A Glance at a Sustainable Solution Using Vertical Constructed Wetland Based on Dewatered Drinking-Water Waste Augmented Nanoparticle Composite Substrate for Wastewater Treatment
Abstract
:1. Introduction
2. Experimental Investigation
2.1. DuPont 1179 Aqueous Solution
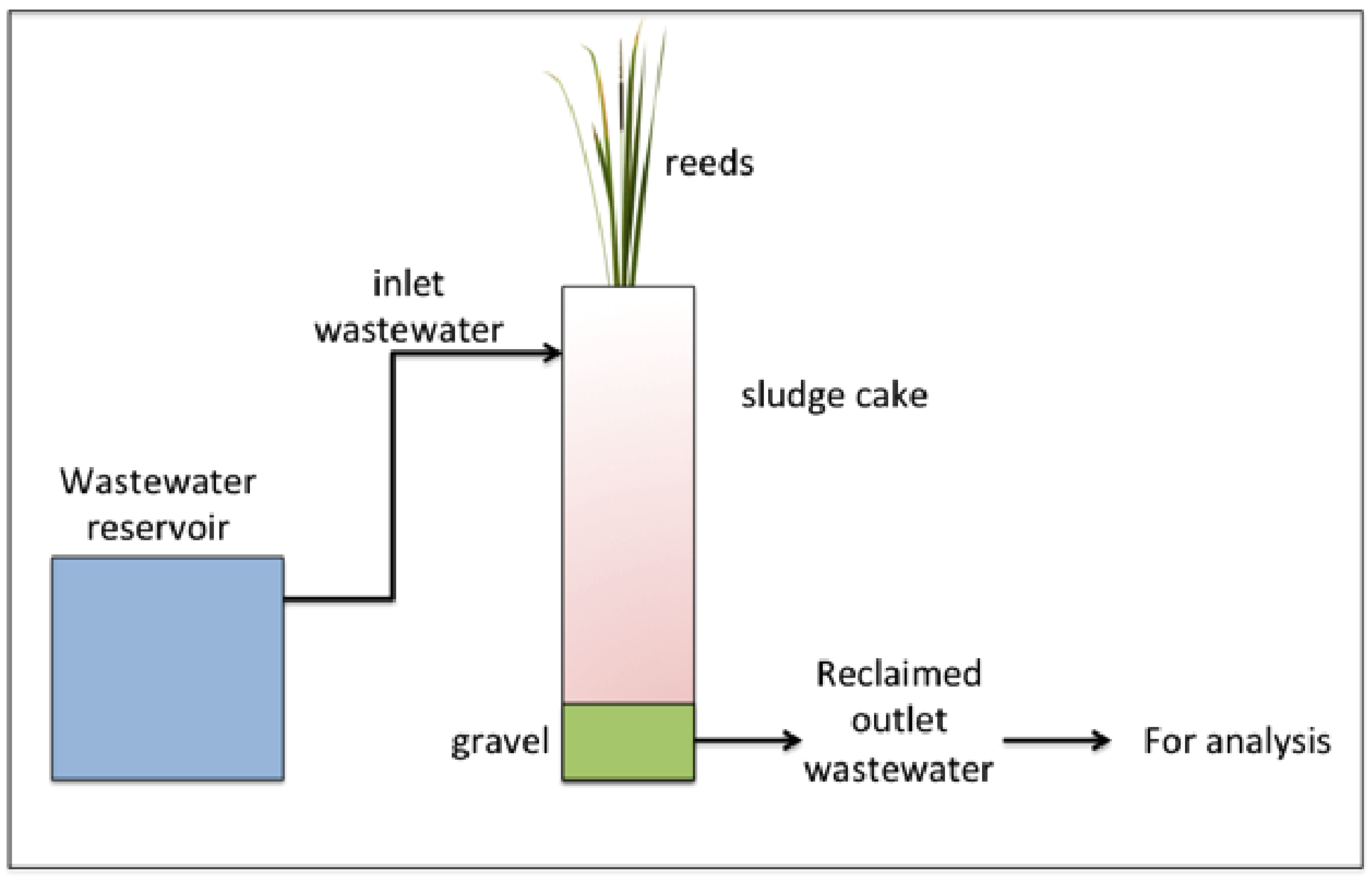
2.2. VFCW System
2.3. AlS–M Composite Characterization
2.4. Analytical Determination
3. Results and Discussion
3.1. Characterization of the AlS–M Composite
3.1.1. XRD Analysis
3.1.2. SEM Images and EDX
3.1.3. FTIR
3.1.4. BET Surface Area
3.1.5. TEM Images
3.2. DuPont 1179 Adsorption on AlS–M Composite Substrate
3.2.1. Effect of Composite Height
3.2.2. Effect of Isotherm Time
3.2.3. Effect of DuPont 1179 Loading in Influent Stream
3.2.4. Effect of Aqueous Stream pH
3.2.5. Effect of Temperature
3.2.6. Kinetic Investigation
3.2.7. Isotherm Models
3.2.8. Comparative Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, Y.Q.; Babatunde, A.O.; Hu, Y.S.; Kumar, J.L.G.; Zhao, X.H. Pilot field-scale demonstration of a novel alum sludge-based constructed wetland system for enhanced wastewater treatment. Process Biochem. 2011, 46, 278–283. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Huynh, K.A.; Padungthon, S.; Pranudta, A.; Amonpattaratkit, P.; Tran, L.B.; Phan, P.T.; Nguyen, N.H. Synthesis of natural flowerlike iron-alum oxide with special interaction of Fe-Si-Al oxides as an effective catalyst for heterogeneous Fenton process. J. Environ. Chem. Eng. 2021, 9, 105732. [Google Scholar] [CrossRef]
- El-Geundi, M.S.; Nassar, M.M.; Farrag, T.E.; Ahmed, M.H. Methomyl adsorption onto Cotton Stalks Activated Carbon (CSAC): Equilibrium and process design. Procedia Environ. Sci. 2013, 17, 630–639. [Google Scholar] [CrossRef]
- Drea, A.A.; Naman, S.N.; Jaffer, B.R. Theoretical degradation study of methomyl. J. Appl. Chem. 2012, 1, 126–137. [Google Scholar]
- Nguyen, D.D.D.; Quang, H.H.P.; Nguyen, X.H.; Nguyen, T.P. The treatment of real dyeing wastewater by the electro-Fenton process using drinking water treatment sludge as a catalyst. RSC Adv. 2021, 11, 27443–27452. [Google Scholar] [CrossRef]
- Tomašević, A.; Mijin, D.; Gašic, S.; Kiss, E. The influence of polychromatic light on methomyl degradation in TiO2 and ZnO aqueous suspension. Desalination Water Treat. 2014, 52, 4342–4349. [Google Scholar] [CrossRef]
- Farhadian, N.; Behin, J. Degradation of 2,4-dichlorophenoxyacetate isopropyl amine (2,4-D IPA) by O/AC/UV in an internally slurry airlift photo-reactor. Environ. Technol. 2017, 38, 3180–3191. [Google Scholar] [CrossRef] [PubMed]
- Rozhkovskaya, A.; Rajapakse, J.; Millar, G.J. Synthesis of high-quality zeolite LTA from alum sludge generated in drinking water treatment plants. J. Environ. Chem. Eng. 2021, 9, 104751. [Google Scholar] [CrossRef]
