The Effect of Low Positive Temperatures on the Formation of Secondary Metabolites in Rhodiola quadrifida (Pall.) Fisch. et C.A. Mey. In Vitro Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Estimation of Growth Characteristics of Hairy Roots and Callus Culture
2.3. Extraction and Preparation of Samples for HPLC-MS/MS Analysis of Flavones
2.4. HPLC-MS/MS Conditions
2.5. Statistical Processing
3. Results
3.1. The Effect of Temperature on the Growth of Calli and Hairy Roots
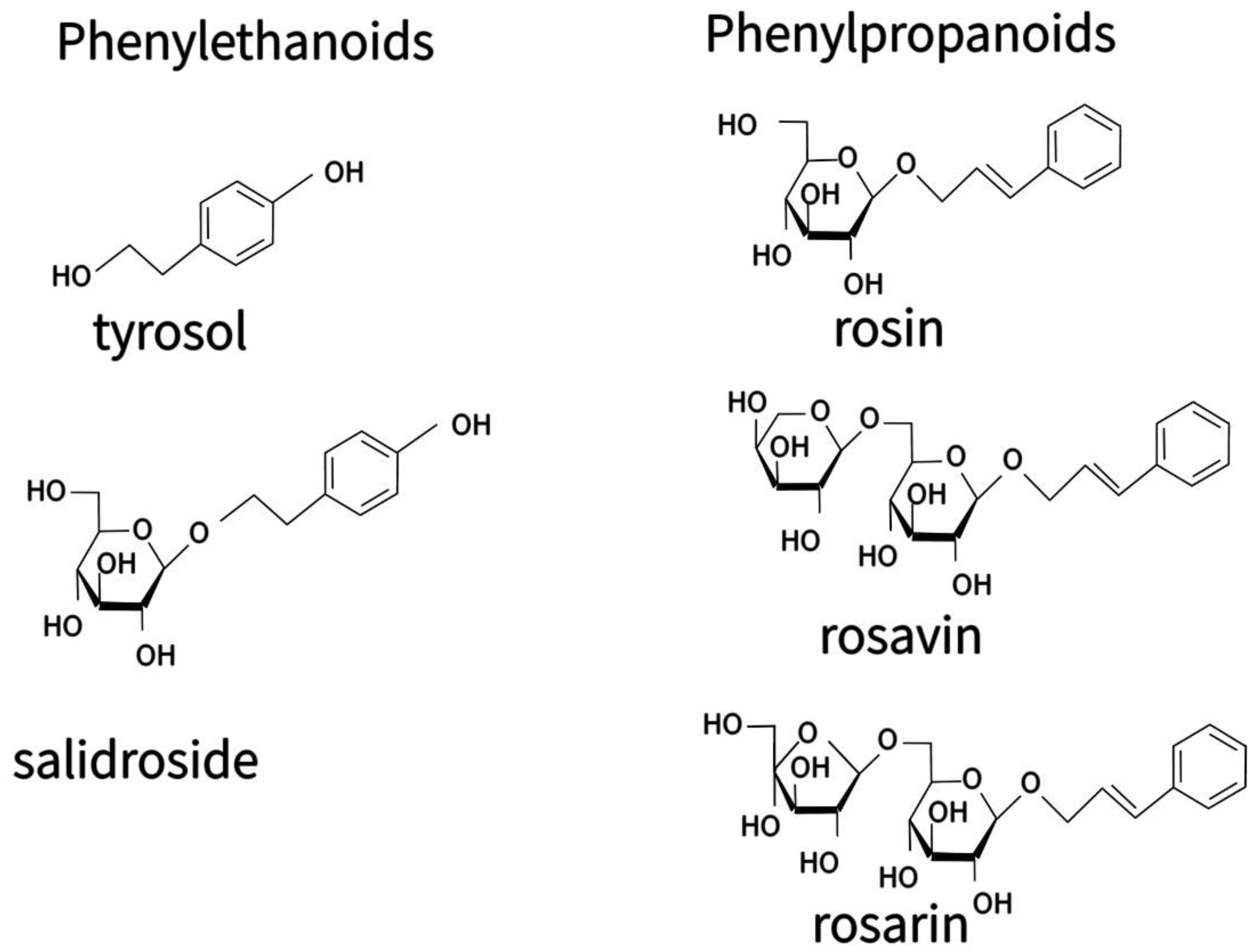
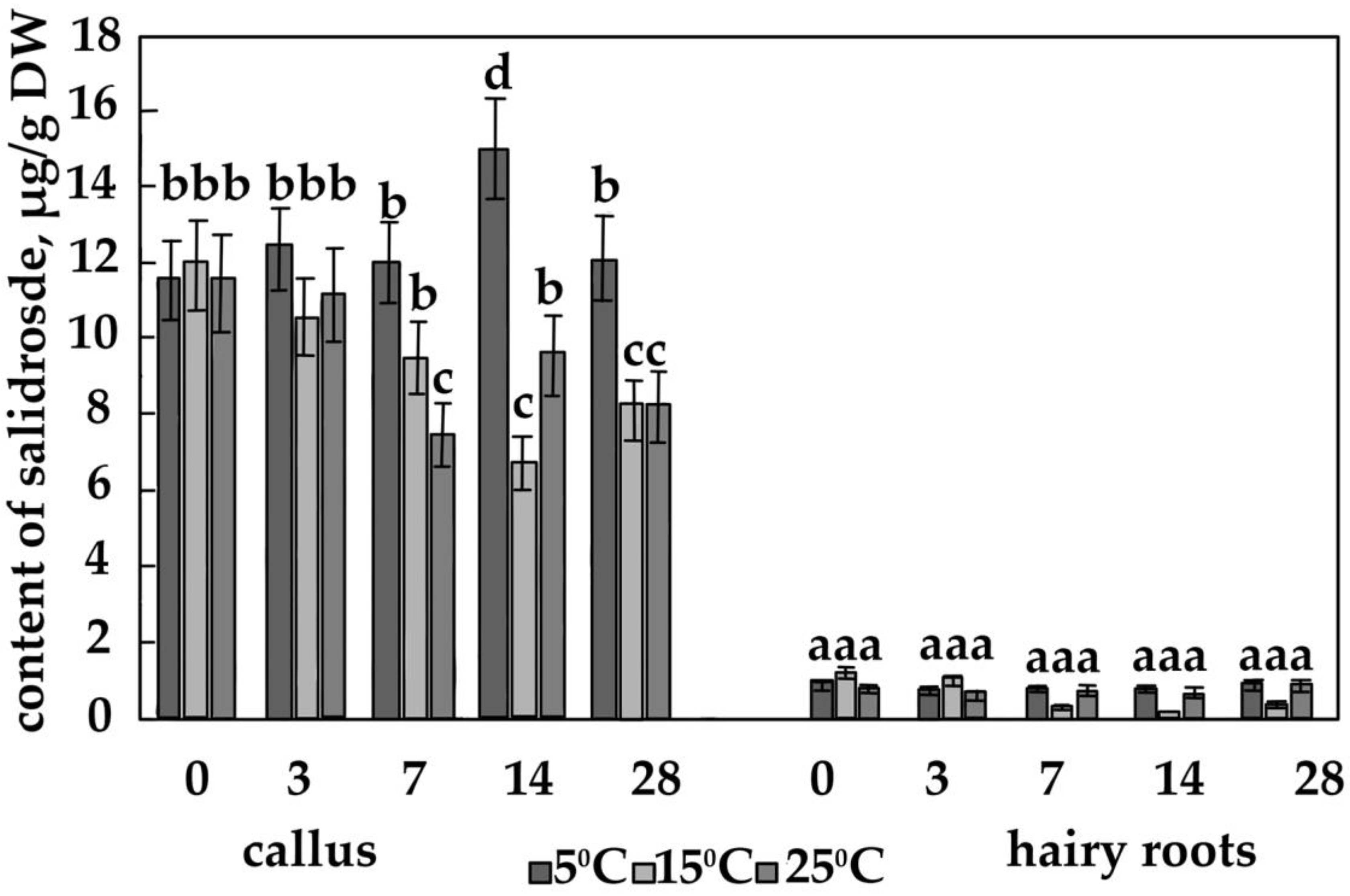
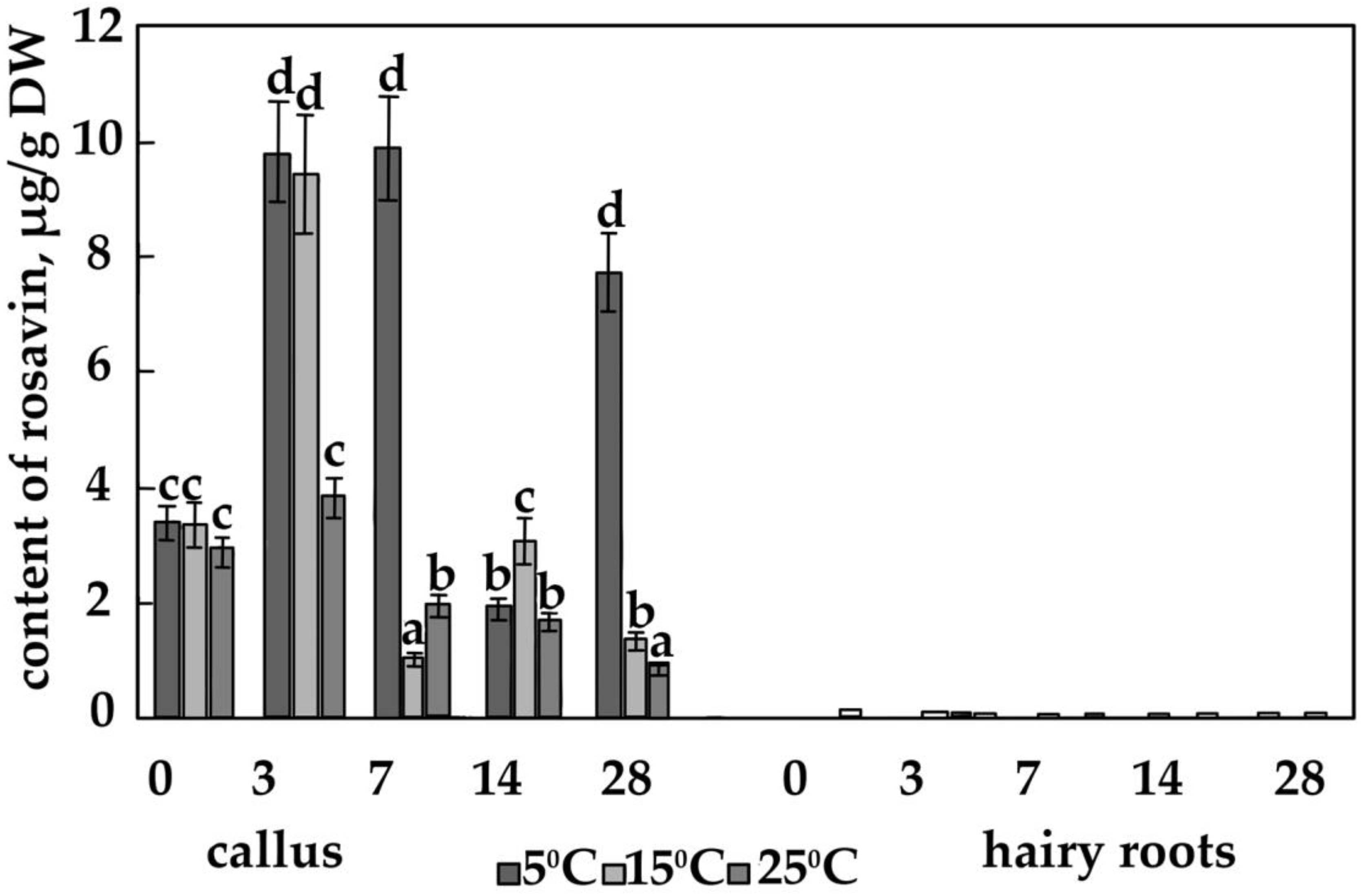
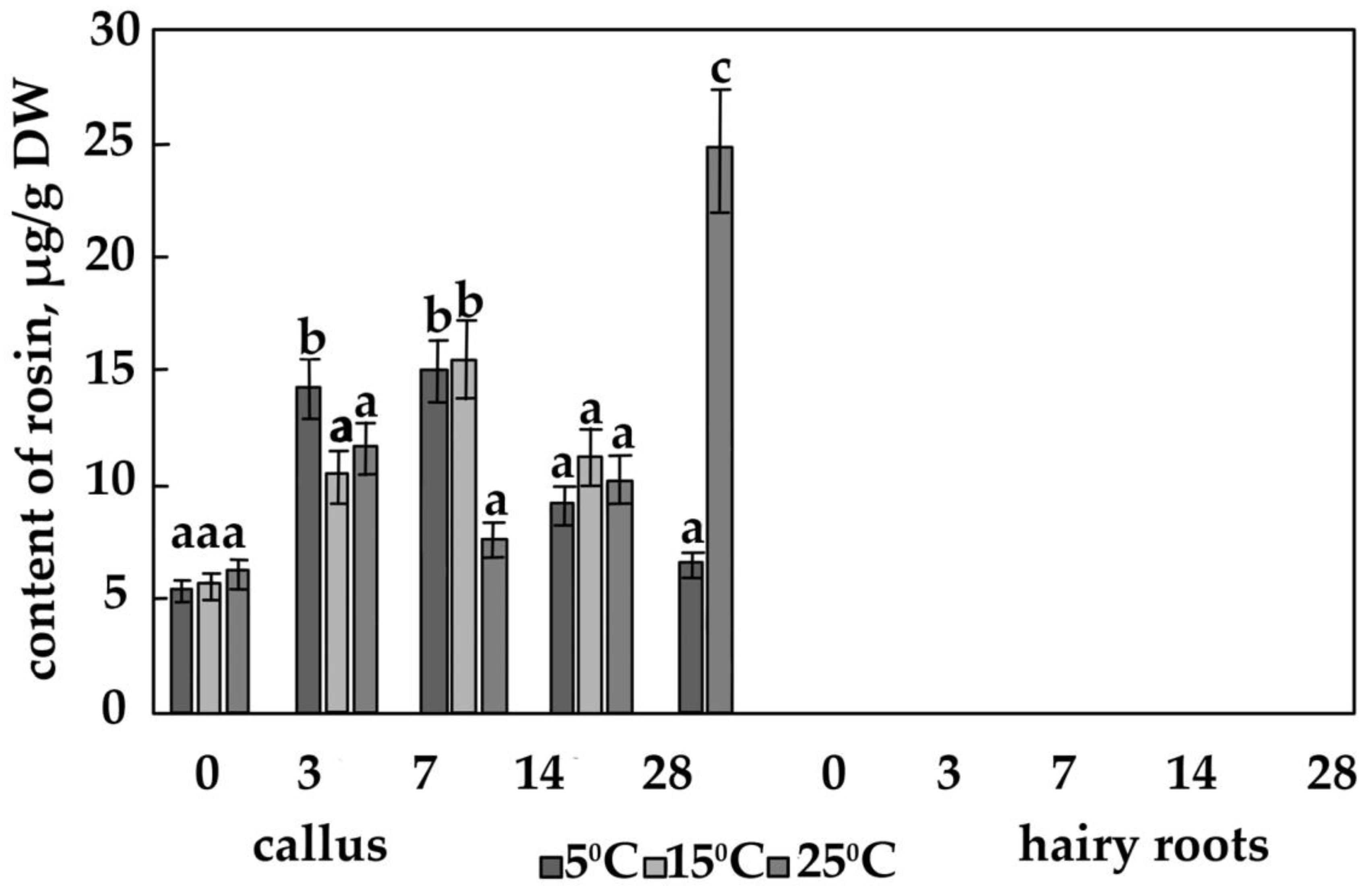
3.2. The Content of the Main Phenolic Compounds in Calli and Hairy Roots
4. Discussion
4.1. Features of In Vitro Growth of Rhodiola Quadrifida Cultures and Its Effect on the Content of Secondary Metabolites
4.2. The Effect of Culture Type on the Content of Secondary Metabolites
4.3. The Effect of Environmental Factors on the Content of Secondary Metabolites in R. quadrifida
4.4. The Effect of Temperature on the Content of Salidroside, Rosin and Rosavin in Calli of R. quadrifida
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. About IPCC (Website). Available online: https://www.ipcc.ch/about/ (accessed on 5 November 2022).
