ELISA Based Immunoreactivity Reduction of Soy Allergens through Thermal Processing
Abstract
:1. Introduction
2. Materials and Methods
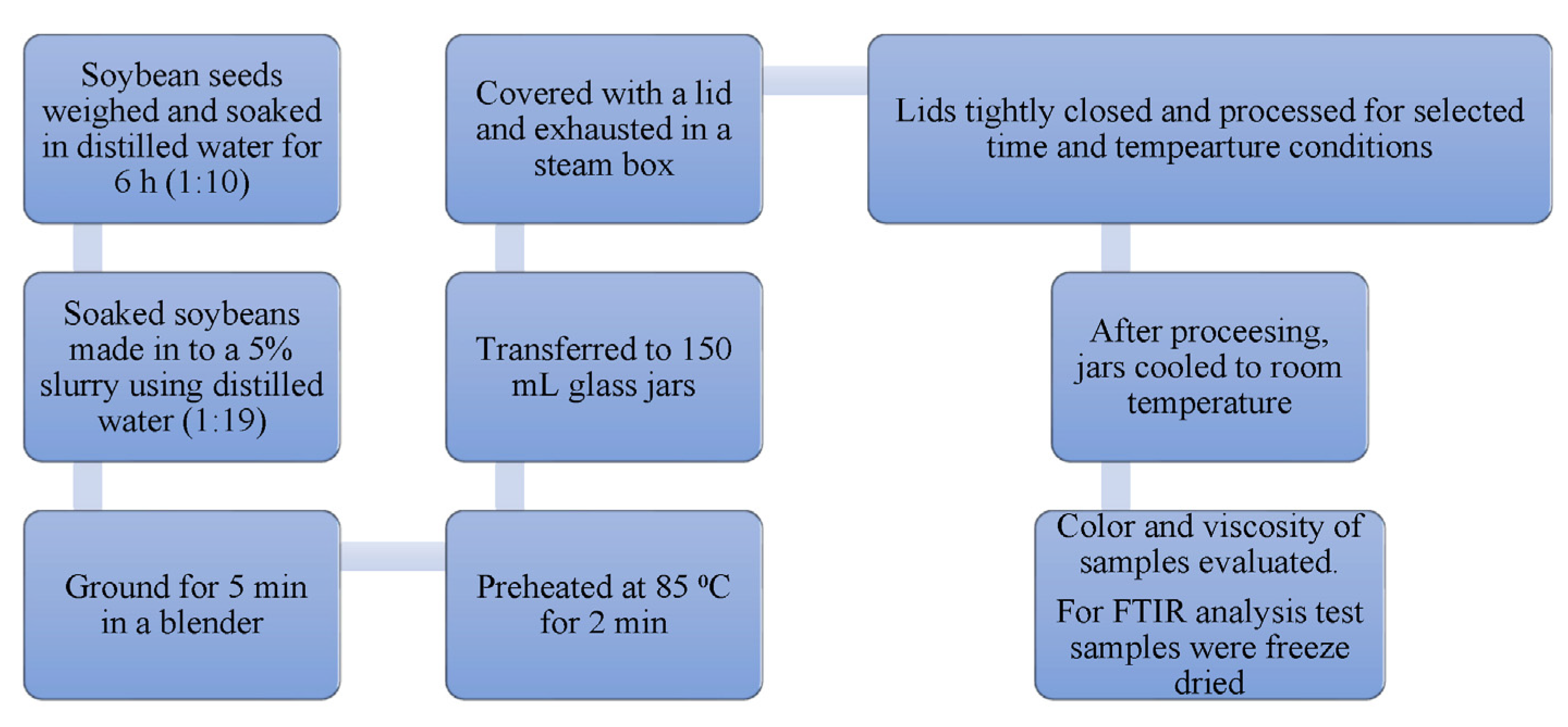
2.1. Soy Slurry Preparation
2.2. Cooking of Soy Slurry Samples
2.3. Commercial Thermal Processing
2.4. Process Lethality and Cook Value
2.5. ELISA Analysis
2.6. FTIR Analysis
2.7. Quality Analysis
2.7.1. Color
2.7.2. Flow Rheology of Soy Slurry
2.7.3. Statistical Analysis
3. Results and Discussion
3.1. Immunoreactivity of Test Samples
3.2. Cooking Treatment
3.3. Thermal Processing at Different Temperatures
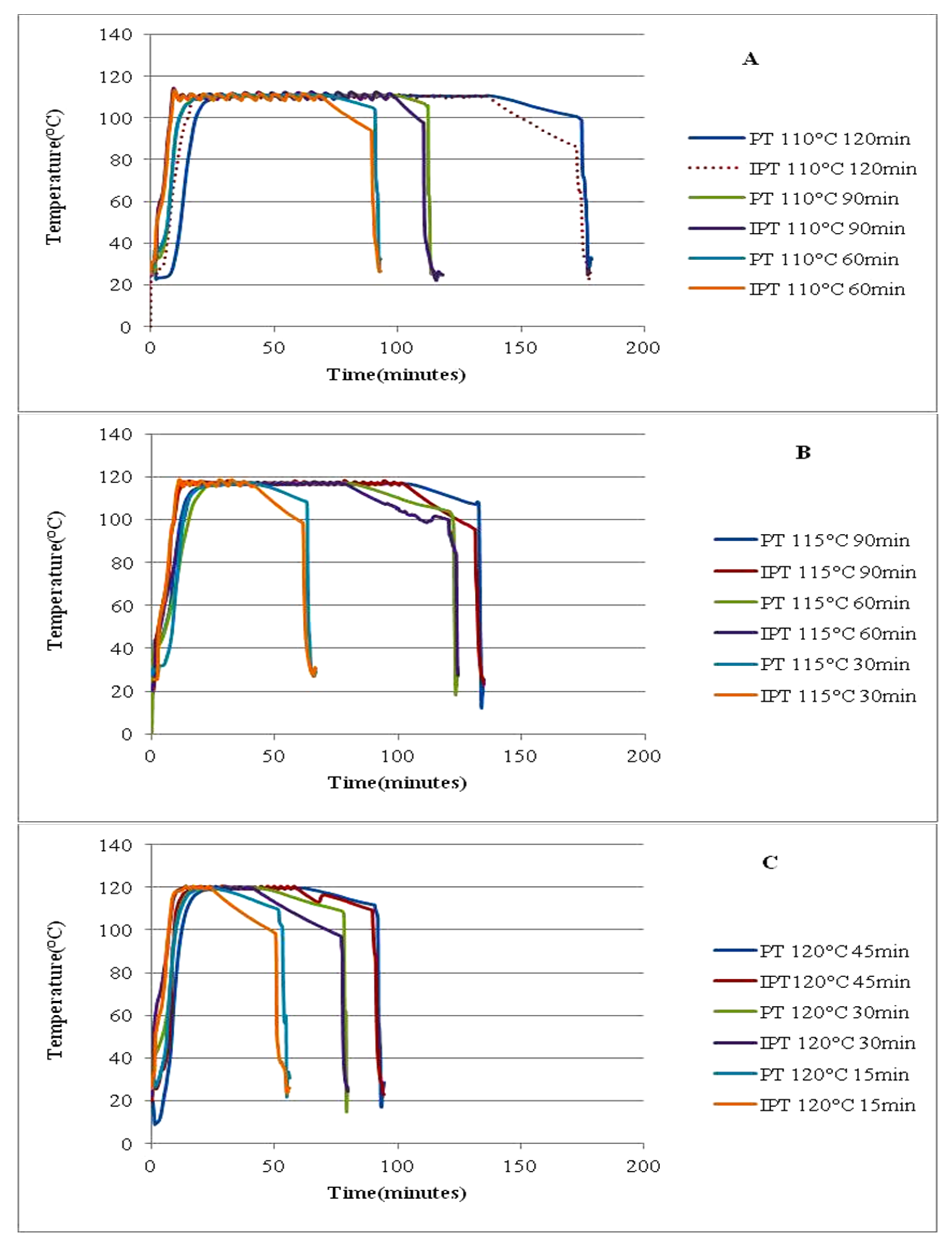
3.3.1. Thermal Processing Conditions
3.3.2. Heat Penetration Profiles
3.3.3. Lethality and Cook Value
3.3.4. Effect of Process Time and Temperature on Allergen Reduction
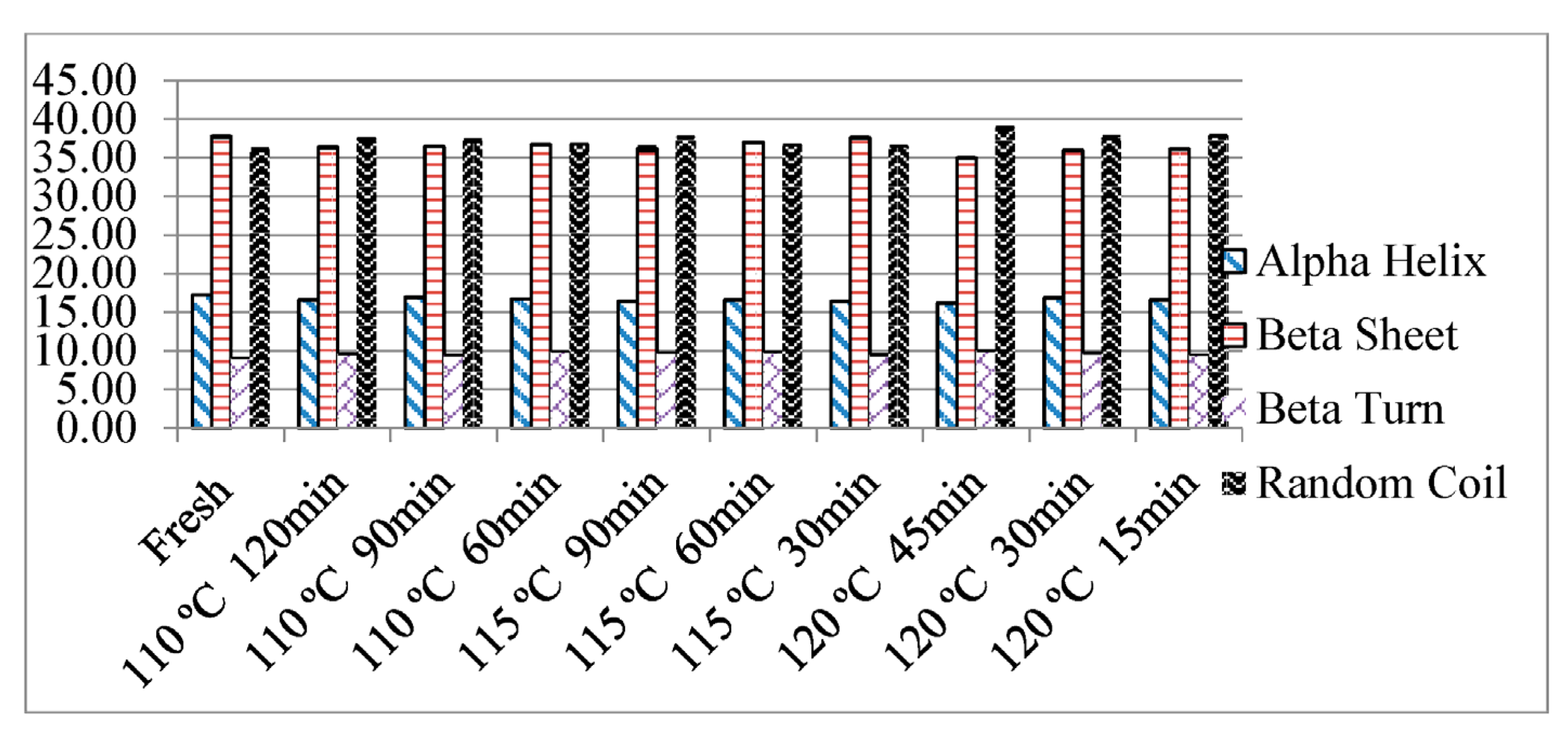
3.4. Effect of Thermal Processing on the Secondary Structure of Proteins
3.5. Effect of Thermal Processing Treatments on Quality Parameters
3.5.1. Effect on Color
3.5.2. Effect on Viscosity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johnson, L.A.; White, P.J.; Galloway, R. Soybeans-Chemistry, Production Processing, and Utilization; AOCS Press: Urbana, IL, USA, 2008; Volume 2, pp. 441–447. [Google Scholar]
- Adachi, M.; Takenaka, Y.; Gidamis, A.B.; Mikami, B.; Utsumi, S. Crystal structure of soybean proglycinin A1aB1b homotrimer. J. Mol. Biol. 2001, 305, 291–305. [Google Scholar] [CrossRef]
- Codina, R.; Lockey, R.F.; Fernández-Caldas, E.; Rama, R. Purification and characterization of a soybean hull allergen responsible for the Barcelona asthma outbreaks. II. Purification and sequencing of the Gly m 2 allergen. Clin. Exp. Allergy 1997, 27, 424–430. [Google Scholar] [CrossRef]
- Crowell, D.N.; John, M.E.; Russell, D.; Amasino, R.M. Characterization of a stress-induced, developmentally regulated gene family from soybean. Plant Mol. Biol. 1992, 18, 459–466. [Google Scholar] [CrossRef] [PubMed]
- González, R.; Zapatero, L.; Caravaca, F.; Carreira, J. Identification of soybean proteins responsible for respiratory allergies. Int. Arch. Allergy Immunol. 