Automated Production at Scale of Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells, Chondrocytes and Extracellular Vehicles: Towards Real-Time Release
Abstract
:1. Introduction
2. Materials and Methods
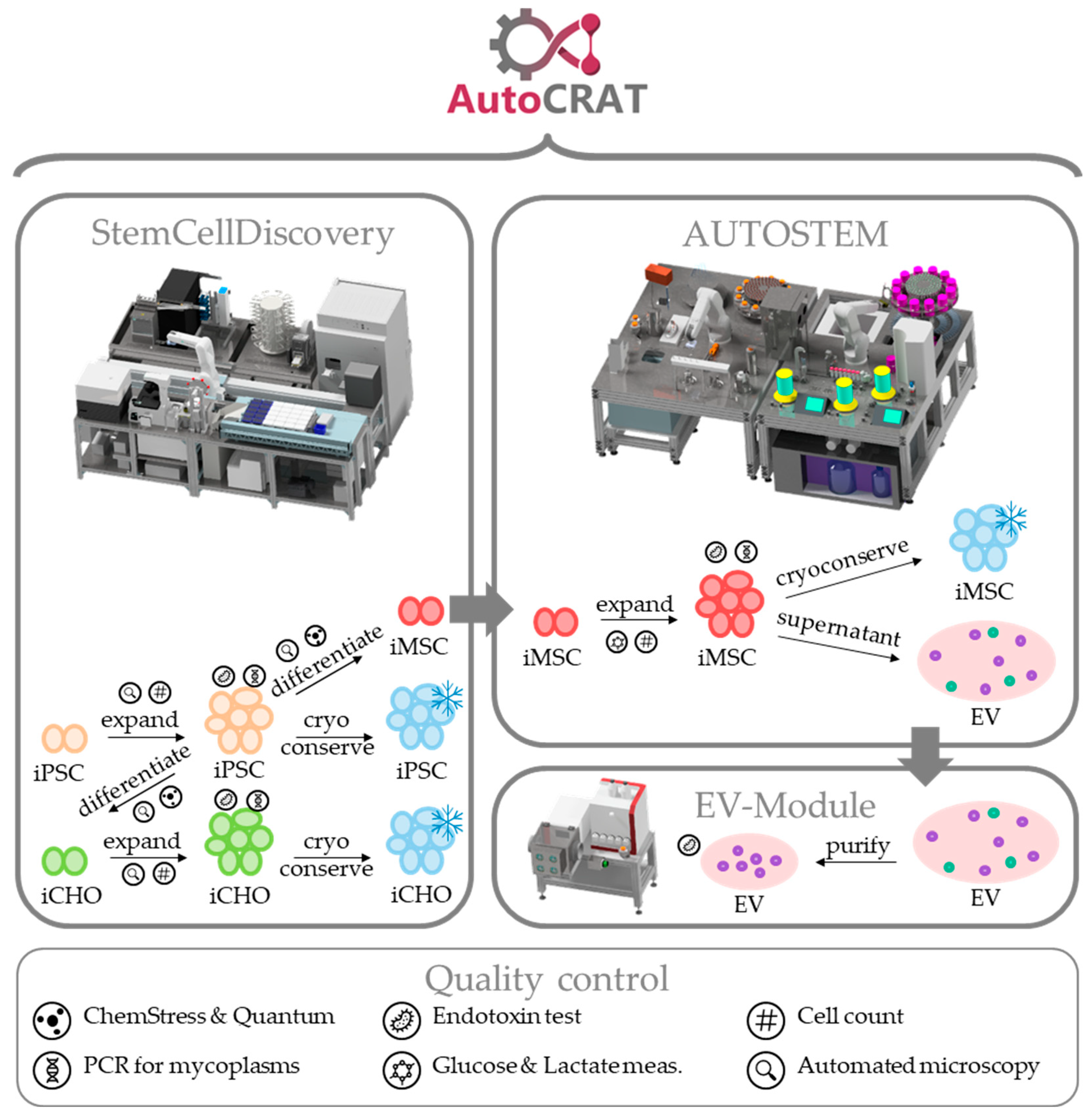
3. Results—The AutoCRAT Platforms
3.1. StemCellDiscovery
3.2. Autostem
3.3. EV Module
3.4. Software
3.5. Process Implementation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burns, L.C.; Ritvo, S.E.; Ferguson, M.K.; Clarke, H.; Seltzer, Z.; Katz, J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: A systematic review. J. Pain Res. 2015, 8, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Yuan, S.; Zeng, Y.; Wang, C.; Yu, N.; Ding, C. New Trends in Pharmacological Treatments for Osteoarthritis. Front. Pharmacol. 2021, 12, 645842. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Lee, W.-S.; Kim, H.J.; Kim, K.-I.; Kim, G.B.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Wickham, J.; Shah, K.; Huguenin, L.; Tenen, A.; Paterson, K.; Boyd, R. Adipose-derived mesenchymal stem cell therapy in the treatment of knee osteoarthritis: A randomized controlled trial. Regen. Med. 2019, 14, 213–230. [Google Scholar] [CrossRef]
- Choudhery, M.S.; Mahmood, R.; Harris, D.T.; Ahmad, F.J. Minimum criteria for defining induced mesenchymal stem cells. Cell Biol. Int. 2022, 46, 986–989. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Sabapathy, V.; Kumar, S. hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J. Cell. Mol. Med. 2016, 20, 1571–1588. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, D.; Liu, Z.; Zhou, F.; Dai, J.; Wu, B.; Zhou, J.; Heng, B.C.; Zou, X.H.; Ouyang, H.; et al. Exosomes from embryonic mesenchymal stem cells alleviate osteoarthritis through balancing synthesis and degradation of cartilage extracellular matrix. Stem Cell Res. Ther. 2017, 8, 189. [Google Scholar] [CrossRef]
- Giebel, B.; Kordelas, L.; Börger, V. Clinical potential of mesenchymal stem/stromal cell-derived extracellular vesicles. Stem Cell Investig. 2017, 4, 84. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef]
- Li, C.; Zhao, H.; Cheng, L.; Wang, B. Allogeneic vs. autologous mesenchymal stem/stromal cells in their medication practice. Cell Biosci. 2021, 11, 187. [Google Scholar] [CrossRef] [PubMed]