- You, J.; Qin, X.; Ranjitkar, S.; Lougheed, S.C.; Wang, M.; Zhou, W.; Ouyang, D.; Zhou, Y.; Xu, J.; Zhang, W.; et al. Response to climate change of montane herbaceous plants in the genus Rhodiola predicted by ecological niche modelling. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogués-Bravo, D.; Araújo, M.B.; Errea, M.P.; Martínez-Rica, J.P. Exposure of global mountain systems to climate warming during the 21st Century. Glob. Environ. Chang. 2007, 17, 420–428. [Google Scholar] [CrossRef]
- Loarie, S.R.; Duffy, P.B.; Hamilton, H.; Asner, G.P.; Field, C.B.; Ackerly, D.D. The velocity of climate change. Nature 2009, 462, 1052–1055. [Google Scholar] [CrossRef]
- Skopińska-Róewska, E.; Bychawska, M.; Białas-Chromiec, B.; Sommer, E. The in vivo effect of Rhodiola rosea and Rhodiola quadrifida hydro-alcoholic extracts on chemokinetic activity of spleen lymphocytes in mice. Cent. J. Immunol. 2009, 34, 42–45. [Google Scholar]
- Wiedenfeld, H.; Dumaa, M.; Malinowski, M.; Furmanowa, M.; Narantuya, S. Phytochemical and analytical studies of extracts from Rhodiola rosea and Rhodiola quadrifida. Pharmazie 2007, 62, 308–311. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Shimada, H.; Shimoda, H.; Murakami, N.; Yamahara, J.; Matsuda, H. Bioactive constituents of Chinese natural medicines. II. Rhodiolae radix. (1). Chemical structures and antiallergic activity of rhodiocyanosides A and B from the underground part of Rhodiola quadrifida (Pall.) Fisch. et Mey. (Crassulaceae). Chem. Pharm. Bull. 1996, 44, 2086–2091. [Google Scholar] [CrossRef]
- Teteryuk, L.V.; Kanev, V.A.; Valuyskikh, O.E.; Teteryuk, B.Y. Rare and protected plants in the flora of the southern part of the “Yugud Va” National park (Komi Republic). Bot. J. 2019, 104, 1283–1298. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Mishra, P.; Mishra, A.K. Effect of climate change on plant secondary metabolism: An ecological perspective. In Evolutionary Diversity as a Source for Anticancer Molecules; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128217108. [Google Scholar]
- Jamloki, A.; Bhattacharyya, M.; Nautiyal, M.C.; Patni, B. Elucidating the relevance of high temperature and elevated CO2 in plant secondary metabolites (PSMs) production. Heliyon 2021, 7, e07709. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Kiss, A.; Ladányi, M.; György, Z. Production of cinnamyl alcohol glycosides by biotransformation in roseroot callus cells. Plant Cell Tissue Organ Cult. 2019, 139, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Mirmazloum, I.; György, Z. Review of the molecular genetics in higher plants towards salidroside and cinnamyl alcohol glycosides biosynthesis in Rhodiola rosea L. Acta Aliment. 2012, 41, 133–146. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Malunova, M.V.; Gladkov, E.A.; Evsyukov, S.V.; Tereshonok, D.V.; Solov’eva, A.I.; Timiryazev, K.A. Collection of Hairy Roots as a Basis for Fundamental and Applied Research. Molecules 2022, 27, 8040. [Google Scholar] [CrossRef] [PubMed]
- Laines-Hidalgo, J.I.; Muñoz-Sánchez, J.A.; Loza-Müller, L.; Vázquez-Flota, F. An Update of the Sanguinarine and Benzophenanthridine Alkaloids Biosynthesis and Their Applications. Molecules 2022, 27, 1378. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, L.; Tripathi, J.N. Role of biotechnology in medicinal plants. Trop. J. Pharm. Res. 2003, 2, 243–253. [Google Scholar] [CrossRef]
- Gladkov, E.A.; Gladkova, O.V. New directions of biology and biotechnology in urban environmental sciences. Hem. Ind. Chemical Ind. 2021, 75, 365–368. [Google Scholar] [CrossRef]
- Stepanova, A.; Malunova, M.; Salamaikina, S.; Selimov, R.; Solov’eva, A. Establishment of Rhodiola quadrifida Hairy Roots and Callus Culture to Produce Bioactive Compounds. Phyton-Int. J. Exp. Bot. 2021, 90, 543–552. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kapoor, S.; Raghuvanshi, R.; Bhardwaj, P.; Sood, H.; Saxena, S.; Chaurasia, O.P. Influence of light quality on growth, secondary metabolites production and antioxidant activity in callus culture of Rhodiola imbricata Edgew. J. Photochem. Photobiol. B Biol. 2018, 183, 258–265. [Google Scholar] [CrossRef]