1991, 95, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Klemans, R.J.; Knol, E.F.; Michelsen-Huisman, A.; Pasmans, S.G.; de Kruijf-Broekman, W.; Bruijnzeel-Koomen, C.A.; van Hoffen, E.; Knulst, A.C. Components in soy allergy diagnostics: Gly m 2S albumin has the best diagnostic value in adults. Allergy 2013, 68, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, N.; Adachi, M.; Takahashi, K.; Yagasaki, K.; Kohno, M.; Takenaka, Y.; Okuda, E.; Nakagawa, S.; Mikami, B.; Utsumi, S. Crystal structures of recombinant and native soybean β-conglycinin β homotrimers. Eur. J. Biochem. 2001, 268, 3595–3604. [Google Scholar] [CrossRef]
- Riascos, J.J.; Weissinger, S.M.; Weissinger, A.K.; Kulis, M.; Burks, A.W.; Pons, L. The Seed Biotinylated Protein of Soybean (Glycine max): A Boiling-Resistant New Allergen (Gly m 7) with the Capacity To Induce IgE-Mediated Allergic Responses. J. Agric. Food Chem. 2016, 64, 3890–3900. [Google Scholar] [CrossRef]
- Rihs, H.P.; Chen, Z.; Ruëff, F.; Petersen, A.; Rozynek, P.; Heimann, H.; Baur, X. IgE binding of the recombinant allergen soybean profilin (rGly m 3) is mediated by conformational epitopes. J. Allergy Clin. Immunol. 1999, 104, 1293–1301. [Google Scholar] [CrossRef]
- Dreau, D.; Lallès, J.P.; Philouze-Rome, V.; Toullec, R.; Salmon, H. Local and systemic immune responses to soybean protein ingestion in early-weaned pigs. J. Anim. Sci. 1994, 72, 2090–2098. [Google Scholar] [CrossRef]
- Helm, R.M.; Cockrell, G.; Connaughton, C.; Sampson, H.A.; Bannon, G.A.; Beilinson, V.; Livingstone, D.; Nielsen, N.C.; Burks, A.W. A soybean G2 glycinin allergen. Int. Arch. Allergy Immunol. 2000, 123, 205–212. [Google Scholar] [CrossRef]
- Holzhauser, T.; Wackermann, O.; Ballmer-Weber, B.K.; Bindslev-Jensen, C.; Scibilia, J.; Perono-Garoffo, L.; Utsumi, S.; Poulsen, L.K.; Vieths, S. Soybean (Glycine max) allergy in Europe: Gly m 5 (β-conglycinin) and Gly m 6 (glycinin) are potential diagnostic markers for severe allergic reactions to soy. J. Allergy Clin. Immunol. 2009, 123, 452–458.e4. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, H.B.; Kim, W.-S.; Jang, S.; Kerley, M.S. All Three Subunits of Soybean β-Conglycinin Are Potential Food Allergens. J. Agric. Food Chem. 2009, 57, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Liu, X.; Wang, Y.-Z.; Liu, J.-X.; Feng, J. Soybean glycinin- and β-conglycinin-induced intestinal immune responses in a murine model of allergy. Food Agric. Immunol. 2013, 24, 357–369. [Google Scholar] [CrossRef]
