Preparation and Characterization of Corn Straw-Based Graphitized Carbon with Ferric Acetylacetonate as Catalyst
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Characterization
2.3. Experimental Methods
2.3.1. CS Liquefied Process
2.3.2. Preparation of Graphitized Carbon
3. Results and Discussion
3.1. Single Factor
3.1.1. Catalyst Concentration
3.1.2. Solid–liquid Ratio of Liquefied Products
3.1.3. Graphitization Temperature
3.2. Characterization of CSBGC
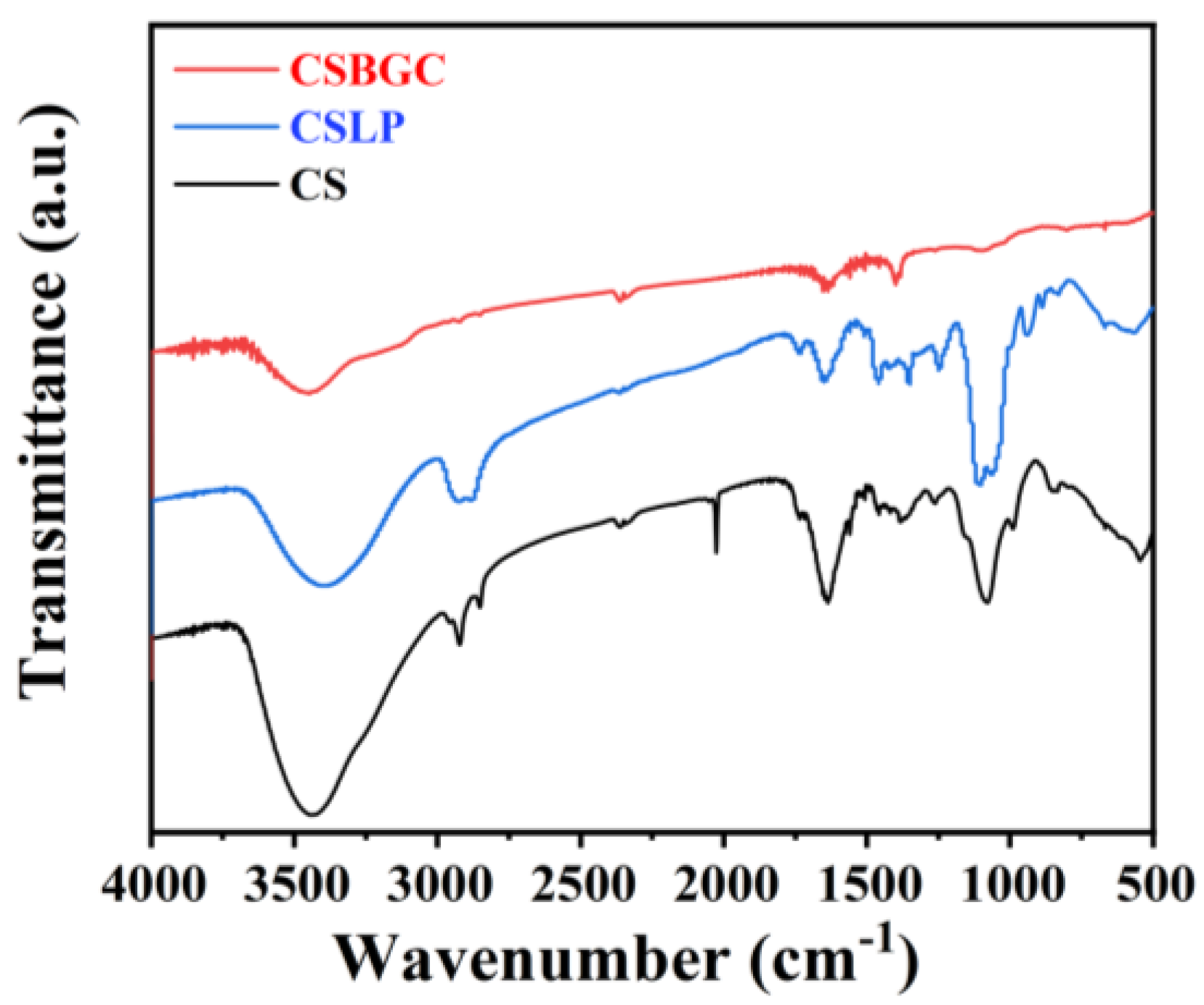
3.2.1. Surface Functional Group Analysis (FT-IR)
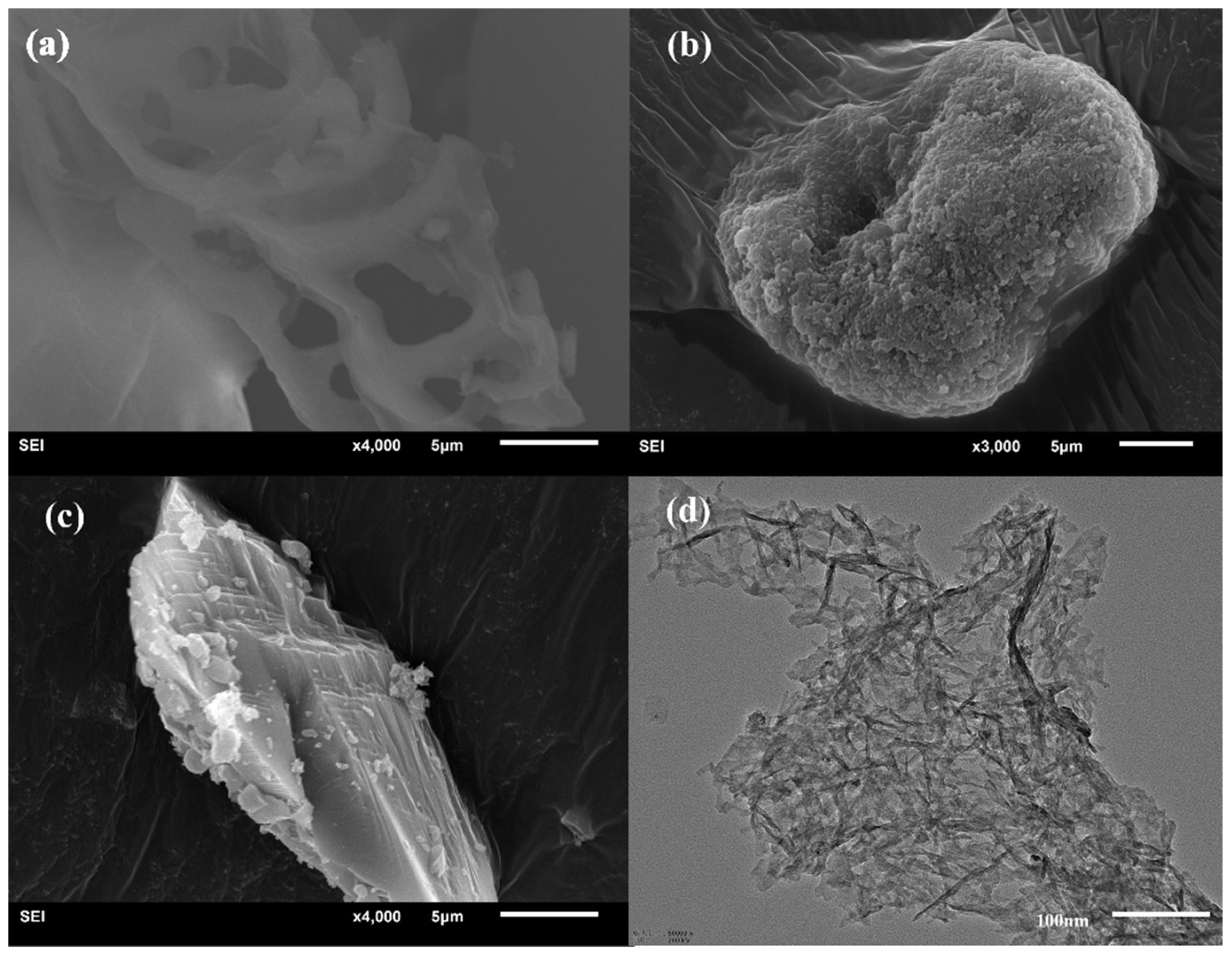
3.2.2. Microstructure Morphology Analysis (SEM and TEM)
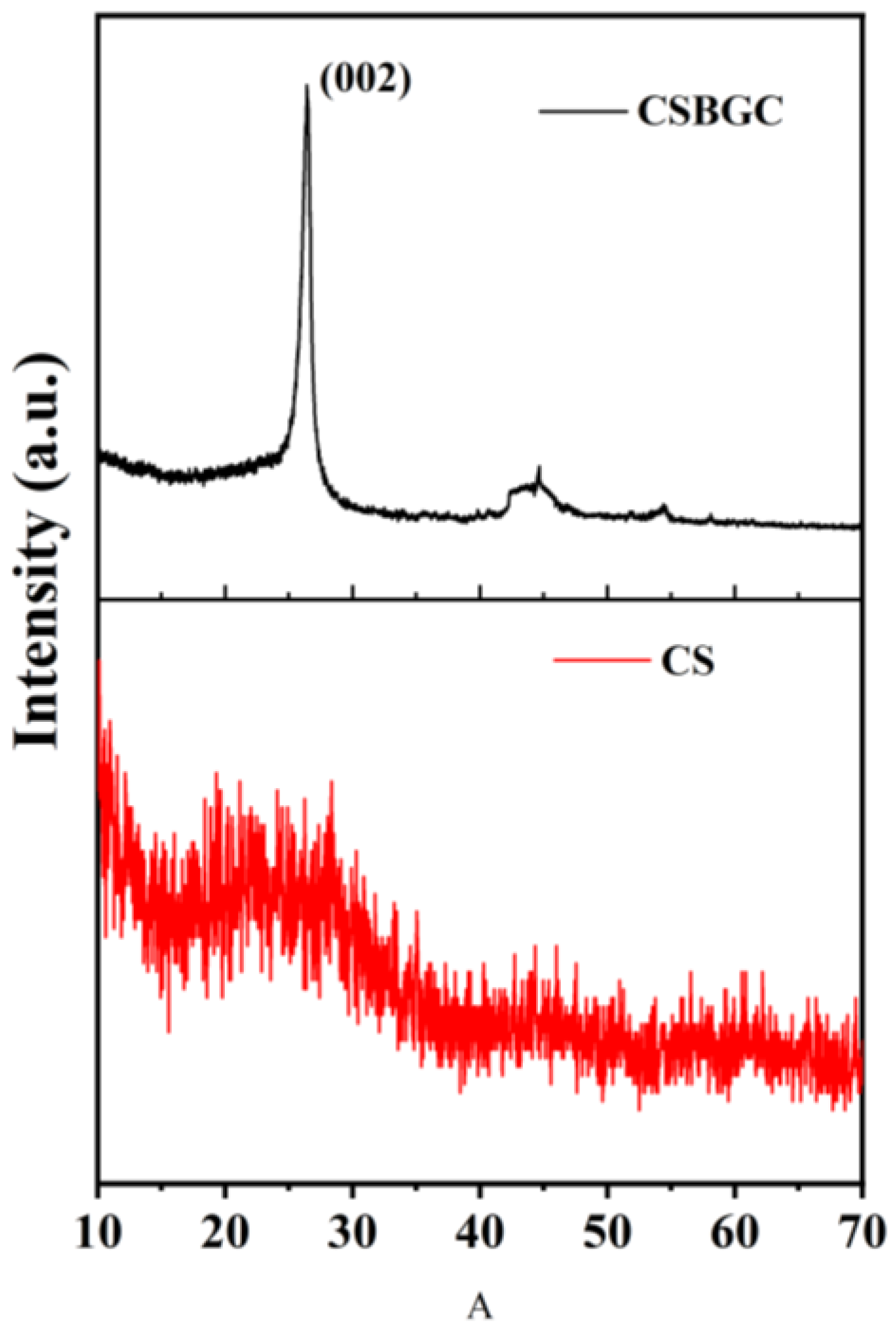
3.2.3. Crystal Structure Analysis (XRD)
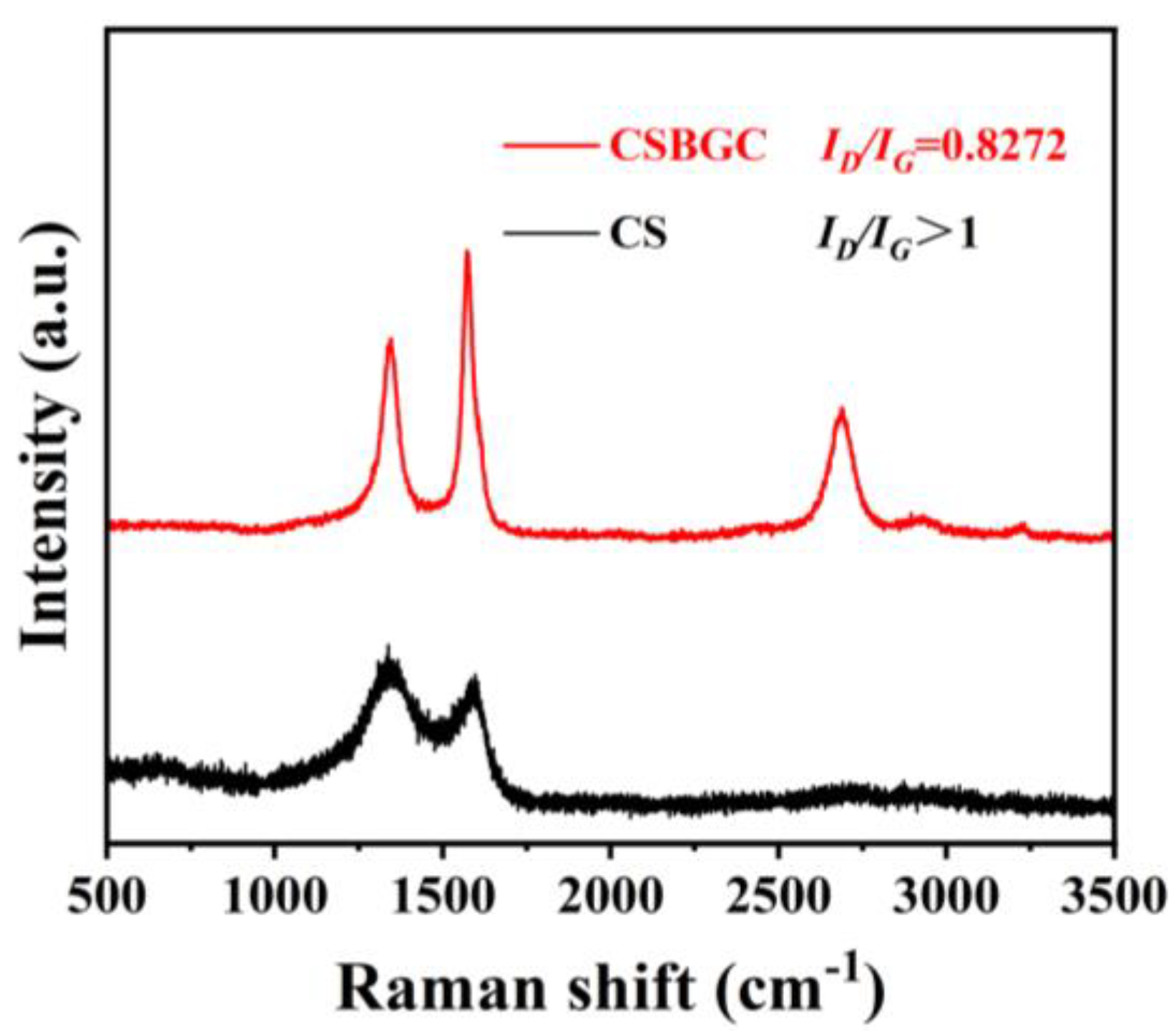
