Supercritical-CO2-Assisted Electrospray for the Production of the PLA-Antibiotic-Sustained Drug-Delivery System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
3.1. Effect of PLA Concentration
3.2. Effect of Pressure
3.3. Effect of CFX Loading
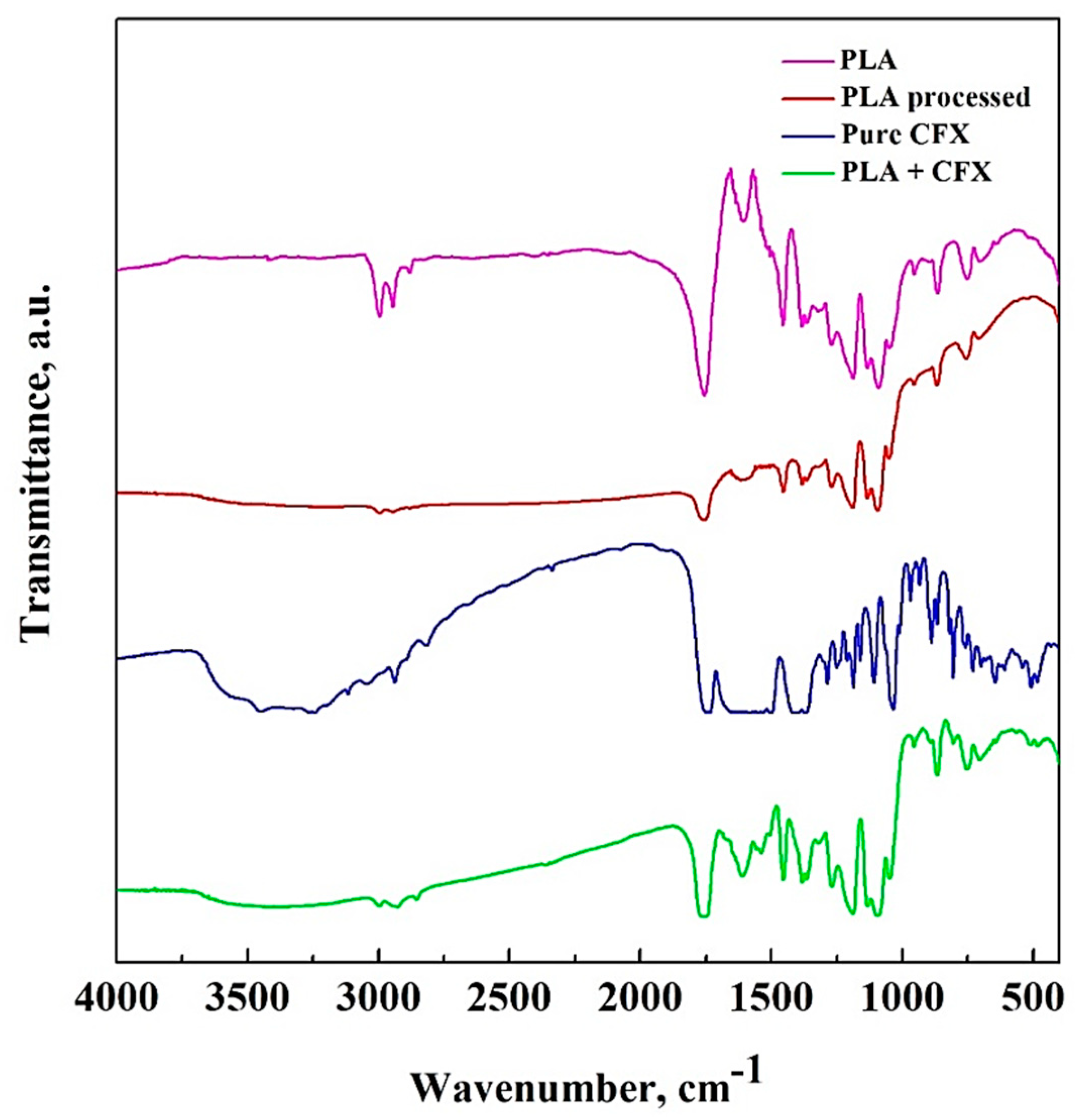
3.4. FT-IR Analysis
3.5. Solvent Residue Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, D.; Shang, P.; Yin, D.C. A Review of Magnet Systems for Targeted Drug Delivery. J. Control. Release 2019, 302, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Salahpour Anarjan, F. Active Targeting Drug Delivery Nanocarriers: Ligands. Nano-Struct. Nano-Objects 2019, 19, 100370. [Google Scholar] [CrossRef]
- Do Amorim Costa Gaspar, L.M.; Dórea, A.C.S.; Droppa-Almeida, D.; de Mélo Silva, I.S.; Montoro, F.E.; Alves, L.L.; Macedo, M.L.H.; Padilha, F.F. Development and Characterization of PLGA Nanoparticles Containing Antibiotics. J. Nanopart. Res. 2018, 20, 289. [Google Scholar] [CrossRef]
- Arana, L.; Gallego, L.; Alkorta, I. Incorporation of Antibiotics into Solid Lipid Nanoparticles: A Promising Approach to Reduce Antibiotic Resistance Emergence. Nanomaterials 2021, 11, 1251. [Google Scholar] [CrossRef] [PubMed]