- Simaria, A.S.; Hassan, S.; Varadaraju, H.; Rowley, J.; Warren, K.; Vanek, P.; Farid, S.S. Allogeneic cell therapy bioprocess economics and optimization: Single-use cell expansion technologies. Biotechnol. Bioeng. 2014, 111, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Lipsitz, Y.Y.; Milligan, W.D.; Fitzpatrick, I.; Stalmeijer, E.; Farid, S.S.; Tan, K.Y.; Smith, D.; Perry, R.; Carmen, J.; Chen, A.; et al. A roadmap for cost-of-goods planning to guide economic production of cell therapy products. Cytotherapy 2017, 19, 1383–1391. [Google Scholar] [CrossRef]
- Malik, N.N.; Durdy, M.B. Chapter 7—Cell Therapy Landscape: Autologous and Allogeneic Approaches. In Translational Regenerative Medicine; Atala, A., Allickson, J.G., Eds.; Academic Press: Boston, MA, USA, 2015; pp. 87–106. ISBN 978-0-12-410396-2. [Google Scholar]
- Jossen, V.; van den Bos, C.; Eibl, R.; Eibl, D. Manufacturing human mesenchymal stem cells at clinical scale: Process and regulatory challenges. Appl. Microbiol. Biotechnol. 2018, 102, 3981–3994. [Google Scholar] [CrossRef]
- Li, H.; Ghazanfari, R.; Zacharaki, D.; Lim, H.C.; Scheding, S. Isolation and characterization of primary bone marrow mesenchymal stromal cells. Ann. N. Y. Acad. Sci. 2016, 1370, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.T.; Reuveny, S.; Oh, S.K.-W. Human mesenchymal stem cell therapy for cartilage repair: Review on isolation, expansion, and constructs. Stem Cell Res. 2020, 44, 101738. [Google Scholar] [CrossRef]
- Jung, Y.; Bauer, G.; Nolta, J.A. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: Progress toward safe clinical products. Stem Cells 2012, 30, 42–47. [Google Scholar] [CrossRef]
- Ochs, J.; Barry, F.; Schmitt, R.; Murphy, J.M. Advances in automation for the production of clinical-grade mesenchymal stromal cells: The AUTOSTEM robotic platform. Cell Gene Ther. Insights 2017, 3, 739–748. [Google Scholar] [CrossRef]
- Madrid, M.; Sumen, C.; Aivio, S.; Saklayen, N. Autologous induced pluripotent stem cell–based cell therapies: Promise, progress, and challenges. Current Protocols 2021, 1, e88. [Google Scholar] [CrossRef] [PubMed]
- Panchalingam, K.M.; Jung, S.; Rosenberg, L.; Behie, L.A. Bioprocessing strategies for the large-scale production of human mesenchymal stem cells: A review. Stem Cell Res. Ther. 2015, 6, 225. [Google Scholar] [CrossRef] [PubMed]
- Cherian, D.S.; Bhuvan, T.; Meagher, L.; Heng, T.S.P. Biological Considerations in Scaling up Therapeutic Cell Manufacturing. Front. Pharmacol. 2020, 11, 654. [Google Scholar] [CrossRef]