- Breesem, K.M.; Faris, F.G.; Abidin, R.Z.; Yusof, N.; Abidin, M.R.Z.; Dom, N.M.; Jassam, S.H.; Abdel-Magid, I.M. Influence of calcination temperatures on microstructures of alum sludge and its pozzolanic properties. Aust. J. Basic Appl. Sci. 2015, 9, 181–188. [Google Scholar]
- Kim, J.-G.; Kim, H.-B.; Yoon, G.-S.; Kim, S.-H.; Min, S.-J.; Tsang, D.C.W.; Baek, K. Simultaneous oxidation and adsorption of arsenic by one-step fabrication of alum sludge and graphitic carbon nitride (g-C3N4). J. Hazard. Mater. 2020, 383, 121138. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, J.; Zhou, J.; Lei, J. Study on adsorption of methylene blue by a novel composite material of TiO2 and alum sludge. RSC Adv. 2018, 8, 32799–32807. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhao, Y.Q.; Babatunde, A.O.; Kearney, P. Two strategies for phosphorus removal from reject water of municipal wastewater treatment plant using alum sludge. Water Sci. Technol. 2009, 60, 3181–3188. [Google Scholar] [CrossRef] [PubMed]
- Kluczka, J.; Zołotajkin, M.; Ciba, J.; Staroń, M. Assessment of aluminum bioavailability in alum sludge for agricultural utilization. Environ. Monit. Assess. 2017, 189, 422. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.Q.; Bache, D.H. Conditioning of alum sludge with polymer and gypsum. Colloids Surf. A Physicochem. Eng. Asp. 2001, 194, 213–220. [Google Scholar] [CrossRef]
- Tony, M.A. Valorization of undervalued aluminum-based waterworks sludge waste for the science of “The 5 Rs’ criteria”. Appl. Water Sci. 2022, 12, 20. [Google Scholar] [CrossRef]
- Kazak, O.; Eker, Y.R.; Akin, I.; Bingol, H.; Tor, A. A novel red mud@ sucrose based carbon composite: Preparation, characterization and its adsorption performance toward methylene blue in aqueous solution. J. Environ. Chem. Eng. 2017, 5, 2639–2647. [Google Scholar] [CrossRef]
- Sharif Zein, S.H.; Boccaccini, A.R. Synthesis and characterization of TiO2 coated multiwalled carbon nanotubes using a sol gel method. Ind. Eng. Chem. Res. 2008, 47, 6598–6606. [Google Scholar] [CrossRef]
- Wu, H.F.; Wang, J.P.; Duan, E.G.; Feng, Y.F.; Wan, Z.Y.; Wu, Y.X.; Lu, Y.Q. Study on the preparation of granular alum sludge adsorbent for phosphorus removal. Water Sci. Technol. 2019, 79, 2378–2386v. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q.; Yang, Y.; Kearney, P. From “fills” to filter: Insights into the reuse of dewatered alum sludge as a filter media in a constructed wetland. J. Residuals Sci. Technol. 2007, 4, 147–152. [Google Scholar]
- Hu, Y.; Zhao, Y.; Zhao, X.; Kumar, J.L.G. High rate nitrogen removal in an alum sludge-based intermittent aeration constructed wetland. Environ. Sci. Technol. 2012, 46, 4583–4590. [Google Scholar] [CrossRef]