- Brigger, I.; Dubernet, C.; Couvreur, P. Nanoparticles in Cancer Therapy and Diagnosis. Adv. Drug Deliv. Rev. 2012, 64, 24–36. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Saha, B.; Mukherjee, A.; Ranjan Santra, C.; Karmakar, P. Gold Nanoparticles Conjugated Antibiotics: Stability and Functional Evaluation. Nanosci. Nanotechnol. 2012, 2, 14–21. [Google Scholar] [CrossRef]
- Endres, H.-J.; Siebert-Raths, A. Engineering Biopolymers: Markets, Manufacturing, Properties, and Applications; Hanser Publishers: Cincinnati, OH, USA, 2011; ISBN 1569904618. [Google Scholar]
- Doppalapudi, S.; Jain, A.; Khan, W.; Domb, A.J. Biodegradable Polymers—An Overview. Polym. Adv. Technol. 2014, 25, 427–435. [Google Scholar] [CrossRef]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Ding, C.; Li, Z. A Review of Drug Release Mechanisms from Nanocarrier Systems. Mater. Sci. Eng. C 2017, 76, 1440–1453. [Google Scholar] [CrossRef]
- Alexis, F. Factors Affecting the Degradation and Drug-Release Mechanism of Poly(Lactic Acid) and Poly[(Lactic Acid)-Co-(Glycolic Acid)]. Polym. Int. 2005, 54, 36–46. [Google Scholar] [CrossRef]