- Tasheva, K.; Kosturkova, G. The Role of Biotechnology for Conservation and Biologically Active Substances Production of Rhodiola rosea: Endangered Medicinal Species. Sci. World J. 2012, 2012, 13. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Li, L.; Xiao, S.; Chen, Z.; Sarsaiya, S.; Zhang, S.; ShangGuan, Y.; Liu, H.; Xu, D. Callus growth kinetics and accumulation of secondary metabolites of Bletilla striata Rchb.f. using a callus suspension culture. PLoS ONE 2020, 15, e0220084. [Google Scholar] [CrossRef] [Green Version]
- Rattan, S.; Kumar, D.; Warghat, A.R. Growth kinetics, metabolite yield, and expression analysis of biosynthetic pathway genes in friable callus cell lines of Rhodiola imbricata (Edgew). Plant Cell Tissue Organ Cult. 2021, 146, 149–160. [Google Scholar] [CrossRef]
- Altantsetseg, K.; Weglarz, Z.; Geszprych, A. Content of biologically active compounds in roseroot [Rhodiola sp.] raw material of different derivation. Herba Pol. 2007, 53, 20–26. [Google Scholar]
- Shi, L.; Wang, C.; Zhou, X.; Zhang, Y.; Liu, Y.; Ma, C. Production of salidroside and tyrosol in cell suspension cultures of Rhodiola crenulata. Plant Cell Tissue Organ Cult. 2013, 114, 295–303. [Google Scholar] [CrossRef]
- Ramachandra Rao, S.; Ravishankar, G.A. Plant cell cultures: Chemical factories of secondary metabolites. Biotechnol. Adv. 2002, 20, 101–153. [Google Scholar] [CrossRef] [PubMed]
- Fazili, M.A.; Bashir, I.; Ahmad, M.; Yaqoob, U.; Geelani, S.N. In vitro strategies for the enhancement of secondary metabolite production in plants: A review. Bull. Natl. Res. Cent. 2022, 46, 35. [Google Scholar] [CrossRef]
- Yazaki, K. Lithospermum erythrorhizon cell cultures: Present and future aspects. Plant Biotechnol. 2017, 34, 131. [Google Scholar] [CrossRef] [Green Version]
- Bozhilova, M. Salidroside content in Rhodiola rosea L., dynamics and variability. Bot. Serbica 2011, 35, 67–70. [Google Scholar]
- Elameen, A.; Kosman, V.M.; Thomsen, M.; Pozharitskaya, O.N.; Shikov, A.N.; Cacciola, F.; Barros, L. Molecules Variability of Major Phenyletanes and Phenylpropanoids in 16-Year-Old Rhodiola rosea L. Clones in Norway. Molecules 2020, 25, 3463. [Google Scholar] [CrossRef]
- Saunders, D.; Poppleton, D.; Struchkov, A.; Ireland, R. Analysis of five bioactive compounds from naturally occurring Rhodiola rosea in eastern Canada. Can. J. Plant Sci. 2014, 94, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Przybył, J.L.; Wȩglarz, Z.; Geszprych, A. Quality of Rhodiola rosea cultivated in Poland. In Proceedings of the Acta Horticulturae. Acta Hortic. 2008, 765, 143–150. [Google Scholar] [CrossRef]
- Buchwald, W.; Mścisz, A.; Krajewska-Patan, A.; Furmanowa, M.; Mielcarek, S.; Mrozikiewicz, P.M. Content of biologically active compounds in Rhodiola rosea roots during vegetation period. Herba Pol. 2006, 54, 39–43. [Google Scholar]
- Platikanov, S.; Evstatieva, L. Introduction of wild golden root (Rhodiola rosea L.) as a potential economic crop in Bulgaria. Econ. Bot. 2008, 62, 621–627. [Google Scholar] [CrossRef]
- Bykov, V.A.; Zapesochnaya, G.G.; Kurkin, V.A. Medicinal plants traditional and biotechnological aspects of obtaining medicinal preparations from Rhodiola rosea L. (a review). Pharm. Chem. J. 1999, 33, 29–40. [Google Scholar] [CrossRef]
- Grech-Baran, M.; Sykłowska-Baranek, K.; Pietrosiuk, A. Biotechnological approaches to enhance salidroside, rosin and its derivatives production in selected Rhodiola spp. in vitro cultures. Phytochem. Rev. 2015, 14, 657–674. [Google Scholar] [CrossRef] [Green Version]
- Kołodziej, B.; Sugier, D. Influence of plants age on the chemical composition of roseroot (Rhodiola rosea L.). Acta Sci. Pol. Hortorum Cultus 2013, 12, 147–160. [Google Scholar]