- Babatunde, A.O.; Zhao, Y.Q. Constructive approaches toward water treatment works sludge management: An international review of beneficial reuses. Crit. Rev. Environ. Sci. Technol. 2007, 37, 129–164. [Google Scholar] [CrossRef]
- Vinita, M.; Dorathi, R.P.J.; Palanivelu, K. Degradation of 2,4,6-trichlorophenol by photo Fenton’s like method using nano heterogeneous catalytic ferric ion. Sol. Energy 2010, 84, 1613–1618. [Google Scholar] [CrossRef]
- Negi, C.; Kandwal, P.; Rawat, J.; Sharma, M.; Sharma, H.; Dalapati, G.; Dwivedi, C. Carbon-doped titanium dioxide nanoparticles for visible light driven photocatalytic activity. Appl. Surf. Sci. 2021, 554, 149553. [Google Scholar] [CrossRef]
- Adams, W.; Blust, R.; Dwyer, R.; Mount, D.; Nordheim, E.; Rodriguez, P.H.; Spry, D. Bioavailability assessment of metals in freshwater environments: A historical review. Environ. Toxicol. Chem. 2020, 39, 48–59. [Google Scholar] [CrossRef] [PubMed]
- Kluczka, J.; Zołotajkin, M.; Ciba, J. Speciation of aluminium in the water and bottom sediment of fish-breeding ponds. Arch. Environ. Prot. 2012, 38, 83–96. [Google Scholar] [CrossRef]
- Nagar, R.; Sarkar, D.; Makris, K.C.; Datta, R. Effect of solution chemistry on arsenic sorption by Fe- and Al-based drinking-water treatment residuals. Chemosphere 2010, 78, 1028–1035. [Google Scholar] [CrossRef]
- Tony, M.A.; Eltabey, M.M. End-of-life waste criteria: Synthesis and utilization of Mn–Zn ferrite nanoparticles as a superparamagnetic photocatalyst for synergistic wastewater remediation. Appl. Water Sci. 2022, 12, 21. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mex. De Física 2007, 53, 18–22. [Google Scholar]
- Duman, O.; Özcan, C.; Polat, T.G.; Tunç, S. Carbon nanotube-based magnetic and non-magnetic adsorbents for the high-efficiency removal of diquat dibromide herbicide from water: OMWCNT, OMWCNT-Fe3O4 and OMWCNT-κ-carrageenan-Fe3O4 nanocomposites. Environ. Pollut. 2019, 244, 723–732. [Google Scholar] [CrossRef]
- Amani, M.; Shakeri, A. Synthesis and Characterization of Water-Based Epoxy-Acrylate/Graphene Oxide Decorated with Fe3O4 Nanoparticles Coatings and Its Enhanced Anticorrosion Properties. Polym.-Plast. Technol. Mater. 2020, 59, 1910–1931. [Google Scholar] [CrossRef]
- Bhalkikar, A.; Gernhart, Z.C.; Cheung, C.L. Recyclable magnetite nanoparticle catalyst for one-pot conversion of cellobiose to 5-hydroxymethylfurfural in water. J. Nanomater. 2015, 2015, 264037. [Google Scholar] [CrossRef]
- Hassan, E.A.; Tony, M.A.; Nabwey, H.; Awad, M.M. Ecologically engineered systems for treating agriculture runoff by integrating “wastes” into constructed wetland. Processes 2023, 11, 396. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; El Sherbiny, S.A.; Tony, M.A. Construction of a hetero-junction recyclable composite photocatalyst from aluminum-based waste/magnetite for efficient carbamate insecticide oxidation. Environ. Sci. Water Res. Technol. 2022, 8, 1874. [Google Scholar] [CrossRef]