- Stipa, P.; Marano, S.; Galeazzi, R.; Minnelli, C.; Mobbili, G.; Laudadio, E. Prediction of Drug-Carrier Interactions of PLA and PLGA Drug-Loaded Nanoparticles by Molecular Dynamics Simulations. Eur. Polym. J. 2021, 147, 110292. [Google Scholar] [CrossRef]
- Grayson, A.C.R.; Voskerician, G.; Lynn, A.; Anderson, J.M.; Cima, M.J.; Langer, R. Differential Degradation Rates In Vivo and In Vitro of Biocompatible Poly(Lactic Acid) and Poly(Glycolic Acid) Homo- and Co-Polymers for Polymeric Drug-Delivery Microchip. J. Biomater. Sci. Polym. Ed. 2004, 15, 1281–1304. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-Co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Buhecha, M.D.; Lansley, A.B.; Somavarapu, S.; Pannala, A.S. Development and Characterization of PLA Nanoparticles for Pulmonary Drug Delivery: Co-Encapsulation of Theophylline and Budesonide, a Hydrophilic and Lipophilic Drug. J. Drug Deliv. Sci. Technol. 2019, 53, 101128. [Google Scholar] [CrossRef]
- Xia, H.; Gao, X.; Gu, G.; Liu, Z.; Hu, Q.; Tu, Y.; Song, Q.; Yao, L.; Pang, Z.; Jiang, X.; et al. Penetratin-Functionalized PEG-PLA Nanoparticles for Brain Drug Delivery. Int. J. Pharm. 