Transport of Steam-Gas Mixture in Hydrodynamic Devices: A Numerical Study of Steam Reforming of Methane
Abstract
:1. Introduction
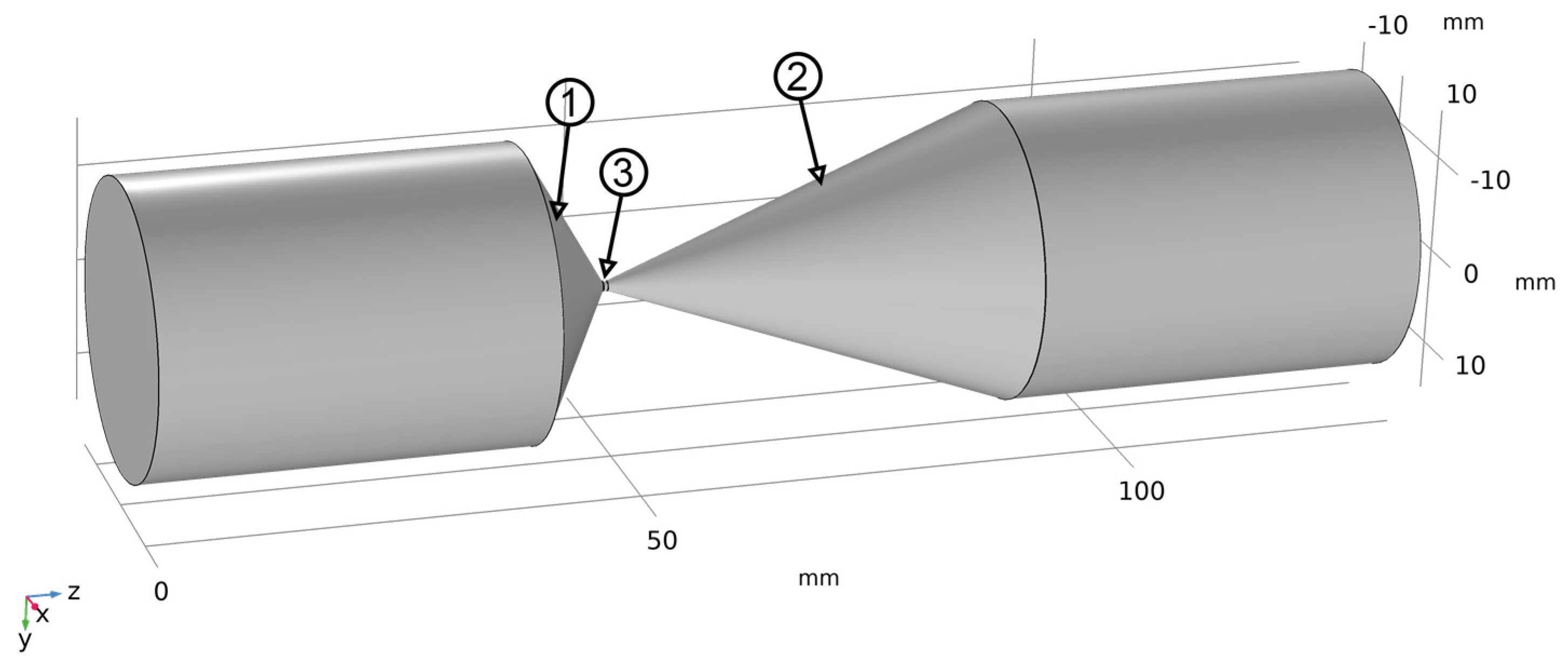
2. Study of the Formation of a Cavitation Zone
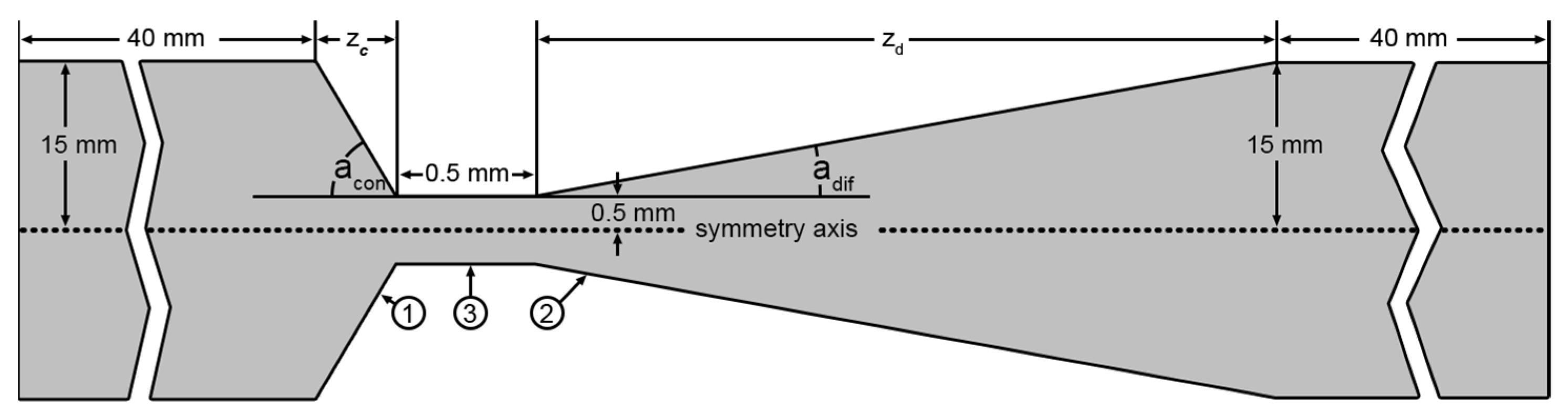