- Thabet, R.H.; Fouad, M.K.; El Sherbiny, S.A.; Tony, M.A. Identifying optimized conditions for developing dewatered alum sludge based photocatalyst to immobilize a wide range of dye contamination. Appl. Water Sci. 2022, 12, 210. [Google Scholar] [CrossRef]
- Rio, S.; Le Coq, L.; Faur, C.; Lecomte, D.; Le Cloirec, P. Preparation of adsorbents from sewage sludge by steam activation for industrial emission treatment. Process Saf. Environ. Prot. 2006, 84, 258–264. [Google Scholar] [CrossRef]
- Lee, C.-I.; Yang, W.-F.; Chiou, C.-S. Utilization of water clarifier sludge for copper removal in a liquid fluidized-bed reactor. J. Hazard. Mater. 2006, 129, 58–63. [Google Scholar] [CrossRef]
- Guo, S.; Dan, Z.; Duan, N.; Chen, G.; Gao, W.; Zhao, W. Zn(II), Pb(II), and Cd(II) adsorption from aqueous solution by magnetic silica gel: Preparation, characterization, and adsorption. Environ. Sci. Pollut. Res. 2018, 25, 30938–30948. [Google Scholar] [CrossRef]
- Sekhula, M.M.; Okonkwo, J.O.; Zvinowanda, C.M.; Agyei, N.N.; Chaudhary, A.J. Fixed bed column adsorption of Cu(II) onto maize tassel-PVA beads. J. Chem. Eng. Process Technol. 2012, 3, 1–5. [Google Scholar] [CrossRef]
- Carvallho, M.N.; da Silva, K.; Sales, D.C.S.; Freire, E.M.P.L.; Sobrinho, M.A.M.; Ghislandi, M.G. Dye removal from textile industrial effluents by adsorption on exfoliated graphite nanoplatelets: Kinetic and equilibrium studies. Water Sci. Technol. 2016, 73, 2189–2198. [Google Scholar] [CrossRef]
- Saleem, M.; Fang, L.; Ruan, H.B.; Wu, F.; Huang, Q.L.; Xu, C.L.; Kong, C.Y. Effect of zinc acetate concentration on the structural and optical properties of ZnO thin films deposited by Sol-Gel method. Int. J. Phys. Sci 2012, 7, 2971. [Google Scholar] [CrossRef]
- Elsayed, S.A.; El-Sayed, I.E.T.; Tony, M.A. Impregnated chitin biopolymer with magnetic nanoparticles to immobilize dye from aqueous media as a simple, rapid and efficient composite photocatalyst. Appl. Water Sci. 2022, 12, 252. [Google Scholar] [CrossRef]
- Tong, D.S.; Liu, M.; Li, L.; Lin, C.X.; Yu, W.H.; Xu, Z.P.; Zhou, C.H. Transformation of alunite residuals into layered double hydroxides and oxides for adsorption of acid red G dye. Appl. Clay Sci. 2012, 70, 1–7. [Google Scholar] [CrossRef]
- Wang, S. A comparative study of Fenton and Fenton-like reaction kinetics in decolourisation of wastewater. Dye Pigment. 2008, 76, 714–720. [Google Scholar] [CrossRef]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, L.A.; Fatta-Kassinos, D. Solar photo-Fenton oxidation against the bioresistant fractions of winery wastewater. J. Environ. Chem. Eng. 2013, 1, 703–712. [Google Scholar] [CrossRef]