2012, 436, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gu, G.; Liu, Z.; Jiang, M.; Kang, T.; Miao, D.; Tu, Y.; Pang, Z.; Song, Q.; Yao, L.; et al. F3 Peptide-Functionalized PEG-PLA Nanoparticles Co-Administrated with TLyp-1 Peptide for Anti-Glioma Drug Delivery. Biomaterials 2013, 34, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Hao, S.; Wang, Y.; Wang, B.; Deng, J.; Liu, X.; Liu, J. Rapid Preparation of PH-Sensitive Polymeric Nanoparticle with High Loading Capacity Using Electrospray for Oral Drug Delivery. Mater. Sci. Eng. C 2013, 33, 4562–4567. [Google Scholar] [CrossRef]
- Zhang, S.; Kawakami, K. One-Step Preparation of Chitosan Solid Nanoparticles by Electrospray Deposition. Int. J. Pharm. 2010, 397, 211–217. [Google Scholar] [CrossRef]
- Chen, J.; Cao, L.; Cui, Y.; Tu, K.; Wang, H.; Wang, L.Q. The Exploration of Endocytic Mechanisms of PLA-PEG Nanoparticles Prepared by Coaxialtri-Capillary Electrospray-Template Removal Method. Colloids Surf. B Biointerfaces 2018, 161, 10–17. [Google Scholar] [CrossRef]
- Ghaffarzadegan, R.; Khoee, S.; Rezazadeh, S. Fabrication, Characterization and Optimization of Berberine-Loaded PLA Nanoparticles Using Coaxial Electrospray for Sustained Drug Release. DARU J. Pharm. Sci. 2020, 28, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Tasci, M.E.; Dede, B.; Tabak, E.; Gur, A.; Sulutas, R.B.; Cesur, S.; Ilhan, E.; Lin, C.