3.2.4. Graphitization Degree Analysis (Raman)
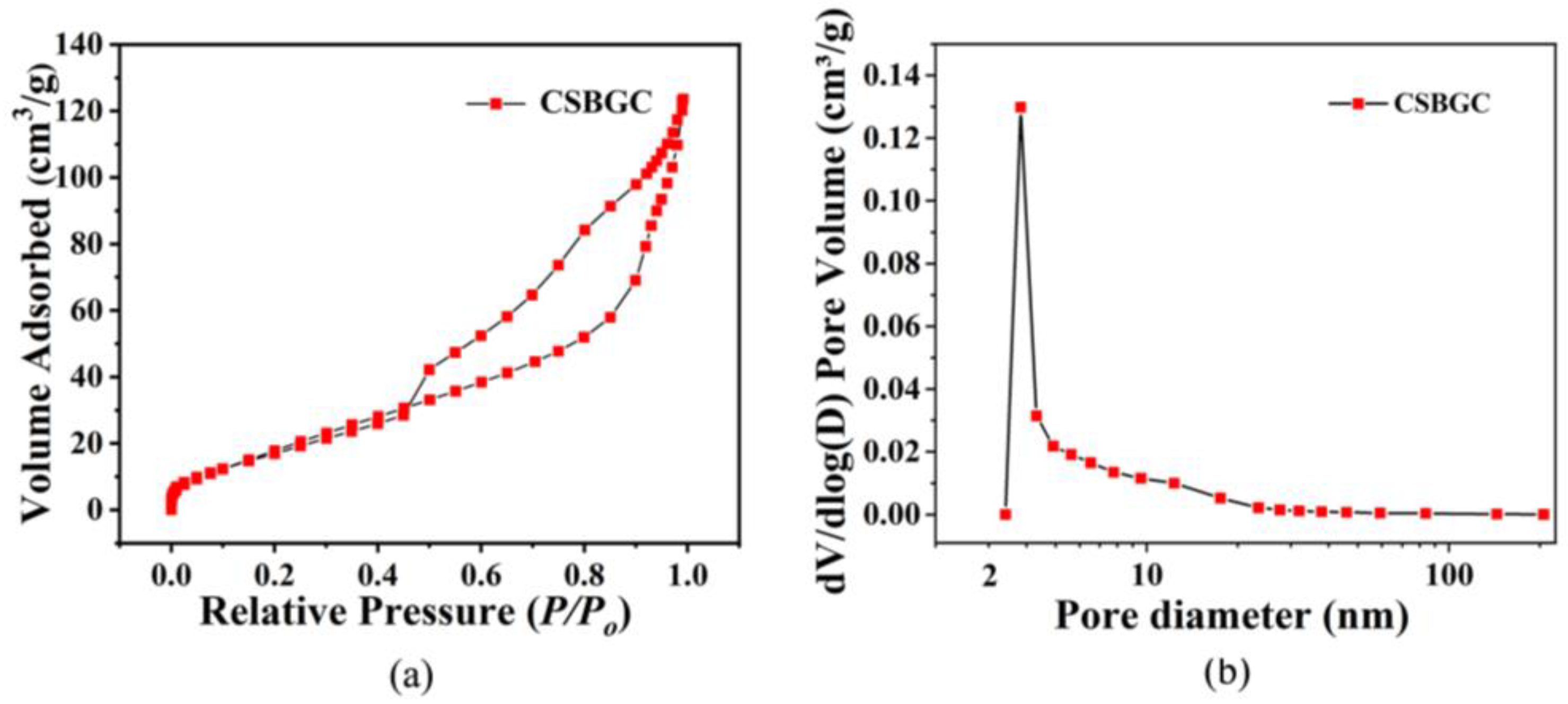
3.2.5. Specific Surface Area and Pore Size Analysis (BET)
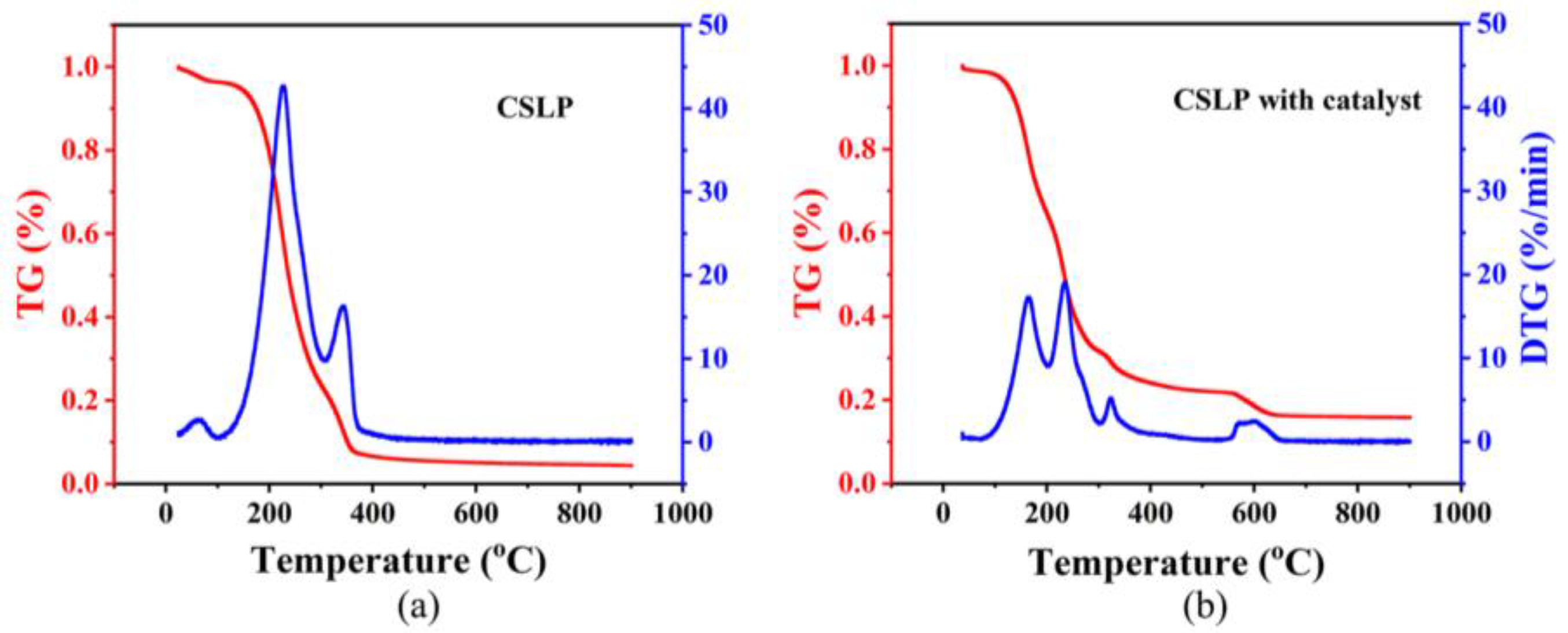
3.2.6. Thermogravimetric Analysis (TG)
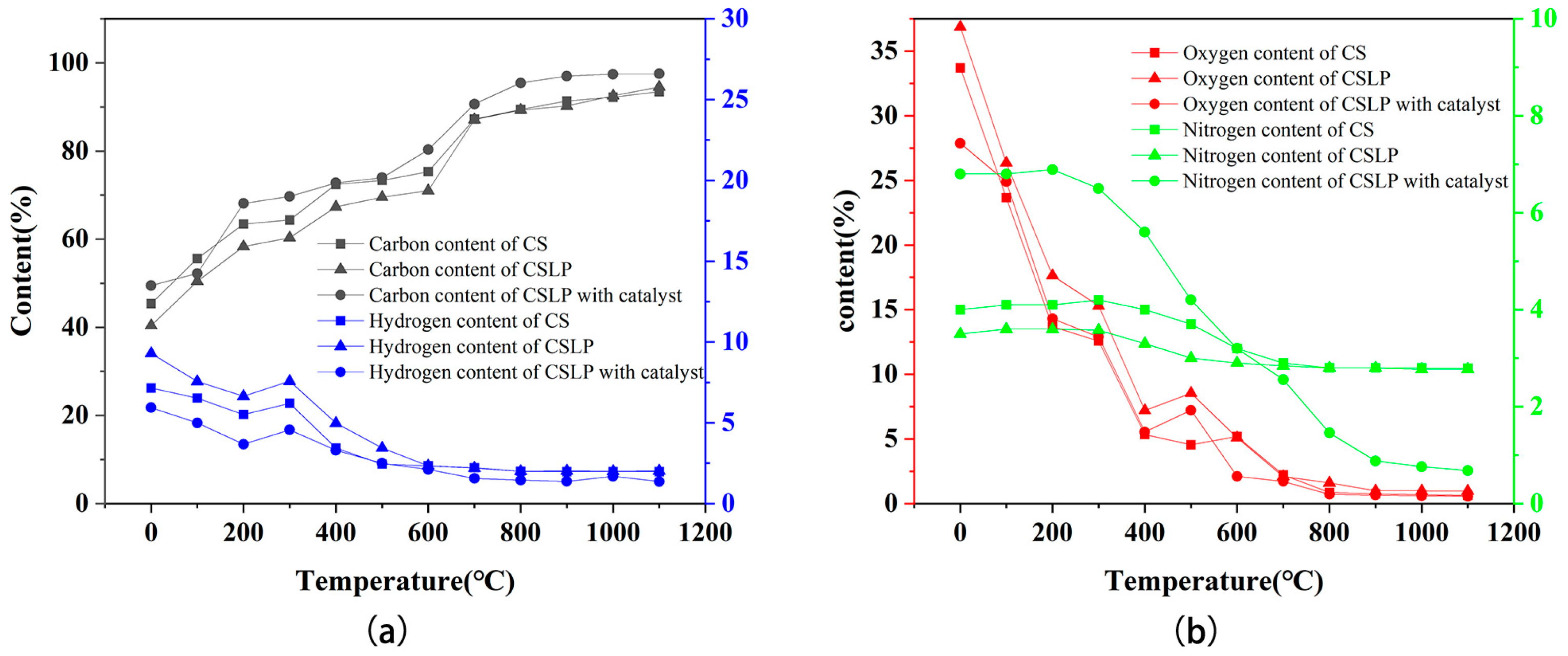
3.2.7. Elemental Analysis
3.2.8. Inductively Coupled Plasma Optical Emission Spectrometer
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yan, Q.; Li, J.; Zhang, X.; Hassan, E.B.; Wang, C.; Zhang, J.; Cai, Z. Catalytic Graphitization of Kraft Lignin to Graphene-Based Structures with Four Different Transitional Metals. J. Nanopart. Res. 2018, 20, 223. [Google Scholar] [CrossRef]
- Senneca, O.; Cerciello, F.; Russo, C.; Wütscher, A.; Muhler, M.; Apicella, B. Thermal Treatment of Lignin, Cellulose and Hemicellulose in Nitrogen and Carbon Dioxide. Fuel 2020, 271, 117656. [Google Scholar] [CrossRef]