- Costa, J.; Amaral, J.S.; Grazina, L.; Oliveira MB, P.P.; Mafra, I. Matrix-normalised real-time PCR approach to quantify soybean as a potential food allergen as affected by thermal processing. Food Chem. 2017, 221, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Kattan, J.D.; Cocco, R.R.; Järvinen, K.M. Milk and Soy Allergy. Pediatr. Clin. N. Am. 2011, 58, 407–426. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, R.S.; Sampson, H.A.; Bock, S.A.; Burks, A.W., Jr.; Harden, K.; Noone, S.; Martin, D.; Leung, S.; Wilson, G. Soy allergy in infants and children with IgE-associated cow’s milk allergy. J. Pediatr. 1999, 134, 614–622. [Google Scholar] [CrossRef]
- Ogawa, T.; Samoto, M.; Takahashi, K. Soybean Allergens and Hypoallergenic Soybean Products. J. Nutr. Sci. Vitaminol. 2000, 46, 271–279. [Google Scholar] [CrossRef] [Green Version]
- Lusas, E.W.; Riaz, M.N. Soy protein products: Processing and use. J. Nutr. 1995, 125 (Suppl. S3), 573S–580S. [Google Scholar]
- Verhoeckx, K.C.M.; Vissers, Y.M.; Baumert, J.L.; Faludi, R.; Feys, M.; Flanagan, S.; Herouet-Guicheney, C.; Holzhauser, T.; Shimojo, R.; van der Bolt, N.; et al. Food processing and allergenicity. Food Chem. Toxicol. 2015, 80, 223–240. [Google Scholar] [CrossRef]
- Wang, T.; Qin, G.X.; Sun, Z.W.; Zhao, Y. Advances of research on glycinin and beta-conglycinin: A review of two major soybean allergenic proteins. Crit. Rev. Food Sci. Nutr. 2014, 54, 850–862. [Google Scholar] [CrossRef]
- Shibasaki, M.; Suzuki, S.; Tajima, S.; Nemoto, H.; Kuroume, T. Allergenicity of Major Component Proteins of Soybean. Int. Arch. Allergy Immunol. 1980, 61, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Cabanillas, B.; Cuadrado, C.; Rodriguez, J.; Dieguez, M.C.; Crespo, J.F.; Novak, N. Boiling and pressure cooking impact on IgE reactivity of soybean allergens. Int. Arch. Allergy Immunol. 2018, 175, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, A.; Boye, J.I. Impact of thermal processing time and cookie size on the detection of casein, egg, gluten and soy allergens in food. Food Res. Int. 2013, 52, 483–489. [Google Scholar] [CrossRef]
- Gomaa, A.; Ribereau, S.; Boye, J. Detection of allergens in a multiple allergen matrix and study of the impact of thermal processing. J. Nutr. Food Sci. S 2012, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Pi, X.; Sun, Y.; Fu, G. Effect of processing on soybean allergens and their allergenicity. Trends Food Sci. Technol. 2021, 118, 316–327. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Guo, X.; Chen, Q.; Cheng, J. Effects of thermal sterilization on the allergenicity of soybeans. LWT 2022, 15415, 112678. [Google Scholar] [CrossRef]