-C.; Paik, P.; Ficai, D.; et al. Production, Optimization and Characterization of Polylactic Acid Microparticles Using Electrospray with Porous Structure. Appl. Sci. 2021, 11, 5090. [Google Scholar] [CrossRef]
- Mai, Z.; Chen, J.; He, T.; Hu, Y.; Dong, X.; Zhang, H.; Huang, W.; Ko, F.; Zhou, W. Electrospray Biodegradable Microcapsules Loaded with Curcumin for Drug Delivery Systems with High Bioactivity. RSC Adv. 2017, 7, 1724–1734. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical CO2 Assisted Electrospray of PVP-Rutin Mixtures Using a Liquid Collector. J. Supercrit. Fluids 2022, 188, 105684. [Google Scholar] [CrossRef]
- Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Assisted Electrospray/Spinning to Produce PVP+quercetin Microparticles and Microfibers. J. Taiwan Inst. Chem. Eng. 2020, 117, 278–286. [Google Scholar] [CrossRef]
- Barbero-Colmenar, E.; Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical CO2 Assisted Electrospray to Produce Poly(lactic-co-glycolic Acid) Nanoparticles. ChemEngineering 2022, 6, 66. [Google Scholar] [CrossRef]
- Guastaferro, M.; Cardea, S.; Baldino, L.; Reverchon, E. Supercritical-CO2 Assisted Electrospray to Produce Cellulose Acetate+Rutin Micro-Carriers. Chem. Eng. Trans. 2022, 93, 109–114. [Google Scholar] [CrossRef]
- Xu, X.; Chen, X.; Wang, Z.; Jing, X. Ultrafine PEG–PLA fibers loaded with both paclitaxel and doxorubicin hydrochloride and their in vitro cytotoxicity. Eur. J. Pharm. Biopharm. 2009, 72, 18–25. [Google Scholar] [CrossRef]
- Tewabe, A.; Marew, T.; Birhanu, G. The Contribution of Nano-Based Strategies in Overcoming Ceftriaxone Resistance: A Literature Review. Pharmacol. Res. Perspect. 2021, 9, e00849. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-Strategies to Fight Multidrug Resistant Bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1441. [Google Scholar] [CrossRef]
- Gull, N.; Khan, S.M.; Zahid Butt, M.T.; Khalid, S.; Shafiq, M.; Islam, A.; Asim, S.; Hafeez, S.; Khan, R.U. In Vitro Study of Chitosan-Based Multi-Responsive Hydrogels as Drug Release Vehicles: A Preclinical Study. RSC Adv. 2019, 9, 31078–31091. [Google Scholar] [CrossRef]
- Hong, S.-H.; Park, J.H.; Kim, O.Y.; Hwang, S.-H. Preparation of Chemically Modified Lignin-Reinforced PLA Biocomposites and Their 3D Printing Performance. Polymers 2021, 13, 667. [Google Scholar] [CrossRef]