- Shikov, A.N.; Pozharitskaya, O.N.; Makarov, V.G.; Wagner, H.; Verpoorte, R.; Heinrich, M. Medicinal Plants of the Russian Pharmacopoeia; their history and applications. J. Ethnopharmacol. 2014, 154, 481–536. [Google Scholar] [CrossRef]
- Galambosi, B. Demand and availability of Rhodiola rosea L. raw material. In Medicinal and Aromatic Plants: Agricultural, Commercial, Ecological, Legal, Pharmacological and Social Aspects; Bogers, R.J., Craker, L.E., Lange, D., Eds.; Springer: Berlin, Germany, 2006; pp. 223–236. [Google Scholar]
- Kucharski, W.A.; Mordalski, R.; Buchwald, W.; Instytut, S.M.; Polskiego, W. Roseroot—The comparison of tillage in conventional and ecological system. J. Res. Appl. Agric. Eng. 2011, 56, 232–235. [Google Scholar]
- Sheng, C.Z.; Hu, T.Q.; Bi, H.; Yuan, Y.J.; Jiang, Y. Effects of plant growth substances on induction and culture of callus from Rhodiola quadrifida. Zhongguo Zhongyao Zazhi 2005, 30, 1237–1240. [Google Scholar]
- Kucinskaite, A.; Pobłocka-Olech, L.; Krauze-Baranowska, M.; Sznitowska, M.; Savickas, A.; Briedis, V. Evaluation of biologically active compounds in roots and rhizomes. Medicina 2007, 43, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Booker, A.; Zhai, L.; Gkouva, C.; Li, S.; Frommenwiler, D.; Reich, E.; Slater, A.; Heinrich, M. Comparison of different Rhodiola species using NMR- metabolomics and HPTLC techniques. Planta Med. 2016, 82, 1050. [Google Scholar] [CrossRef] [Green Version]
- Jochum, G.M.; Mudge, K.W.; Thomas, R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentrations in the understory herb Panax quinquefolius (Araliaceae). Am. J. Bot. 2007, 94, 819–826. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecule 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tavakoli, F.; Rafieiolhossaini, M.; Ravash, R.; Ebrahimi, M. Subject: UV-B radiation and low temperature promoted hypericin biosynthesis in adventitious root culture of Hypericum perforatum. Plant Signal. Behav. 2020, 15, 1764184. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Kang, T.; Jin, L.; Liu, Z.; Zhang, Z.; Xing, H.; Sun, P.; Li, M. Temperature-dependent growth and hypericin biosynthesis in Hypericum perforatum. Plant Physiol. Biochem. PPB 2019, 139, 613–619. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhai, J.; Shao, L.; Lin, W.; Peng, C. Accumulation of Anthocyanins: An Adaptation Strategy of Mikania micrantha to Low Temperature in Winter. Front. Plant Sci. 2019, 10, 1049. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanova, A.Y.; Gladkov, E.A.; Tereshonok, D.V.; Selimov, R.N.; Goncharova, E.N.; Solov’eva, A.I. The Effect of Low Positive Temperatures on the Formation of Secondary Metabolites in Rhodiola quadrifida (Pall.) Fisch. et C.A. Mey. In Vitro Cultures. Processes 2023, 11, 28. https://doi.org/10.3390/pr11010028
Stepanova AY, Gladkov EA, Tereshonok DV, Selimov RN, Goncharova EN, Solov’eva AI. The Effect of Low Positive Temperatures on the Formation of Secondary Metabolites in Rhodiola quadrifida (Pall.) Fisch. et C.A. Mey. In Vitro Cultures. Processes. 2023; 11(1):28. https://doi.org/10.3390/pr11010028
Chicago/Turabian StyleStepanova, Anna Y., Evgeny A. Gladkov, Dmitry V. Tereshonok, Renat N. Selimov, Elisaveta N. Goncharova, and Aleksandra I. Solov’eva. 2023. "The Effect of Low Positive Temperatures on the Formation of Secondary Metabolites in Rhodiola quadrifida (Pall.) Fisch. et C.A. Mey. In Vitro Cultures" Processes 11, no. 1: 28. https://doi.org/10.3390/pr11010028
APA StyleStepanova, A. Y., Gladkov, E. A., Tereshonok, D. V., Selimov, R. N., Goncharova, E. N., & Solov’eva, A. I. (2023). The Effect of Low Positive Temperatures on the Formation of Secondary Metabolites in Rhodiola quadrifida (Pall.) Fisch. et C.A. Mey. In Vitro Cultures. Processes, 11(1), 28. https://doi.org/10.3390/pr11010028