- Kerezsi, A.D.; Jacquet, N.; Blecker, C. Advances on physical treatments for soy allergens reduction—A review. Trends Food Sci. Technol. 2022, 122, 24–39. [Google Scholar] [CrossRef]
- Chen, J.; Wang, J.; Song, P.; Ma, X. Determination of glycinin in soybean and soybean products using a sandwich enzyme-linked immunosorbent assay. Food Chem. 2014, 162, 27–33. [Google Scholar] [CrossRef]
- Hei, W.; Li, Z.; Ma, X.; He, P. Determination of beta-conglycinin in soybean and soybean products using a sandwich enzyme-linked immunosorbent assay. Anal. Chim. Acta 2012, 734, 62–68. [Google Scholar] [CrossRef]
- Alpaslan, M.; Hayta, M. Effect of soymilk substitution on the rheological and sensory properties of salep (traditional Turkish milk beverage). Int. J. Food Prop. 2007, 10, 413–420. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Effects of high-intensity ultrasound processing on the physiochemical and allergenic properties of shrimp. Innov. Food Sci. Emerg. Technol. 2020, 65, 102441. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Raghavan, V. Critical reviews and recent advances of novel non-thermal processing techniques on the modification of food allergens. Crit. Rev. Food Sci. Nutr. 2021, 61, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Okwunodulu, I.; Abasiekong, K. Rheological behaviour of fortified soymilk from sprouted soybean for complementary feeding: A response surface analysis. J. Mol. Stud. Med. Res. 2015, 1, 26–33. [Google Scholar] [CrossRef]
- Xiang, B.Y.; Simpson, M.V.; Ngadi, M.O.; Simpson, B.K. Effect of pulsed electric field on the rheological and colour properties of soy milk. Int. J. Food Sci. Nutr. 2011, 62, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Bandekar, J. Amide modes and protein conformation. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1992, 1120, 123–143. [Google Scholar] [CrossRef]
- Baronio, C.M.; Baldassarre, M.; Barth, A. Insight into the internal structure of amyloid-β oligomers by isotope-edited Fourier transform infrared spectroscopy. Phys. Chem. Chem. Phys. 2019, 21, 8587–8597. [Google Scholar] [CrossRef] [Green Version]