- Morosawa, F.; Hayashi, S.; Terakawa, M. Femtosecond Laser-Induced Graphitization of Transparent Cellulose Nanofiber Films. ACS Sustain. Chem. Eng. 2021, 9, 2955–2961. [Google Scholar] [CrossRef]
- Li, Z.; Chen, Z.; Liu, C.; Hu, Z.; Zhao, W.; Zhao, G. Study on Pyrolysis Characteristics of Corn Straw. Int. J. Chem. React. Eng. 2011, 9. [Google Scholar] [CrossRef]
- Li, L.; You, Q.; Wu, S.; Huang, S.; Wu, Y.; Gao, J. Co-Liquefaction Behavior of Corn Straw and Shengli Lignite. J. Energy Inst. 2016, 89, 335–345. [Google Scholar] [CrossRef]
- Hudakorn, T.; Sritrakul, N. Biogas and Biomass Pellet Production from Water Hyacinth. Energy Rep. 2020, 6, 532–538. [Google Scholar] [CrossRef]
- Yelverton, T.L.B.; Brashear, A.T.; Nash, D.G.; Brown, J.E.; Singer, C.F.; Kariher, P.H.; Ryan, J.V.; Burnette, P. Characterization of Emissions from a Pilot-Scale Combustor Operating on Coal Blended with Woody Biomass. Fuel 2020, 264, 116774. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xie, X.; Wang, J.; Jiang, J. Directional Liquefaction Coupling Fractionation of Lignocellulosic Biomass for Platform Chemicals. Green Chem. 2016, 18, 3124–3138. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, Q.; Lin, X.; Jiang, K.; Han, D. The Degradation and Repolymerization Analysis on Solvolysis Liquefaction of Corn Stalk. Polymers 2020, 12, 2337. [Google Scholar] [CrossRef] [PubMed]
- Nizamuddin, S.; Baloch, H.A.; Mubarak, N.M.; Riaz, S.; Siddiqui, M.T.H.; Takkalkar, P.; Tunio, M.M.; Mazari, S.; Bhutto, A.W. Solvothermal