- Jørgensen, A.; Stengaard-Pedersen, K.; Simonsen, O.; Pfeiffer-Jensen, M.; Eriksen, C.; Bliddal, H.; Pedersen, N.W.; Bødtker, S.; Hørslev-Petersen, K.; Snerum, L.Ø.; et al. Intra-articular hyaluronan is without clinical effect in knee osteoarthritis: A multicentre, randomised, placebo-controlled, double-blind study of 337 patients followed for 1 year. Ann. Rheum. Dis. 2010, 69, 1097–1102. [Google Scholar] [CrossRef] [PubMed]
- Beswick, A.D.; Wylde, V.; Gooberman-Hill, R.; Blom, A.; Dieppe, P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? A systematic review of prospective studies in unselected patients. BMJ Open 2012, 2, e000435. [Google Scholar] [CrossRef]
- Bjordal, J.M.; Ljunggren, A.E.; Klovning, A.; Slørdal, L. Non-steroidal anti-inflammatory drugs, including cyclo-oxygenase-2 inhibitors, in osteoarthritic knee pain: Meta-analysis of randomised placebo controlled trials. BMJ 2004, 329, 1317. [Google Scholar] [CrossRef]
- Ochs, J.; Biermann, F.; Piotrowski, T.; Erkens, F.; Nießing, B.; Herbst, L.; König, N.; Schmitt, R.H. Fully Automated Cultivation of Adipose-Derived Stem Cells in the StemCellDiscovery—A Robotic Laboratory for Small-Scale, High-Throughput Cell Production Including Deep Learning-Based Confluence Estimation. Processes 2021, 9, 575. [Google Scholar] [CrossRef]
- Ochs, J.; Hanga, M.P.; Shaw, G.; Duffy, N.; Kulik, M.; Tissin, N.; Reibert, D.; Biermann, F.; Moutsatsou, P.; Ratnayake, S.; et al. Needle to needle robot-assisted manufacture of cell therapy products. Bioeng. Transl. Med. 2022, 7, e10387. [Google Scholar] [CrossRef]
- Sugita, S.; Hono, A.; Fujino, S.; Futatsugi, Y.; Yunomae, Y.; Shimizu, N.; Takahashi, M. Detection of Mycoplasma Contamination in Transplanted Retinal Cells by Rapid and Sensitive Polymerase Chain Reaction Test. Int. J. Mol. Sci. 2021, 22, 12555. [Google Scholar] [CrossRef]
- Szaraz, P.; Mander, P.; Davies, S.; Gasner, A.; Clifford, J.; Librach, C. Multiplex Functional Testing of Bioreactor-Upscaled First Trimester Human Umbilical Cord Perivascular Cells (FTM HUCPVC) and Bone Marrow-Derived Mesencymal Stem Cells (BMSC) using the chemstress® fingerprinting assay, reveals hidden differences between cell therapy candidates. Cytotherapy 2020, 22, S159–S160. [Google Scholar] [CrossRef]
- ISO 14644-1:2015; NA 041-02-21 AA—Reinraumtechnik. Reinräume und Zugehörige Reinraumbereiche—Teil 1: Klassifizierung der Luftreinheit Anhand der Partikelkonzentration. Beuth: Berlin, Germany, 2016.