- Mahmoud, M.E.; El-Ghanam, A.M.; Mohamed, R.H.A.; Saad, S.R. Enhanced Adsorption of Levofloxacin and Ceftriaxone Antibiotics from Water by Assembled Composite of Nanotitanium Oxide/Chitosan/Nano-Bentonite. Mater. Sci. Eng. C 2020, 108, 110199. [Google Scholar] [CrossRef] [PubMed]
- Antolín, E.M.; Quiñónez, Y.B.; Canavaciolo, V.G.; Cruz, E.R. Validation of an Analytical Method for Quality Control of Residual Solvents (n-Hexane and Acetone) in D-002: New Active Ingredient from Beeswax. J. Pharm. Biomed. Anal. 2008, 47, 646–650. [Google Scholar] [CrossRef]


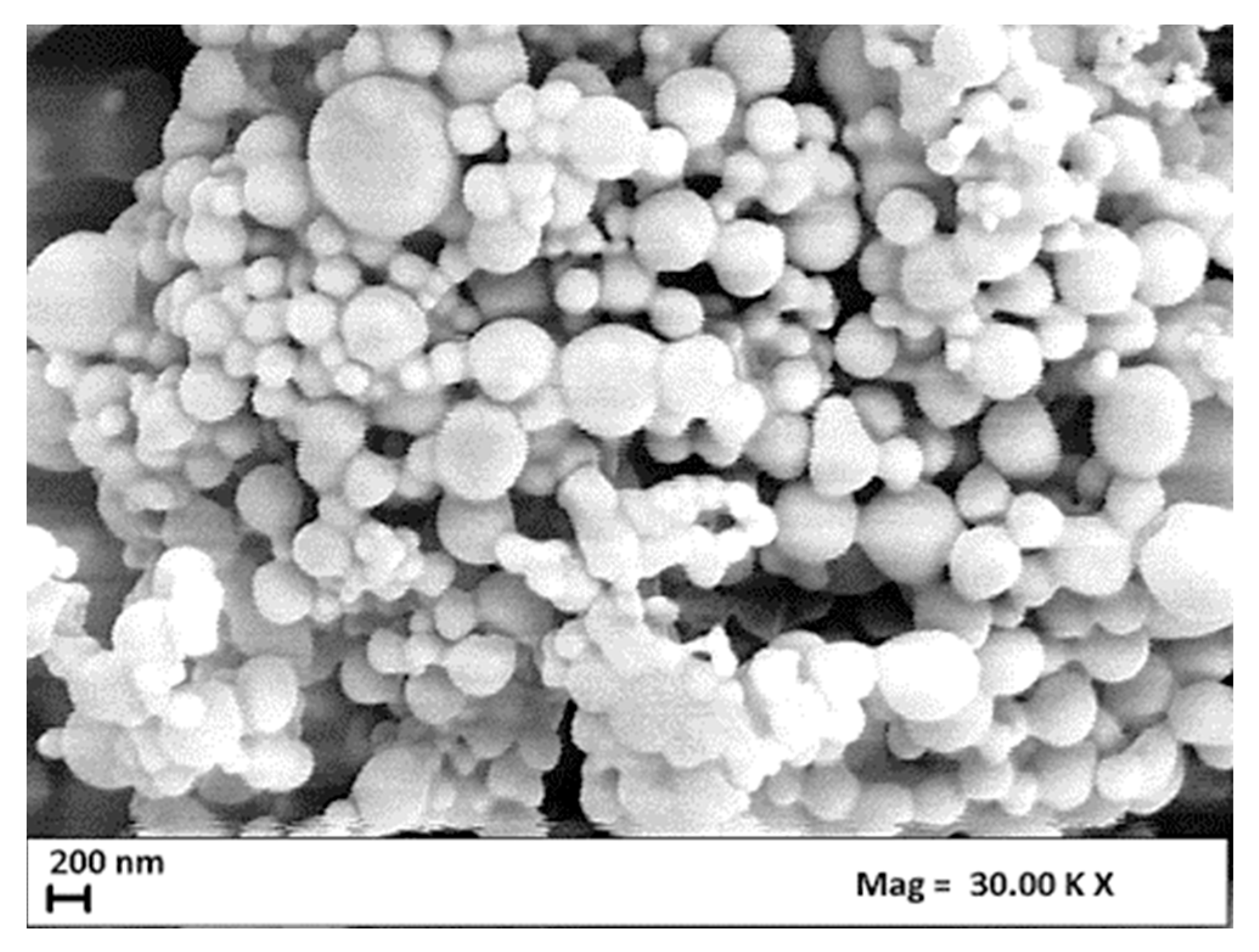
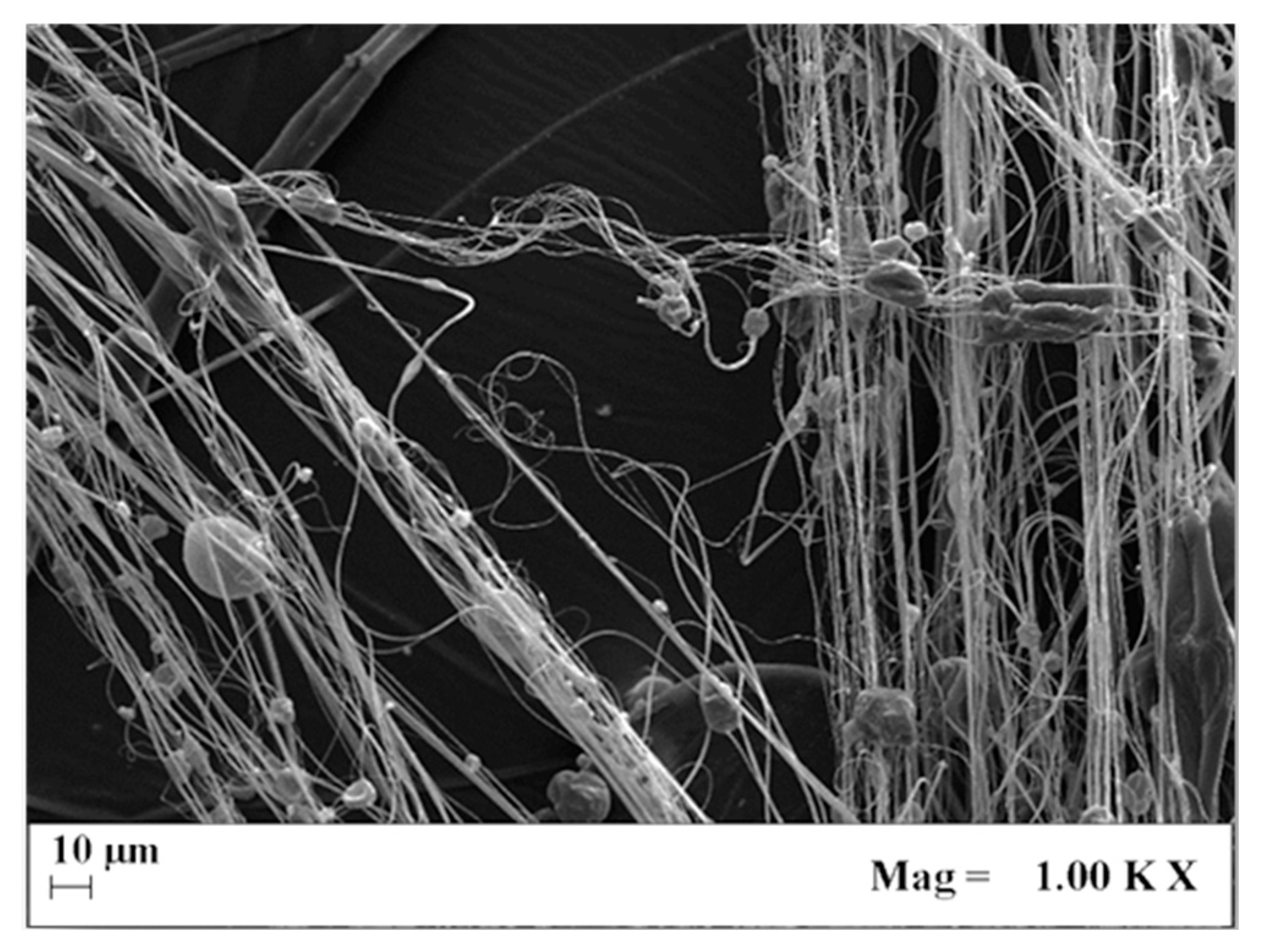
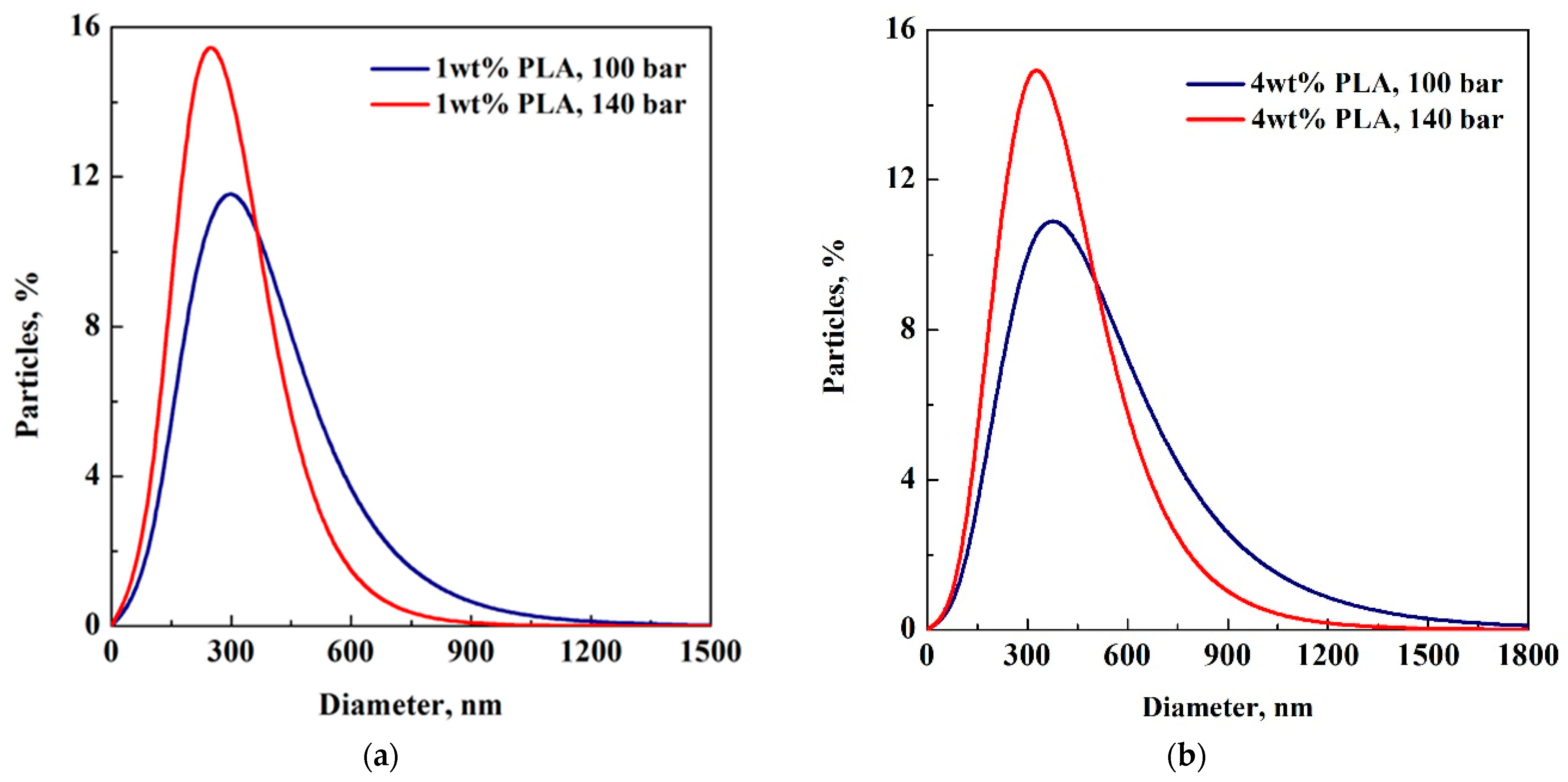
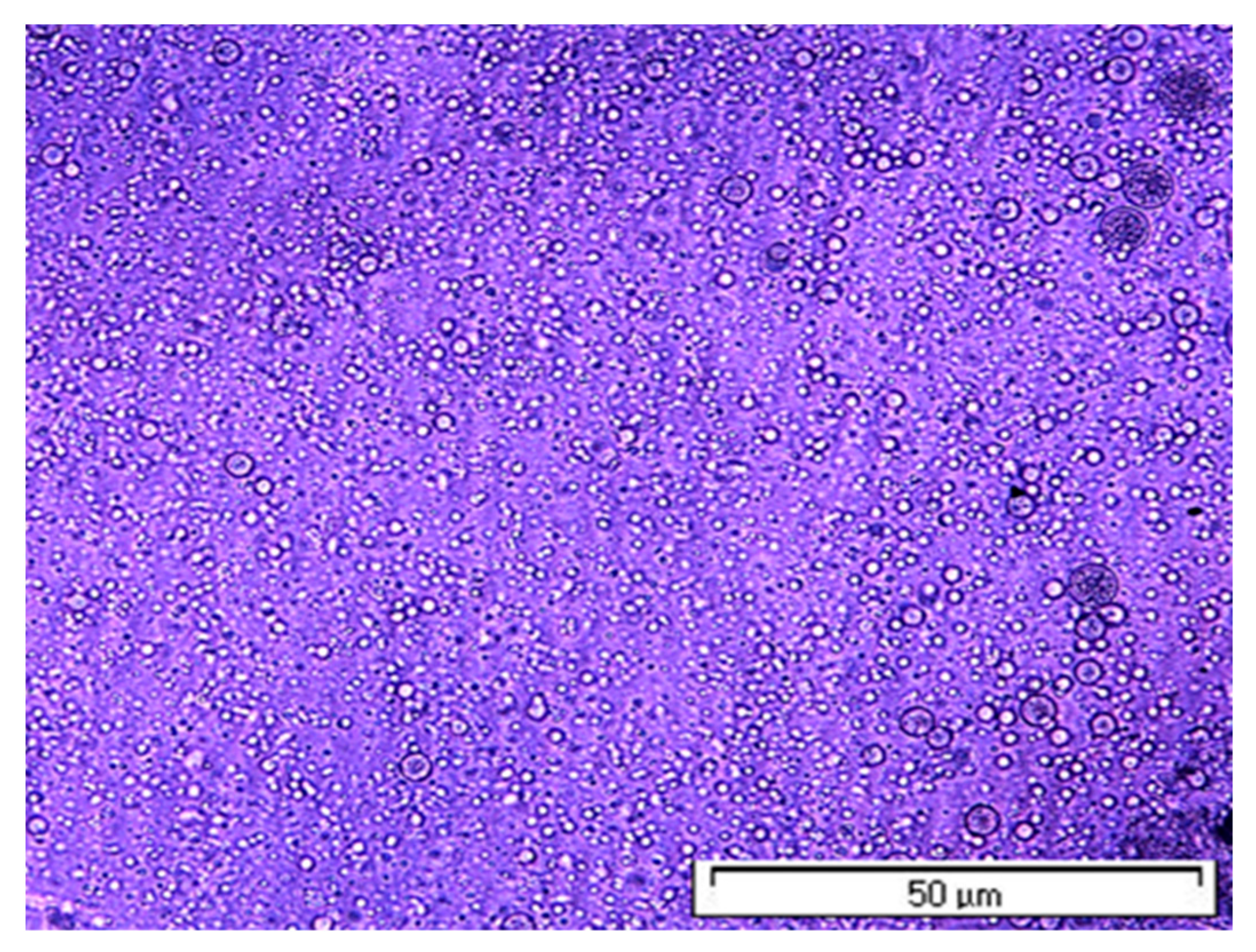



| Concentration PLA, wt% | Average Diameter, nm P = 100 bar | Average Diameter, nm P = 140 bar |
|---|---|---|
| 1 | 270 ± 70 | 250 ± 50 |
| 2 | 310 ± 70 | 290 ± 80 |
| 3 | 330 ± 100 | 290 ± 90 |
| 4 | 350 ± 110 | 310 ± 80 |
| Concentration PLA, wt% | Morphology | Average Diameter, nm P = 100 bar | Average Diameter, nm P = 140 bar |
|---|---|---|---|
| 2 (CFX/PLA = 10 wt%) | P | 210 ± 40 | 180 ± 30 |
| 4 (CFX/PLA = 5 wt%) | P | 240 ± 40 | 190 ± 30 |