Liquefaction of Corn Stalk: Physico-Chemical Properties of Bio-oil and Biochar. Waste Biomass Valoriz. 2019, 10, 1957–1968. [Google Scholar] [CrossRef]
- Mun, S.P.; Cai, Z.; Zhang, J. Fe-Catalyzed Thermal Conversion of Sodium Lignosulfonate to Graphene. Mater. Lett. 2013, 100, 180–183. [Google Scholar] [CrossRef]
- Sun, L.; Tian, C.; Li, M.; Meng, X.; Wang, L.; Wang, R.; Yin, J.; Fu, H. From Coconut Shell to Porous Graphene-Like Nanosheets for High-Power Supercapacitors. J. Mater. Chem. A 2013, 1, 6462–6470. [Google Scholar] [CrossRef]
- Shen, J.; Li, T.; Long, Y.; Shi, M.; Li, N.; Ye, M. One-Step Solid State Preparation of Reduced Graphene Oxide. Carbon 2012, 50, 2134–2140. [Google Scholar] [CrossRef]
- Niu, M.; Zhao, G.J.; Alma, M.H. Thermogravimetric Studies on Condensed Wood Residues in Polyhydric Alcohols Liquefaction. BioResources 2011, 6, 615–630. [Google Scholar] [CrossRef]
- Yamada, T.; Ono, H. Rapid Liquefaction of Lignocellulosic Waste by Using Ethylene Carbonate. Bioresour. Technol. 1999, 70, 61–67. [Google Scholar] [CrossRef]
- Cheng, G.; Varanasi, P.; Li, C.; Liu, H.; Melnichenko, Y.B.; Simmons, B.A.; Kent, M.S.; Singh, S. Transition of Cellulose Crystalline Structure and Surface Morphology of Biomass as a Function of Ionic Liquid Pretreatment and Its Relation to Enzymatic Hydrolysis. Biomacromolecules 2011, 12, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Tai, Z.; Zhang, Q.; Liu, Y.; Liu, H.; Dou, S. Activated Carbon from the Graphite with Increased Rate Capability for the Potassium Ion Battery. Carbon 2017, 123, 54–61. [Google Scholar] [CrossRef]