- European Commission. The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use: Annex 1: Manufacture of Sterile Medicinial Products, C. In EudraLex; European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Patel, D.B.; Luthers, C.R.; Lerman, M.J.; Fisher, J.P.; Jay, S.M. Enhanced extracellular vesicle production and ethanol-mediated vascularization bioactivity via a 3D-printed scaffold-perfusion bioreactor system. Acta Biomater. 2019, 95, 236–244. [Google Scholar] [CrossRef]
- Kordelas, L.; Schwich, E.; Dittrich, R.; Horn, P.A.; Beelen, D.W.; Börger, V.; Giebel, B.; Rebmann, V. Individual Immune-Modulatory Capabilities of MSC-Derived Extracellular Vesicle (EV) Preparations and Recipient-Dependent Responsiveness. Int. J. Mol. Sci. 2019, 20, 1642. [Google Scholar] [CrossRef]
- Witwer, K.W.; van Balkom, B.W.M.; Bruno, S.; Choo, A.; Dominici, M.; Gimona, M.; Hill, A.F.; de Kleijn, D.; Koh, M.; Lai, R.C.; et al. Defining mesenchymal stromal cell (MSC)-derived small extracellular vesicles for therapeutic applications. J. Extracell. Vesicles 2019, 8, 1609206. [Google Scholar] [CrossRef]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef]
- Jung, S.; Grunert, D.; Schmitt, R. Service-oriented Communication and Control System Architecture for Dynamically Interconnected Assembly Systems. In Tagungsband des 3. Kongresses Montage Handhabung Industrieroboter, [1. Auflage]; Schüppstuhl, T., Tracht, K., Franke, J., Eds.; Springer Vieweg: Berlin, Germany, 2018; pp. 223–229. ISBN 978-3-662-56713-5. [Google Scholar]
- Schüppstuhl, T.; Tracht, K.; Franke, J. (Eds.) Tagungsband des 3. Kongresses Montage Handhabung Industrieroboter; [1. Auflage]; Springer Vieweg: Berlin, Germany, 2018; ISBN 978-3-662-56713-5. [Google Scholar]
- Biermann, F.; Mathews, J.; Nießing, B.; König, N.; Schmitt, R.H. Automating Laboratory Processes by Connecting Biotech and Robotic Devices—An Overview of the Current Challenges, Existing Solutions and Ongoing Developments. Processes 2021, 9, 966. [Google Scholar] [CrossRef]
- Egri, P.; Csáji, B.C.; Kis, K.B.; Monostori, L.; Váncza, J.; Ochs, J.; Jung, S.; König, N.; Schmitt, R.; Brecher, C.; et al. Bio-inspired control of automated stem cell production. Procedia CIRP 2020, 88, 600–605. [Google Scholar] [CrossRef]