- Munu, N.; Kigozi, J.; Zziwa, A.; Kambugu, R.; Wasswa, J.; Tumutegyereize, P. Effect of ambient-soaking time on soybean characteristics fortraditional soy milk extraciton. J. Adv. Food Sci. Technol. 2016, 3, 119–128. [Google Scholar]
- Shi, L.; Mu, K.; Arntfield, S.D.; Nickerson, M.T. Changes in levels of enzyme inhibitors during soaking and cooking for pulses available in Canada. J. Food Sci. Technol. 2017, 54, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Dwivedi, M.; Ramaswamy, H.S. Comparative study of wireless versus standard thermocouples for data gathering and analyses in rotary cookers. J. Food Process. Preserv. 2010, 34, 557–574. [Google Scholar] [CrossRef]
- Abatemarco, C.A.; Ramaswamy, H.S. Heating behavior and quality factor retention in a canned model food as influenced by thermal processing in a rotary retort. J. Food Qual. 1993, 16, 273–285. [Google Scholar] [CrossRef]
- Meinlschmidt, P.; Ueberham, E.; Lehmann, J.; Schweiggert-Weisz, U.; Eisner, P. Immunoreactivity, sensory and physicochemical properties of fermented soy protein isolate. Food Chem. 2016, 205, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Dudley, R.; Edwards, P.; Ekins, R.; Finney, D.; McKenzie, I.; Raab, G.; Rodbard, D.; Rodgers, R. Guidelines for immunoassay data processing. Clin. Chem. 1985, 31, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, W. FTIR analysis of protein structure. Course Man. Chem. 2009, 455. [Google Scholar]
- Alharaty, G.; Ramaswamy, H.S. The Effect of Sodium Alginate-Calcium Chloride Coating on the Quality Parameters and Shelf Life of Strawberry Cut Fruits. J. Compos. Sci. 2020, 4, 123. [Google Scholar] [CrossRef]
- Ramaswamy, H.S.; Gundurao, A. Effect of Soluble Solids and High Pressure Treatment on Rheological Properties of Protein Enriched Mango Puree. FOODS 2019, 8, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbott, M.; Hayward, S.; Ross, W.; Godefroy, S.B.; Ulberth, F.; Van Hengel, A.J.; Roberts, J.; Akiyama, H.; Popping, B.; Yeung, J.M. Validation procedures for quantitative food allergen ELISA methods: Community guidance and best practices. J. AOAC Int. 2010, 93, 442–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke-Taylor, S. Development of VITAL Allergen Actions Levels Grid Explanatory notes. 2007. Available online: https://www.bezpecnostpotravin.cz/UserFiles/File/Kvasnickova/VITAL_brozura.pdf (accessed on 5 December 2022).