- Pan, C.; Liu, Y.; Cao, F.; Wang, J.; Ren, Y. Synthesis and Growth Mechanism of Carbon Nanotubes and Nanofibers from Ethanol Flames. Micron 2004, 35, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Mendieta-Taboada, O.; Sobral, P.J.d.A.; Carvalho, R.A.; Habitante, A.M.B.Q. Thermomechanical Properties of Biodegradable Films Based on Blends of Gelatin and Poly(Vinyl Alcohol). Food Hydrocoll. 2008, 22, 1485–1492. [Google Scholar] [CrossRef]
- Yagmur, E.; Inal, I.I.G.; Gokce, Y.; Ulusoy Ghobadi, T.G.; Aktar, T.; Aktas, Z. Examination of Gas and Solid Products during the Preparation of Activated Carbon Using Phosphoric Acid. J. Environ. Manag. 2018, 228, 328–335. [Google Scholar] [CrossRef] [PubMed]


| Sample | Test 1 | Test 2 | Test 3 | Average |
|---|---|---|---|---|
| (wt.%) | (wt.%) | (wt.%) | (wt.%) | |
| CSBGC | 0.022 | 0.011 | 0.013 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, L.; He, P.; Liu, P. Preparation and Characterization of Corn Straw-Based Graphitized Carbon with Ferric Acetylacetonate as Catalyst. Processes 2023, 11, 2884. https://doi.org/10.3390/pr11102884
Mu L, He P, Liu P. Preparation and Characterization of Corn Straw-Based Graphitized Carbon with Ferric Acetylacetonate as Catalyst. Processes. 2023; 11(10):2884. https://doi.org/10.3390/pr11102884
Chicago/Turabian StyleMu, Li, Peng He, and Pengfei Liu. 2023. "Preparation and Characterization of Corn Straw-Based Graphitized Carbon with Ferric Acetylacetonate as Catalyst" Processes 11, no. 10: 2884. https://doi.org/10.3390/pr11102884