2.1. Problem Statement and Mathematical Model
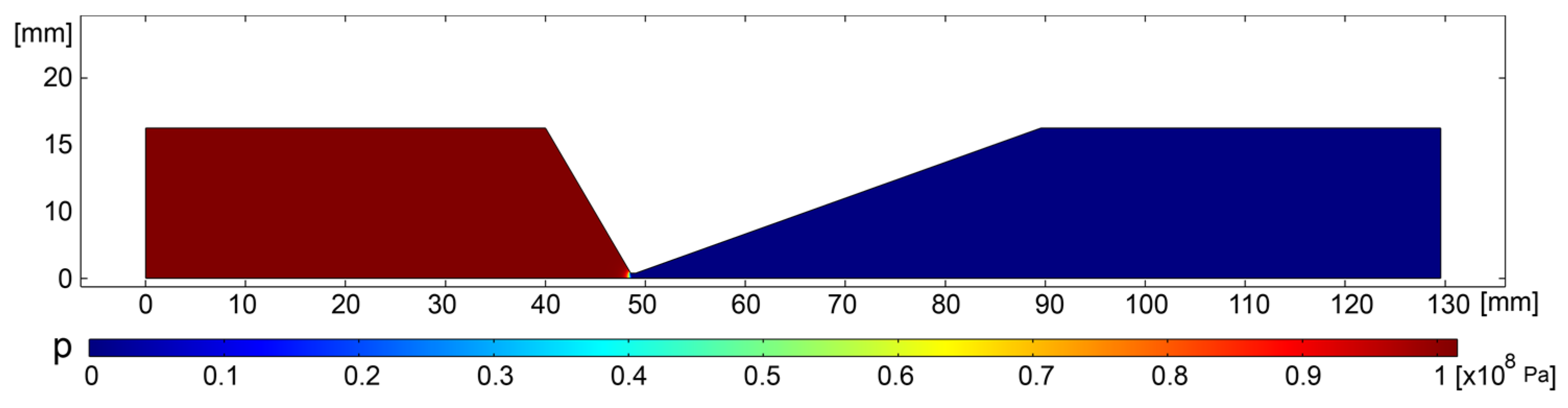
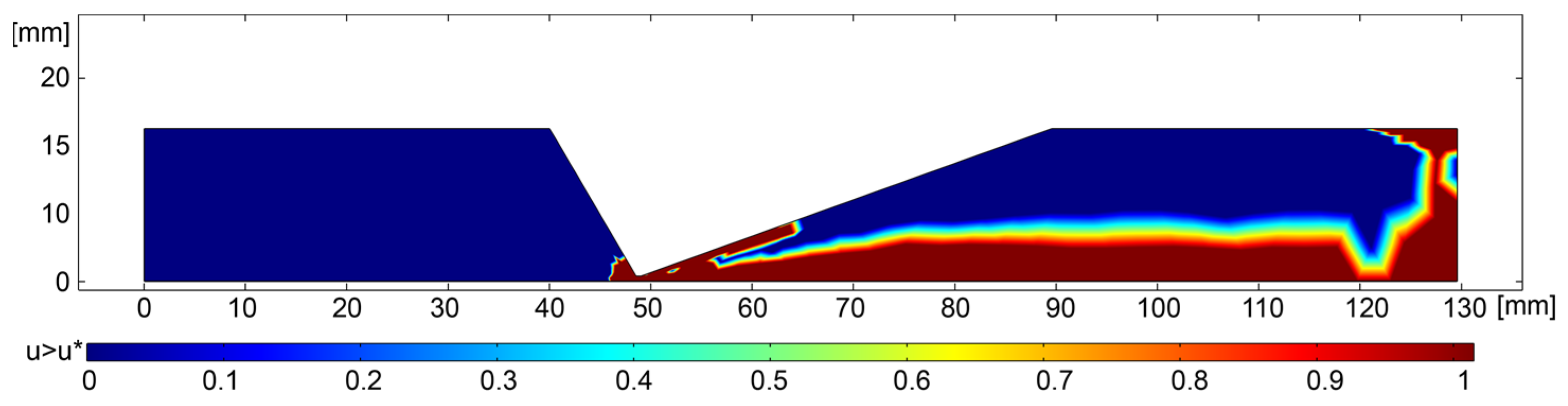
2.2. Results and Discussion