- Gibbons, M.K.; Mortula, M.M.; Gagnon, G.A. Phosphorus adsorption on water treatment residual solids. J. Water Supply: Res. Technol. AQUA 2009, 58, 1–10. [Google Scholar] [CrossRef]
- Tony, M.A. Zeolite-based adsorbent from alum sludge residue for textile wastewater treatment. Int. J. Environ. Sci. Technol. 2020, 17, 2485–2498. [Google Scholar] [CrossRef]
- Hovsepyan, A.; Bonzongo, J.-C.J. Aluminum drinking water treatment residuals (Al-WTRs) as sorbent for mercury: Implications for soil remediation. J. Hazard. Mater. 2009, 164, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F.; Haynes, R.J. Removal of Pb(II), Cr(III) and Cr(VI) from aqueous solutions using alum-derived water treatment sludge. Water Air Soil Pollut. 2011, 215, 631–643. [Google Scholar] [CrossRef]
- Silvetti, M.; Castaldi, P.; Garau, G.; Demurtas, D.; Deiana, S. Sorption of cadmium (II) and zinc (II) from aqueous solution by water treatment residuals at different pH values. Water Air Soil Pollut. 2015, 226, 313. [Google Scholar] [CrossRef]
- Jiao, J.; Zhao, J.; Pei, Y. Adsorption of Co(II) from aqueous solutions by water treatment residuals. J. Environ. Sci. 2017, 52, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Chavoshi, S.; Bagheri, M.; Yetilmezsoy, K.; Samadi, M.T. Alum-based sludge (AbS) recycling for turbidity removal in drinking water treatment: An insight into statistical, technical, and health-related standpoints. J. Mater. Cycles Waste Manag. 2018, 20, 1999–2017. [Google Scholar] [CrossRef]
- Tony, M. Win-win wastewater treatment to sustain world: Porous adsorbents from waste waterworks sludge for phenol remediation. In Proceedings of the Anaerobic Digestion Conference AD16, the International Water Association, IWA, Delft, The Netherlands, 23–27 June 2019. [Google Scholar]
- Cheng, W.-P.; Chen, P.-H.; Yu, R.-F.; Ho, W.-N. Treating ammonium-rich wastewater with sludge from water treatment plant to produce ammonium alum. Sustain. Environ. Res. 2016, 26, 63–69. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, Y. Extending the use of dewatered alum sludge as a P-trapping material in effluent purification: Study on two separate water treatment sludges. J. Environ. Sci. Health Part A 2010, 45, 1234–1239. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, H.; Fu, B.; Liu, H. feasibility of sludge deep-dewatering with sawdust conditioning for incineration disposal without energy put. Chem. Eng. J. 2017, 313, 655–662. [Google Scholar] [CrossRef]
- Xiao, Z.; Yuan, X.; Jiang, L.; Chen, X.; Li, H.; Zeng, G.; Leng, L.; Wang, H.; Huang, H. Energy recovery and secondary pollutant emission form the combustion of co-pelletized fuel from municipal sewage sludge and wood sawdust. Energy 2015, 91, 441–450. [Google Scholar] [CrossRef]