| Sample | Concentration, ppm |
|---|---|
| 3 wt% PLA_100 bar (Particles) | 422 |
| 7 wt% PLA_100 bar (Particles and Fibers) | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guastaferro, M.; Baldino, L.; Cardea, S.; Reverchon, E. Supercritical-CO2-Assisted Electrospray for the Production of the PLA-Antibiotic-Sustained Drug-Delivery System. Processes 2023, 11, 2957. https://doi.org/10.3390/pr11102957
Guastaferro M, Baldino L, Cardea S, Reverchon E. Supercritical-CO2-Assisted Electrospray for the Production of the PLA-Antibiotic-Sustained Drug-Delivery System. Processes. 2023; 11(10):2957. https://doi.org/10.3390/pr11102957
Chicago/Turabian StyleGuastaferro, Mariangela, Lucia Baldino, Stefano Cardea, and Ernesto Reverchon. 2023. "Supercritical-CO2-Assisted Electrospray for the Production of the PLA-Antibiotic-Sustained Drug-Delivery System" Processes 11, no. 10: 2957. https://doi.org/10.3390/pr11102957
APA StyleGuastaferro, M., Baldino, L., Cardea, S., & Reverchon, E. (2023). Supercritical-CO2-Assisted Electrospray for the Production of the PLA-Antibiotic-Sustained Drug-Delivery System. Processes, 11(10), 2957. https://doi.org/10.3390/pr11102957









