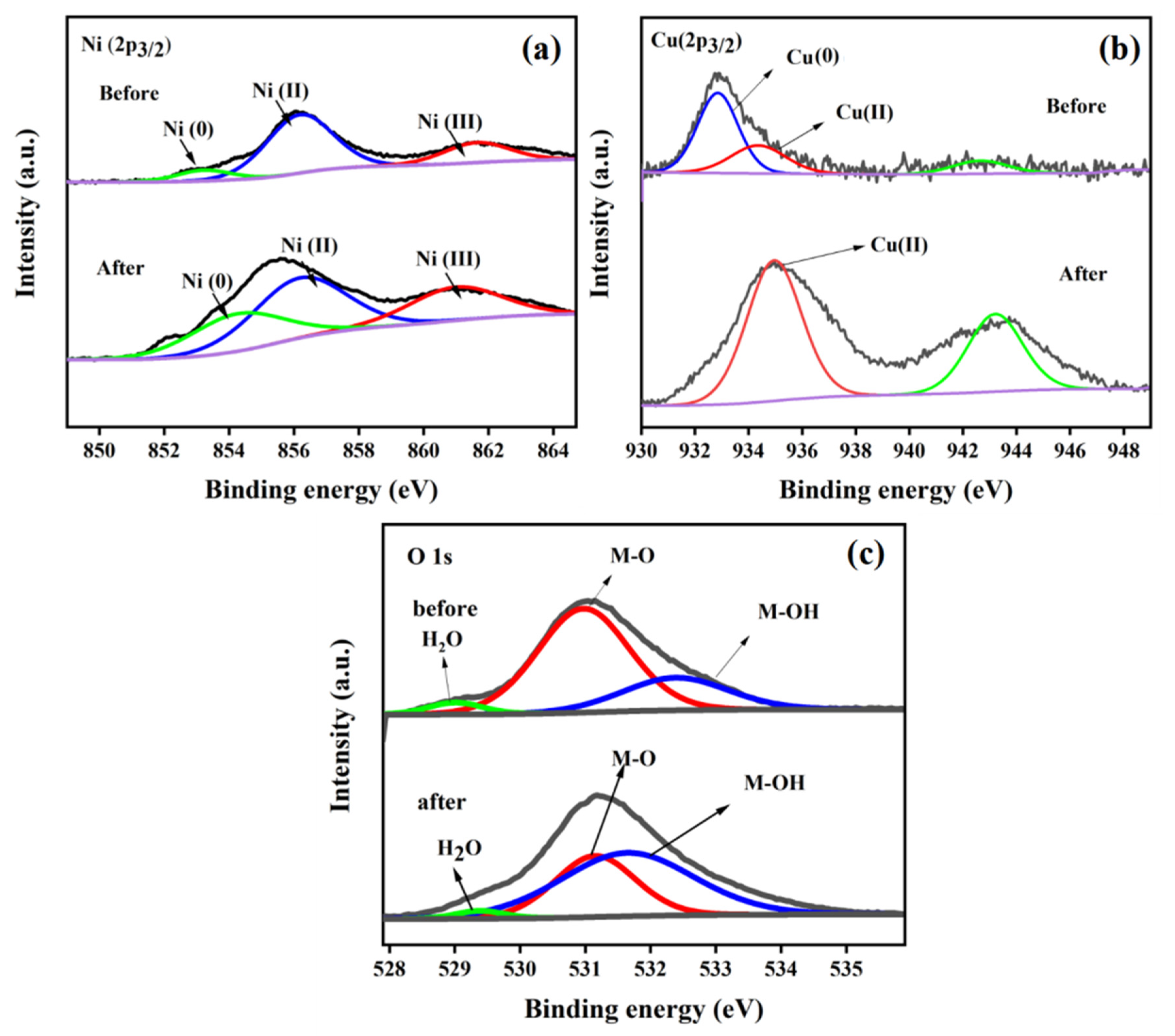
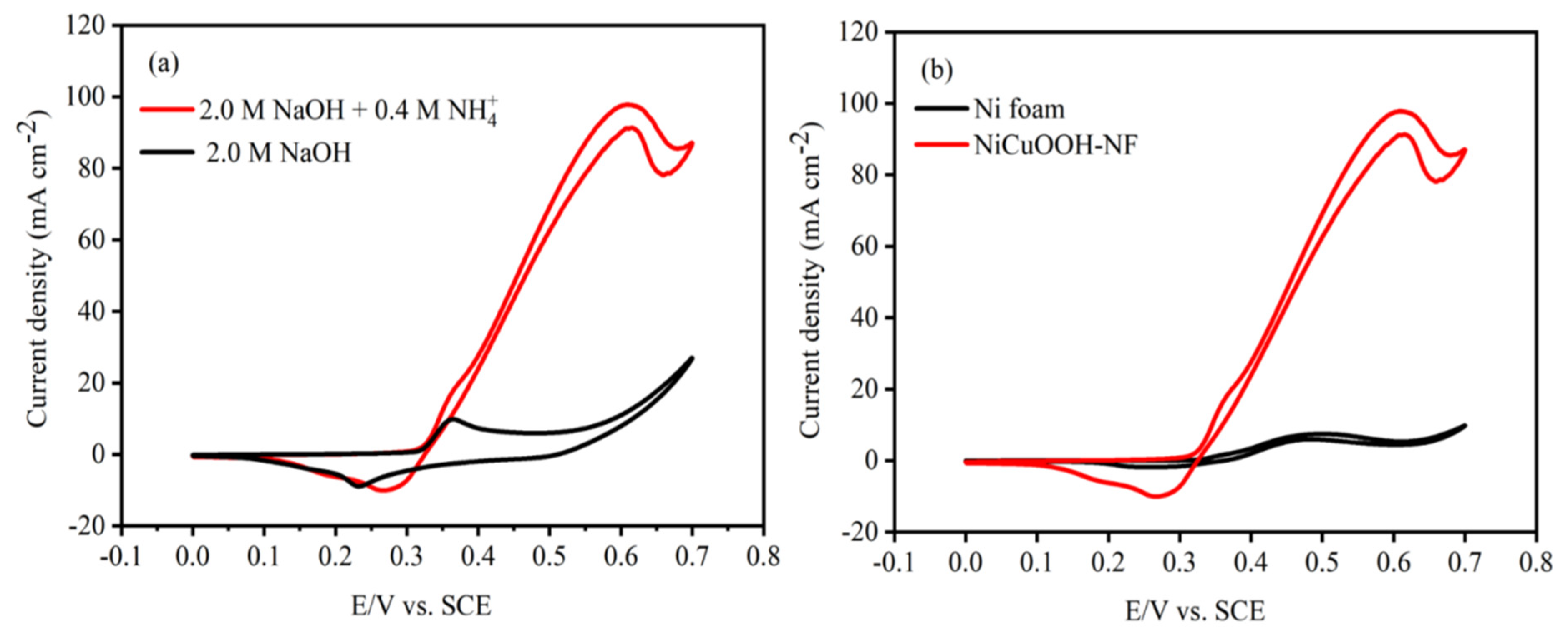
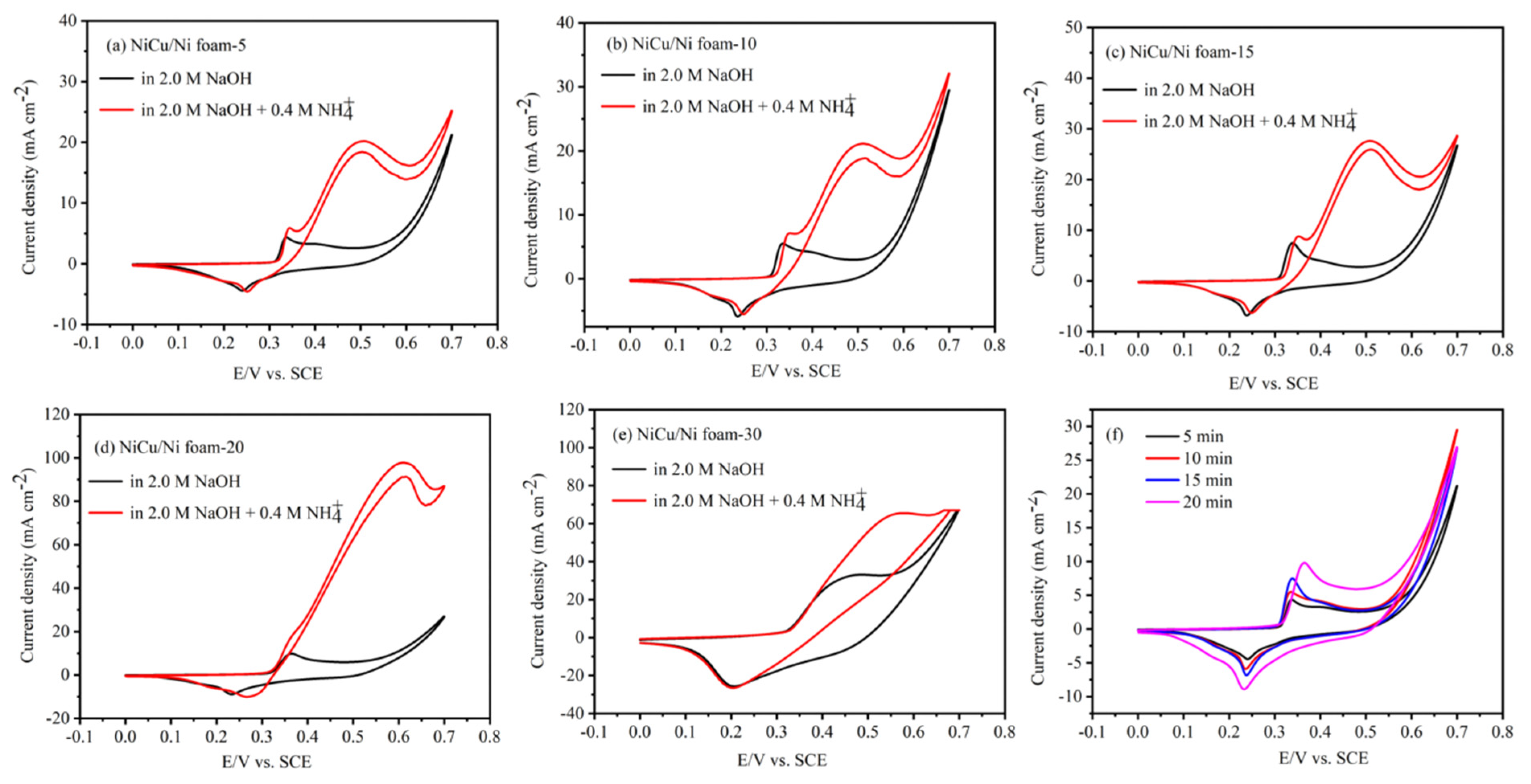

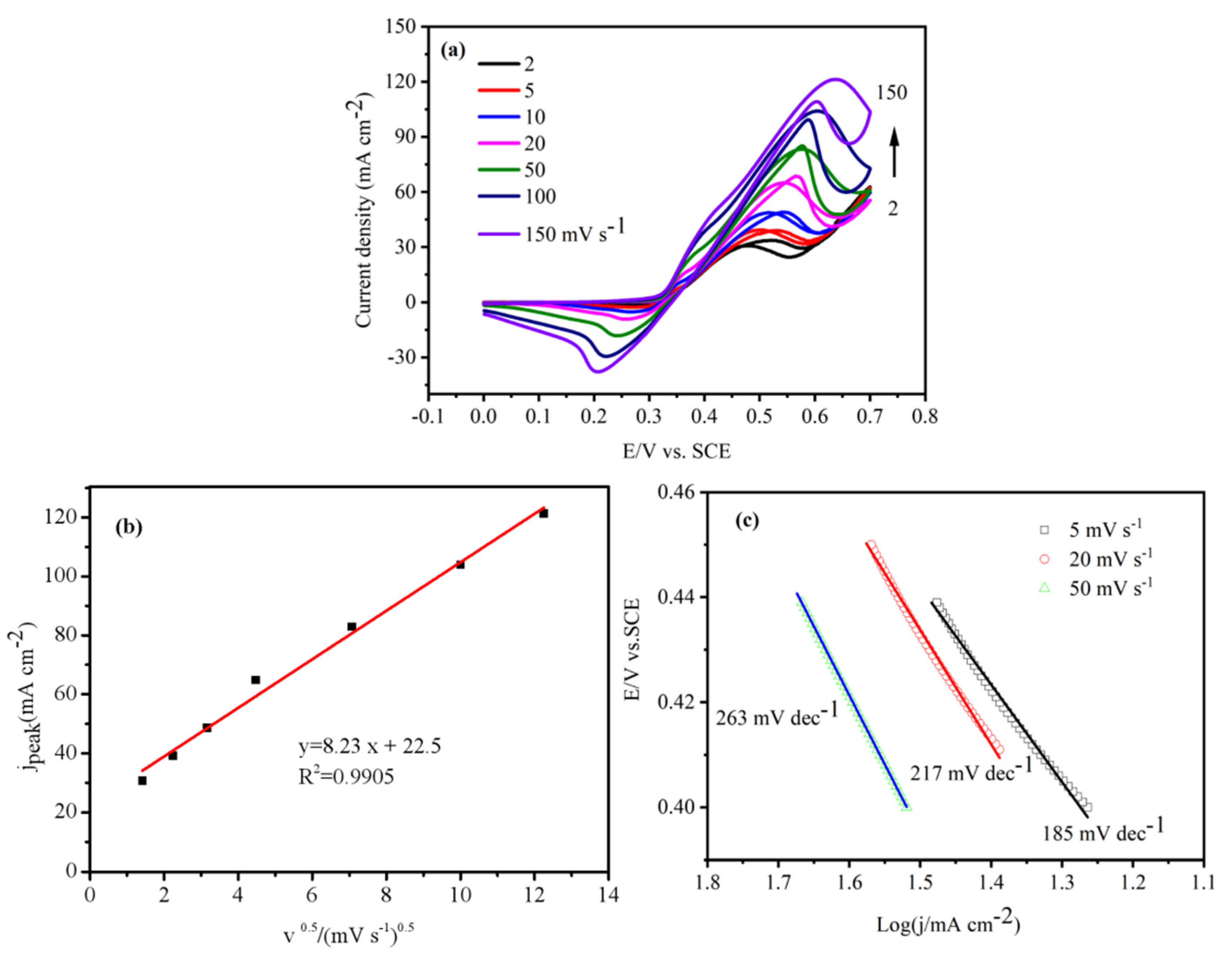


| Electrode | Elements | Atomic % | Electrode | Elements | Atomic % |
|---|---|---|---|---|---|
| NiCu-NF | O | 20.7 | NiCuOOH-NF | O | 62.8 |
| Cl | 7.9 | Cl | 0.54 | ||
| Ni | 39.4 | Ni | 20.8 | ||
| Cu | 28.6 | Cu | 15.9 |