| Kinetic Model | Kinetics Parameters | 100% Composite VFCW | 75% Composite VFCW | 50% Composite VFCW | 40% Composite VFCW |
|---|---|---|---|---|---|
| Lagergren’s first-order | qe, mg/g | 0.27 | 0.09 | 0.29 | 0.28 |
| k1, min−1 | 4.67 | 4.29 | 4.77 | 4.82 | |
| R2 | 0.67 | 0.97 | 0.63 | 0.62 | |
| Pseudo second-order | qe, mg/g | 25,000 | 33,333 | 2000 | 2000 |
| k2 × 10−5, g·mg/min | 53.3 | 22.5 | 41.6 | 25.0 | |
| R2 | 0.99 | 0.99 | 0.97 | 0.95 |
| Isotherm Model | Isotherm Parameters | 100% Composite VFCW | 75% Composite VFCW | 50% Composite VFCW | 40% Composite VFCW |
|---|---|---|---|---|---|
| Langmuir | aL (L/mg) | 0.0099 | 0.0041 | 0.0088 | 0.01668 |
| KL | 0.7674 | 0.8907 | 0.4587 | 1.2004 | |
| Qo (mg/g) | 76.92 | 217.39 | 52.08 | 71.94 | |
| R2 | 0.96 | 0.96 | 0.98 | 0.99 | |
| Freundlich | KF | 14.8797 | 1.9151 | 11.8224 | 7.5007 |
| n | 3.22580 | 2.1276 | 2.9841 | 2.7778 | |
| R2 | 0.89 | 0.92 | 0.84 | 0.95 |
| Alum Sludge Dose | Pollutant in Wastewater | Operating pH | Treatment Time | Removals | Ref. |
|---|---|---|---|---|---|
| Adsorption column | DuPont 1179 Insecticide | 6.9 | 5 h | Complete removal | Current study |
| 4 g/L | Phosphorus | 6.2 | 12 d | 0.89 mg/g | [46] |
| 8 mg/kg | Mercury | 6.5 | 7 d | 79 mg/g | [48] |
| 250 mg/L | Arsenic | 8.1 | 20 h | 0.001–0.003 mg/g | [46] |
| Adsorption column | Lead | 5–8 | NA | 0.21–0.22 mmol/g | [49] |
| 0.1 g | Zinc and copper | 4.5 | 24 h | 0.040 mmol/g | [50] |
| Adsorption column | Cobalt | 6.0 | 48 h | 17.31 mg/g | [51] |
| 19.71 g/L | Humic acid | 5.56 | NA | 0.47 mg/g | [52] |
| 0.5 g/L | Phosphorus | 7.0 | 24 h; | 0.90 mg/g | [18] |
| Adsorption column | Phenol | NA | 1 h | 275 mg/g | [53] |
| 300 g/L | Ammonium | NA | 60 min | 11.3 mg/g | [54] |
| 2 g/L | Textile dye | 7.0 | 1 h | 6.5 mg/g | [47] |
| Adsorption column | Phosphorus | 4.3 | NA | 22.4 mg/g | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nabwey, H.A.; Tony, M.A. A Glance at a Sustainable Solution Using Vertical Constructed Wetland Based on Dewatered Drinking-Water Waste Augmented Nanoparticle Composite Substrate for Wastewater Treatment. Processes 2023, 11, 2836. https://doi.org/10.3390/pr11102836
Nabwey HA, Tony MA. A Glance at a Sustainable Solution Using Vertical Constructed Wetland Based on Dewatered Drinking-Water Waste Augmented Nanoparticle Composite Substrate for Wastewater Treatment. Processes. 2023; 11(10):2836. https://doi.org/10.3390/pr11102836
Chicago/Turabian StyleNabwey, Hossam A., and Maha A. Tony. 2023. "A Glance at a Sustainable Solution Using Vertical Constructed Wetland Based on Dewatered Drinking-Water Waste Augmented Nanoparticle Composite Substrate for Wastewater Treatment" Processes 11, no. 10: 2836. https://doi.org/10.3390/pr11102836