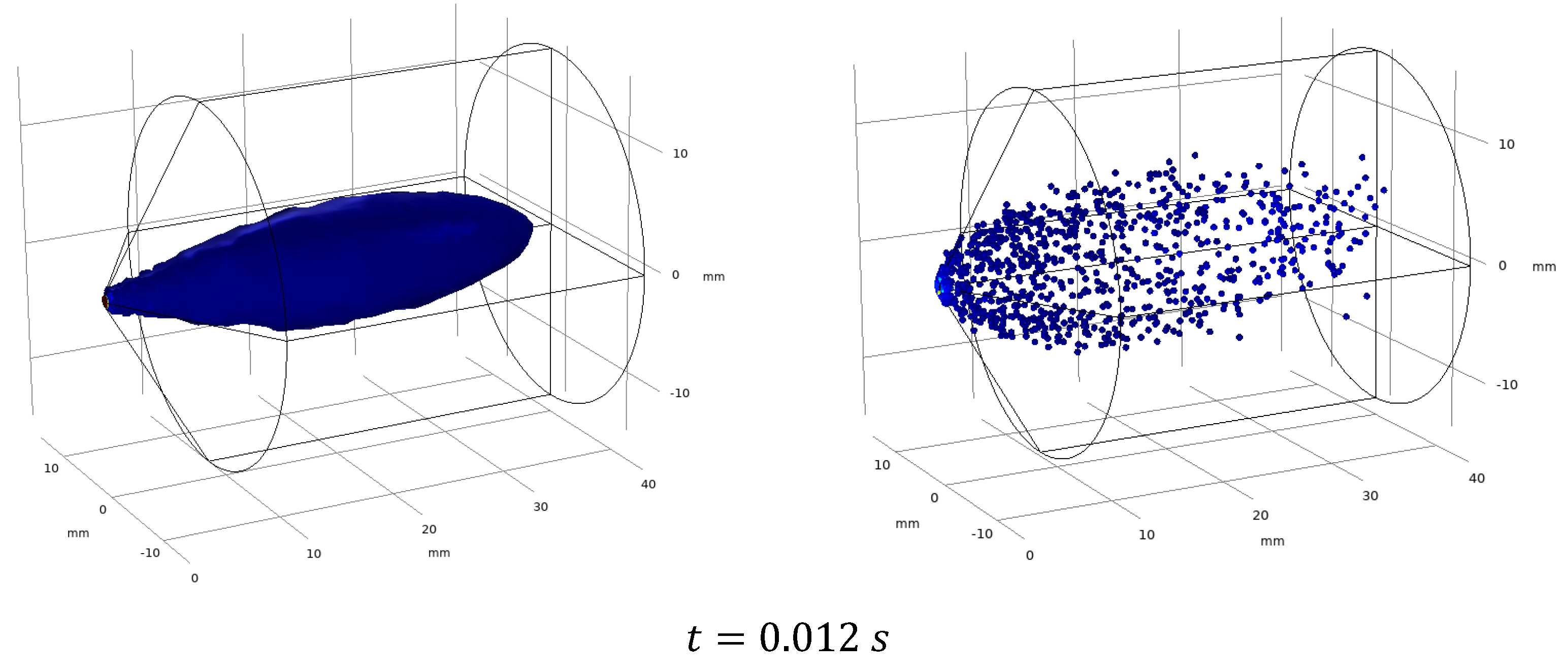
3. Study of the Motion of Aerosol Particles of Methane in Water Steam
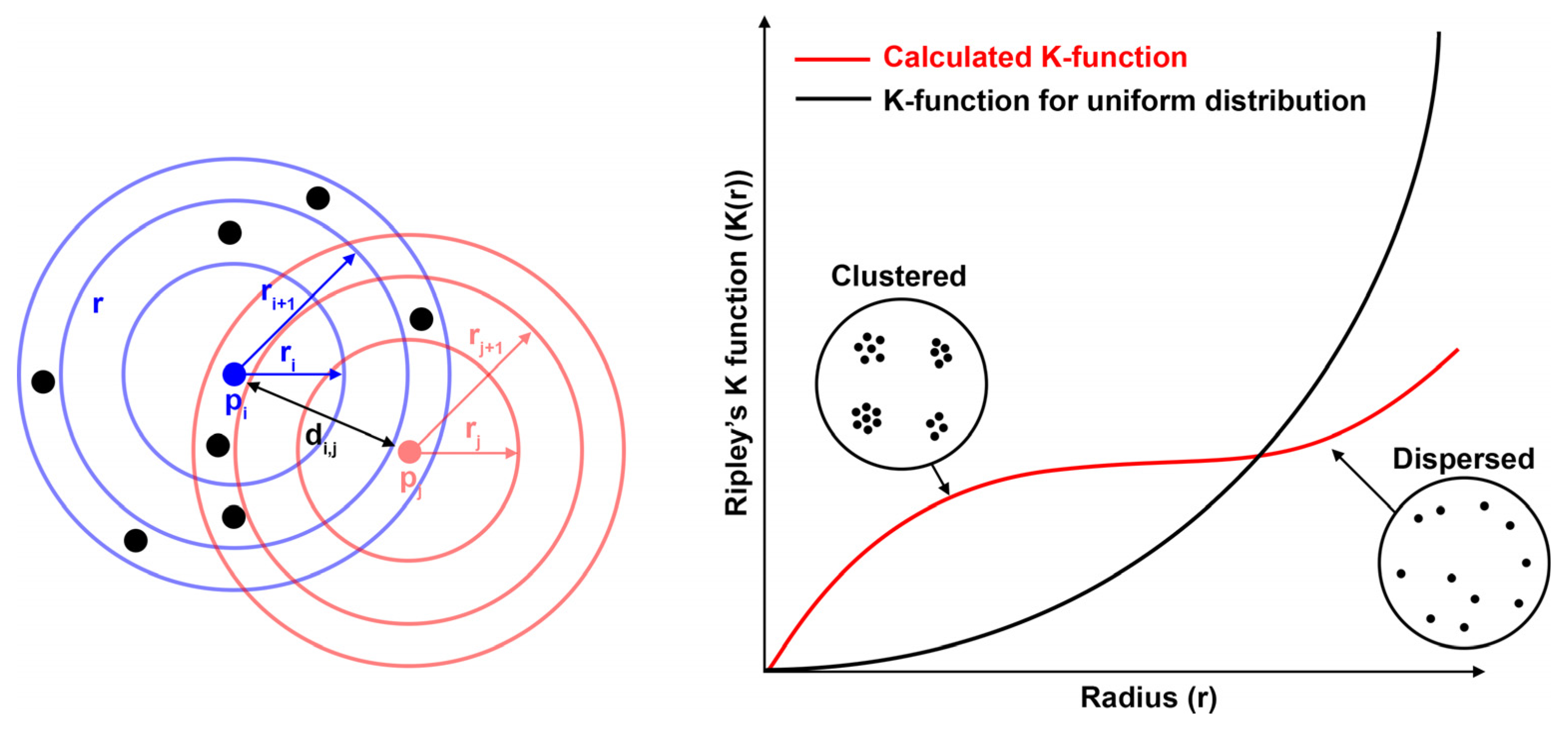