- FDA U.S. Food and Drug Administration. In Draft Threshold report Approaches to Establish Thresholds for Major Food Allergens and for Gluten in Food.; 2006. Available online: https://www.fda.gov/files/food/published/Approaches-to-Establish-Thresholds-for-Major-Food-Allergens.pdf (accessed on 5 December 2022).
- Mills, E.; Madsen, C.; Shewry, P.; Wichers, H. Food allergens of plant origin—Their molecular and evolutionary relationships. Trends Food Sci. Technol. 2003, 14, 145–156. [Google Scholar] [CrossRef]
- Kleber, N.; Maier, S.; Hinrichs, J. Antigenic response of bovine β-lactoglobulin influenced by ultra-high pressure treatment and temperature. Innov. Food Sci. Emerg. Technol. 2007, 8, 39–45. [Google Scholar] [CrossRef]
- Krimm, S.; Bandekar, J. Vibrational spectroscopy and conformation of peptides, polypeptides, and proteins. Adv. Protein Chem. 1986, 38, 181–364. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Cameron, D.G.; Moffatt, D.J. A Generalized Approach to Derivative Spectroscopy. Appl. Spectrosc. 1987, 41, 539–544. [Google Scholar] [CrossRef]
- Jiménez-Saiz, R.; Benedé, S.; Molina, E.; López-Expósito, I. Effect of processing technologies on the allergenicity of food products. Crit. Rev. Food Sci. Nutr. 2015, 55, 1902–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, E.; Papadopoulou, A.; Ledward, D. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- L’Hocine, L.; Boye, J.I.; Jouve, S. Ionic strength and pH-induced changes in the immunoreactivity of purified soybean glycinin and its relation to protein molecular structure. J. Agric. Food Chem. 2007, 55, 5819–5826. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Zheng, Z.; Zheng, H. Effect of heat treatment on the antigenicity of bovine α-lactalbumin and β-lactoglobulin in whey protein isolate. Food Agric. Immunol. 2009, 20, 195–206. [Google Scholar] [CrossRef]
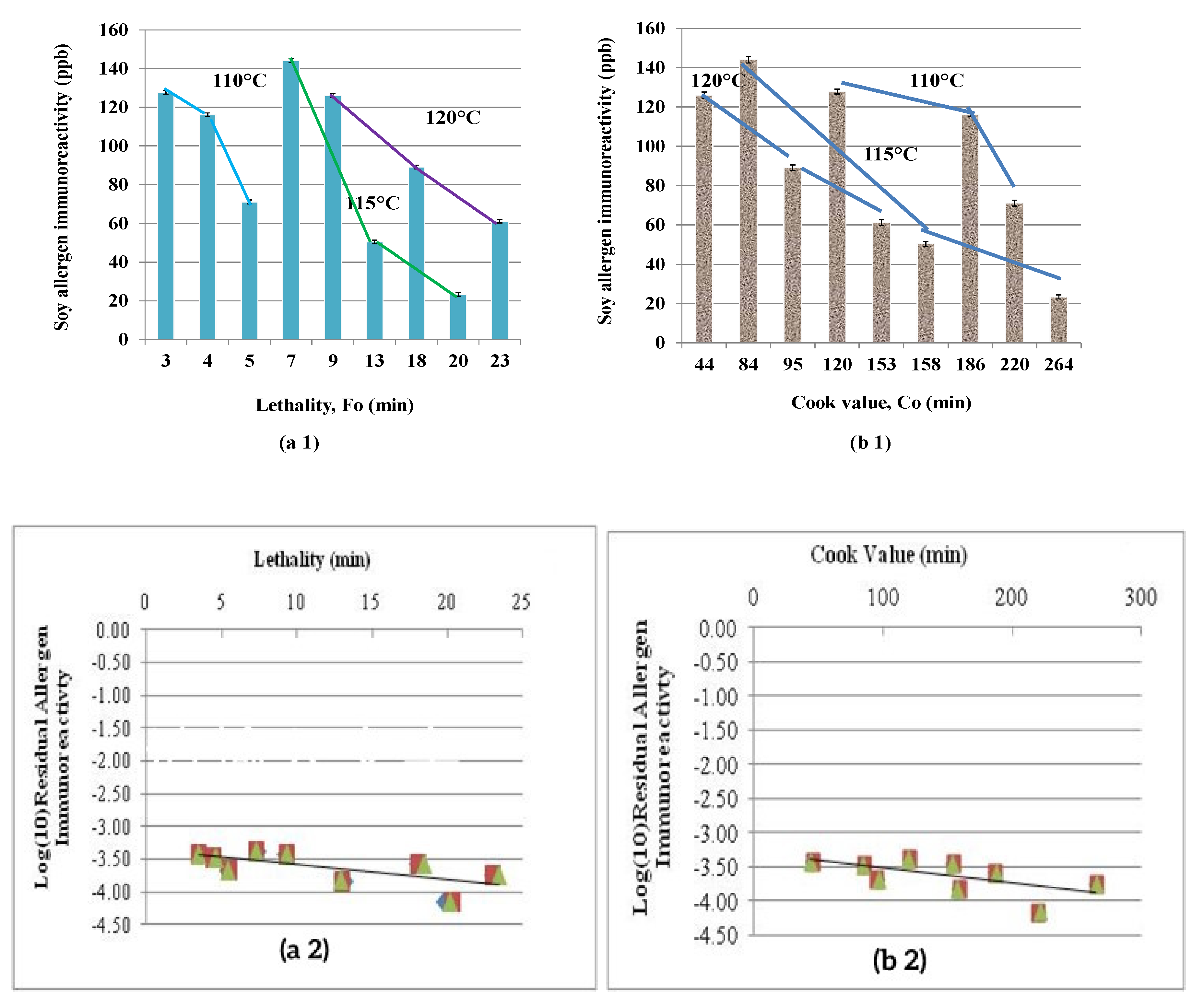

| Thermal Treatment | Nominal Temperature (°C) | Time (min) | Targeted Lethality, Fo min | Delivered Lethality, Fo min | Cook Value min | Co/Fo Ratio |