| Electrodes | Electrolytes | Anode Potential | Removal Efficiency (%) | Ref. |
|---|---|---|---|---|
| NiCuOOH-NF | 0.20 M NaOH + 616 mg/L NH4+-N | 0.60 V vs. SCE | 96.53 | This work |
| Ni0.8Cu0.2 oxyhydroxide | 0.1 M KOH + 10 mM NH4+ | 1.53 V vs. RHE | 72 | [25] |
| Cu0.5Co0.5/NF | 10 mM Na2SO4 + 0.032 M NH3 | 1.10 V vs. Ag/AgCl | 72 | [40] |
| NiCuFe | 0.5 M NaOH + 0.055 M NH4Cl | 0.55 V vs. SCE | 90 | [41] |
| Pt/C | 1 M KOH + 0.2 M NH4OH | - | 66 | [42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhou, L.; Chen, W.; Tong, X.; Wang, Y.; Zhang, H. A Petal-like Structured NiCuOOH-NF Electrode by a Sonochemical Combined with the Electrochemical Method for Ammonia Oxidation Reaction. Processes 2023, 11, 228. https://doi.org/10.3390/pr11010228
Wang H, Zhou L, Chen W, Tong X, Wang Y, Zhang H. A Petal-like Structured NiCuOOH-NF Electrode by a Sonochemical Combined with the Electrochemical Method for Ammonia Oxidation Reaction. Processes. 2023; 11(1):228. https://doi.org/10.3390/pr11010228
Chicago/Turabian StyleWang, Hailong, Luanqi Zhou, Wenyi Chen, Xing Tong, Yifei Wang, and Huimin Zhang. 2023. "A Petal-like Structured NiCuOOH-NF Electrode by a Sonochemical Combined with the Electrochemical Method for Ammonia Oxidation Reaction" Processes 11, no. 1: 228. https://doi.org/10.3390/pr11010228