- Doulgkeroglou, M.-N.; Di Nubila, A.; Niessing, B.; König, N.; Schmitt, R.H.; Damen, J.; Szilvassy, S.J.; Chang, W.; Csontos, L.; Louis, S.; et al. Automation, Monitoring, and Standardization of Cell Product Manufacturing. Front. Bioeng. Biotechnol. 2020, 8, 811. [Google Scholar] [CrossRef] [PubMed]
- Nießing, B.; Kiesel, R.; Herbst, L.; Schmitt, R.H. Techno-Economic Analysis of Automated iPSC Production. Processes 2021, 9, 240. [Google Scholar] [CrossRef]
- Elanzew, A.; Nießing, B.; Langendoerfer, D.; Rippel, O.; Piotrowski, T.; Schenk, F.; Kulik, M.; Peitz, M.; Breitkreuz, Y.; Jung, S.; et al. The StemCellFactory: A Modular System Integration for Automated Generation and Expansion of Human Induced Pluripotent Stem Cells. Front. Bioeng. Biotechnol. 2020, 8, 580352. [Google Scholar] [CrossRef]
- Ng, J.; Hynes, K.; White, G.; Sivanathan, K.N.; Vandyke, K.; Bartold, P.M.; Gronthos, S. Immunomodulatory Properties of Induced Pluripotent Stem Cell-Derived Mesenchymal Cells. J. Cell. Biochem. 2016, 117, 2844–2853. [Google Scholar] [CrossRef]
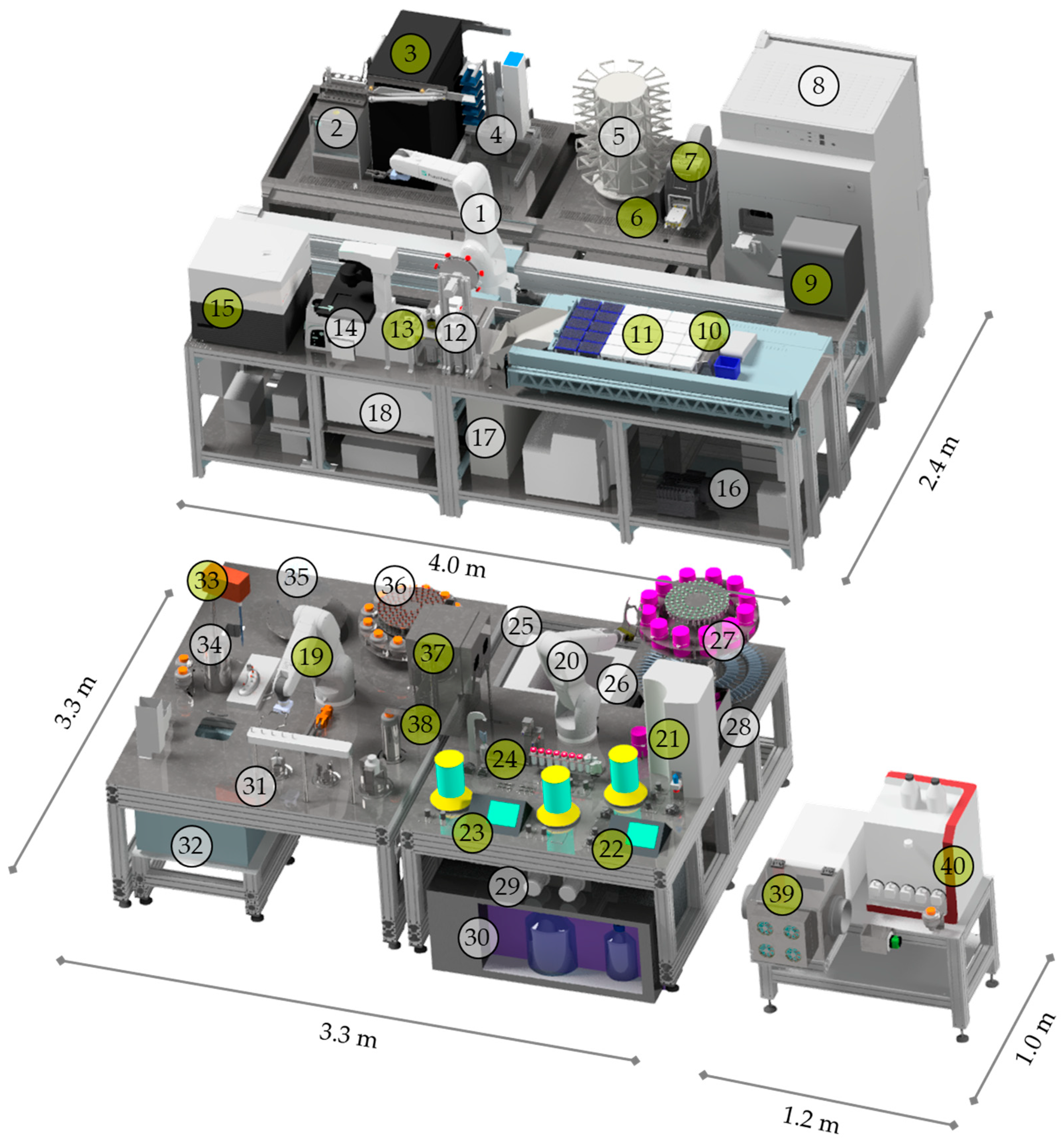
| Device Name | Manufacturer | |
|---|---|---|
| StemCellDiscovery | Six-axes robotic arm (VS-087) | Denso, Kariya, Japan |
| Linear Axis (Toothed belt axis EGC-185) | Festo, Esslingen am Neckar, Germany | |
| Incubator (Cytomat 24 C-IG) | Thermo Fisher, Waltham, MA, USA | |
| Liquid handling unit (Microlab STAR) | Hamilton Company, Reno, NV, USA | |
| Centrifuge (4–16 KRL) | Sigma Laborzentrifugen, Osterode am Harz, Germany | |
| Decapper for 50 mL tubes | Fraunhofer IPT, Aachen, Germany | |
| Microscope (Eclipse Ti2) | Nikon, Minato, Japan | |
| Storage Hotels (50 mL tubes, MTPs, pipette tips) | Fraunhofer IPT, Aachen, Germany | |
| Cooler (4 °C, 50 mL tubes) | Fraunhofer IPT, Aachen, Germany | |
| Waste | Fraunhofer IPT, Aachen, Germany | |
| Endotoxin Test (Endosafe® nexgen-PTS™) * | Charles River Laboratories, Wilmington, MA, USA | |
| PCR (qTOWER³ auto) * | Analytik Jena GmbH, Jena, Germany | |
| Plate Sealer (ALPS 3000) * | Fisher Scientific GmbH, Schwerte, Germany | |
| Plate Reader (Spark® Cyto) * | Tecan, Männedorf, Switzerland | |
| Storge hotels for 2 mL, 5 mL tubes, endotoxin cartridges * | Fraunhofer IPT, Aachen, Germany | |
| Freezer (−20 °C, 50 mL, 5 mL tubes) | Fraunhofer IPT, Aachen, Germany | |
| Autostem | Two six-axes robotic arm (VS-087) | Denso, Kariya, Japan |
| Two applikon bioreactor controler (in-Control) | Getinge, Göteborg, Sweden | |
| Two bioreactors (Mobius 3L) | Merck Millipore, Burlington, MA, USA | |