|---|---|---|---|---|---|---|
| Minimal lethality | 110 | 60 | 3–5 | 3.4 ± 0.16 | 120 ± 1.58 | 35.1 |
| 110 | 90 | 3–5 | 4.4 ± 0.12 | 186 ± 1.61 | 42.3 | |
| Moderate lethality | 110 | 120 | 5–10 | 5.4 ± 0.22 | 220 ± 2.88 | 40.7 |
| 115 | 30 | 5–10 | 7.3 ± 0.57 | 44 ± 0.09 | 6.0 | |
| 120 | 15 | 5–10 | 9.2 ± 0.06 | 84 ± 2.06 | 9.1 | |
| Severe lethality | 115 | 60 | 10–20 | 13 ± 0.77 | 158 ± 156 | 12.1 |
| 115 | 90 | 10–20 | 20 ± 0.17 | 264 ± 0.64 | 13.2 | |
| 120 | 30 | 10–20 | 18 ± 0.21 | 95 ± 0.87 | 5.2 | |
| 120 | 45 | 10–20 | 23 ± 0.56 | 153 ± 0.06 | 6.7 | |
| Cooking (boiling water bath) | 100 | 10 | <0.5 | 0.08 ± 0.01 | ~10 | 125 |
| 100 | 20 | <0.5 | 0.15 ± 0.01 | ~20 | 133 | |
| 100 | 30 | <0.5 | 0.23± 0.03 | ~30 | 130 | |
| 100 | 40 | <0.5 | 0.31± 0.05 | ~40 | 111 | |
| 100 | 50 | <0.5 | 0.36± 0.05 | ~50 | 138 | |
| 100 | 60 | <0.5 | 0.48± 0.08 | ~60 | 120 |
| Type of Processing | Nominal Temperature (°C) | Time (min) | Allergen Concentration Based on IR (ppb) | Percentage Reduction in soy IR (%) (Log Reduction) |
|---|---|---|---|---|
| Unprocessed soy slurry | 333 × 103 ± 1.12 a | 100 | ||
| Minimal lethality | 110 | 60 | 123 ± 0.18 c | 99.96 (3.42 ± 0.01) |
| 110 | 90 | 116 ± 0.16 d | 99.97 (3.46 ± 0.02) | |
| Moderate lethality | 110 | 120 | 71.8 ± 0.99 ef | 99.98 (3.67 ± 0.01) |
| 115 | 30 | 144 ± 0.11 b | 99.96 (3.36 ± 0.02) | |
| 120 | 15 | 124 ± 0.76 cd | 99.96 (3.42 ± 0.01) | |
| Severe lethality | 115 | 60 | 50.7 ± 0.52 gh | 99.98 (3.82 ± 0.02) |
| 115 | 90 | 23.6 ± 0.34 i | 99.99 (4.15 ± 0.01) | |
| 120 | 30 | 89 ± 0.14 e | 99.97 (3.57 ± 0.02) | |
| 120 | 45 | 61.1 ± 0.06 g | 99.98 (3.74 ± 0.02) | |
| Normal cooking | 100 | 10 | 123 × 102 ±1.63 A | 96.30 (1.43 ± 0.01) |
| 20 | 120 × 102 ±2.01 AB | 96.39 (1.44 ± 0.02) | ||
| 30 | 112 × 102 ±1.65 C | 96.63 (1.47 ± 0.01) | ||
| 40 | 109 × 102 ±1.72 D | 96.73 (1.48 ± 0.01) | ||
| 50 | 104 × 102 ±1.23 DE | 96.88 (1.50 ± 0.02) | ||
| 60 | 990 × 101 ±1.01 F | 97.02 (1.53 ± 0.01) |
| Nominal Temperature (°C) | Nominal Time (min) | Come-Up Time (min) | Cook Time (min) | Cook Temperature (°C) |
|---|---|---|---|---|
| 110 | 60 | 16.8 ± 2.19 | 59.5 ± 0.28 | 109.6 ± 0.07 |
| 90 | 15.9 ± 0.78 | 89.7 ± 0.14 | 109.6 ± 0.14 | |
| 120 | 16.4 ± 1.48 | 119.7 ± 0.21 | 109.7 ± 0.07 | |
| 115 | 30 | 182 ± 3.32 | 29.7 ± 0.21 | 114.8 ± 0.07 |
| 60 | 16.9 ± 0.57 | 59.2 ± 0.42 | 114.6 ± 0.14 | |
| 90 | 18.8 ± 1.98 | 89.9 ± 0.07 | 114.8 ± 0.21 | |
| 120 | 15 | 18.8 ± 2.05 | 14.8 ± 0.14 | 119.7 ± 0.21 |
| 30 | 16.4 ± 0.78 | 29.7 ± 0.14 | 119.6 ± 0.14 | |
| 45 | 15.2 ± 0.71 | 44.7 ± 0.28 | 119.7 ± 0.21 |
|
Temperature
(°C) |
Time
(min) | L * | a * | b * | ∆E | Apparent Viscosity (cP) |
|---|---|---|---|---|---|---|
| Fresh | - | 37.63± 0.25 | 0.32 ±0.01 | 1.19 ±0.05 | - | 4.48 ± 0.01 |
| 110 | 60 | 11.65 ±0.21 | −0.53 ±0.02 | 5.18 ±0.11 | 25.1 ± 0.03 bc | 3.27 ± 0.03 DE |
| 90 | 12.26 ±0.21 | −0.61 ±0.01 | 6.12 ±0.03 | 25.9 ± 0.43 b | 3.83 ± 0.01 D | |
| 120 | 13.15± 0.22 | −0.65 ±0.01 | 6.59 ±0.05 | 26.3 ± 0.04 a | 3.89 ± 0.01 D | |
| 115 | 30 | 20.53± 0.28 | −0.25 ±0.06 | 3.00 ±0.01 | 21.2 ± 0.23 b | 4.08 ± 0.01 CD |
| 60 | 16.65± 0.21 | −0.43 ±0.02 | 3.19 ±0.03 | 21.1 ± 0.04 b | 7.18 ± 0.01 B | |
| 90 | 16.71 ±0.01 | −0.34 ±0.03 | 4.31 ±0.03 | 17.2 ± 0.03 d | 8.47 ± 0.03 A | |
| 120 | 15 | 33.11± 0.11 | 0.21 ±0.02 | 1.41 ±0.06 | 9.52 ± 0.49 e | 4.12 ± 0.01 CD |
| 30 | 30.88± 0.08 | 0.14 ±0.02 | 1.95 ±0.08 | 6.80 ± 0.17 ef | 4.33 ± 0.01 C | |
| 45 | 28.27± 0.26 | −0.32 ±0.02 | 2.82 ±0.05 | 4.52 ± 0.36 f | 4.06 ± 0.01 CD |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravindran, A.; Ramaswamy, H.S. ELISA Based Immunoreactivity Reduction of Soy Allergens through Thermal Processing. Processes 2023, 11, 93. https://doi.org/10.3390/pr11010093
Ravindran A, Ramaswamy HS. ELISA Based Immunoreactivity Reduction of Soy Allergens through Thermal Processing. Processes. 2023; 11(1):93. https://doi.org/10.3390/pr11010093
Chicago/Turabian StyleRavindran, Akshaya, and Hosahalli S. Ramaswamy. 2023. "ELISA Based Immunoreactivity Reduction of Soy Allergens through Thermal Processing" Processes 11, no. 1: 93. https://doi.org/10.3390/pr11010093