| Cell counter (NC-3000) | ChemoMetec, Gydevang, Denmark | |
| Centrifuge (4–16 KRL) | Sigma Laborzentrifugen, Osterode am Harz, Germany | |
| −80 °C freezer (Arctiko ULTF 80) | M.u.T. GmbH, Berlin, Germany | |
| 4 °C Fridge (Gamko AV/MS131) | Kaeltetechnik Rauschenbach GmbH, Bergneustadt, Germany | |
| Decentralised Pumping Station * (Pumps: 15KS, Valves: ASCO S126.01-Z130A-24VDC) | Boxer GmbH, Ottobeuren, Germany Emerson Automation Solutions, Wiener Neudorf, Austria | |
| Sampling Station | Fraunhofer IPT, Aachen, Germany | |
| Hatch | Fraunhofer IPT, Aachen, Germany | |
| Decapper (500 mL centrifuge flask, 5 mL tubes) | Fraunhofer IPT, Aachen, Germany | |
| Serological pipette | Fraunhofer IPT, Aachen, Germany | |
| Storage hotels (serological pipettes, 500 mL centrifuge flask, 5 mL tubes, CoolContainer, cassettes for NC-3000) | Fraunhofer IPT, Aachen, Germany | |
| Bioreactor Eppendorf (BioFlo 320) * | Eppendorf SE, Hamburg, Germany | |
| EV-module | FPLC (ÄKTA pure 150 M) | Cytiva, Marlborough, MA, USA |
| Isolation Housing | Fraunhofer IPT, Aachen, Germany |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbst, L.; Groten, F.; Murphy, M.; Shaw, G.; Nießing, B.; Schmitt, R.H. Automated Production at Scale of Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells, Chondrocytes and Extracellular Vehicles: Towards Real-Time Release. Processes 2023, 11, 2938. https://doi.org/10.3390/pr11102938
Herbst L, Groten F, Murphy M, Shaw G, Nießing B, Schmitt RH. Automated Production at Scale of Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells, Chondrocytes and Extracellular Vehicles: Towards Real-Time Release. Processes. 2023; 11(10):2938. https://doi.org/10.3390/pr11102938
Chicago/Turabian StyleHerbst, Laura, Ferdinand Groten, Mary Murphy, Georgina Shaw, Bastian Nießing, and Robert H. Schmitt. 2023. "Automated Production at Scale of Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells, Chondrocytes and Extracellular Vehicles: Towards Real-Time Release" Processes 11, no. 10: 2938. https://doi.org/10.3390/pr11102938
APA StyleHerbst, L., Groten, F., Murphy, M., Shaw, G., Nießing, B., & Schmitt, R. H. (2023). Automated Production at Scale of Induced Pluripotent Stem Cell-Derived Mesenchymal Stromal Cells, Chondrocytes and Extracellular Vehicles: Towards Real-Time Release. Processes, 11(10), 2938. https://doi.org/10.3390/pr11102938