3.1. Investigation of Approaches
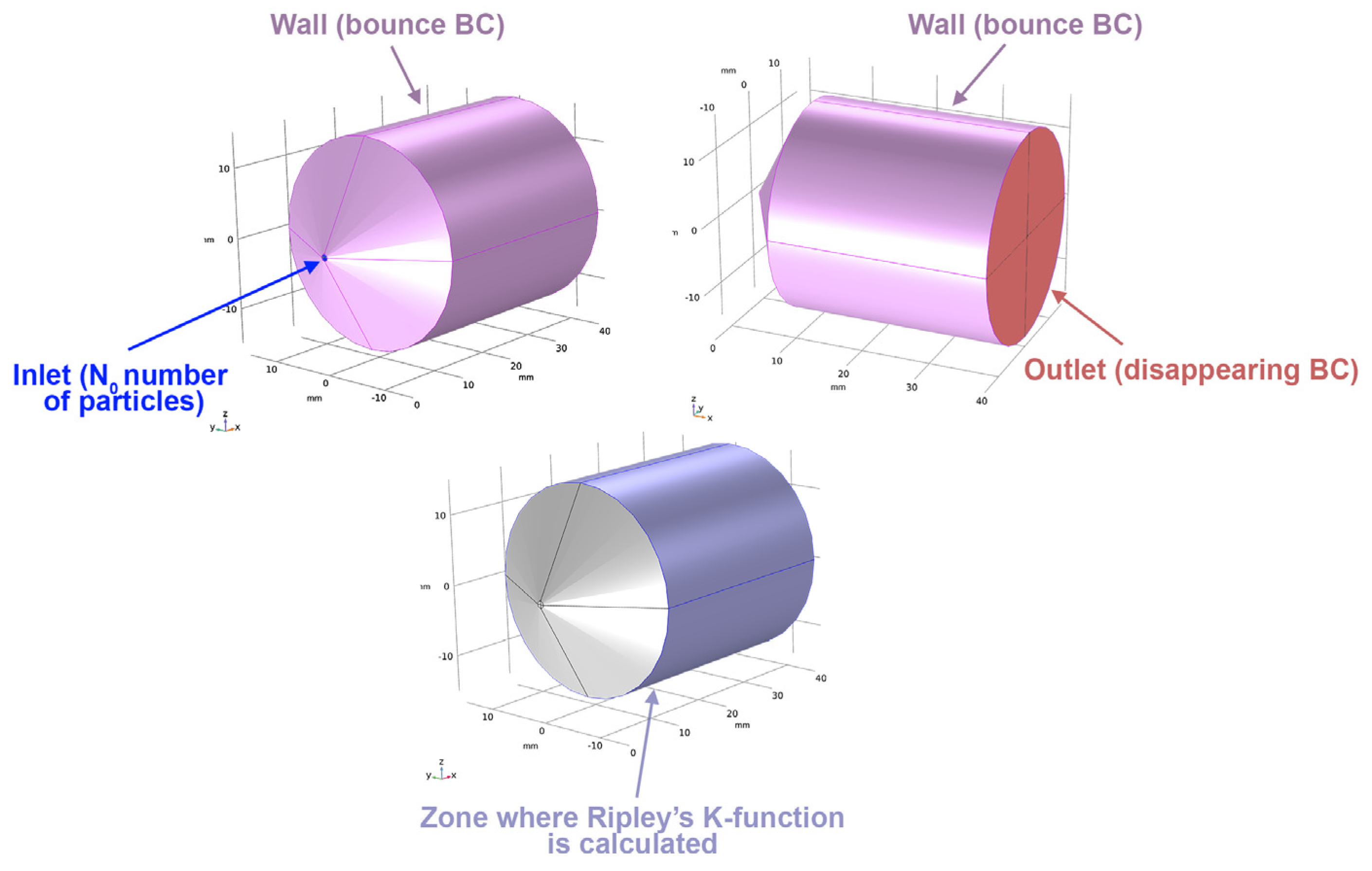
3.2. Problem Statement and Mathematical Model
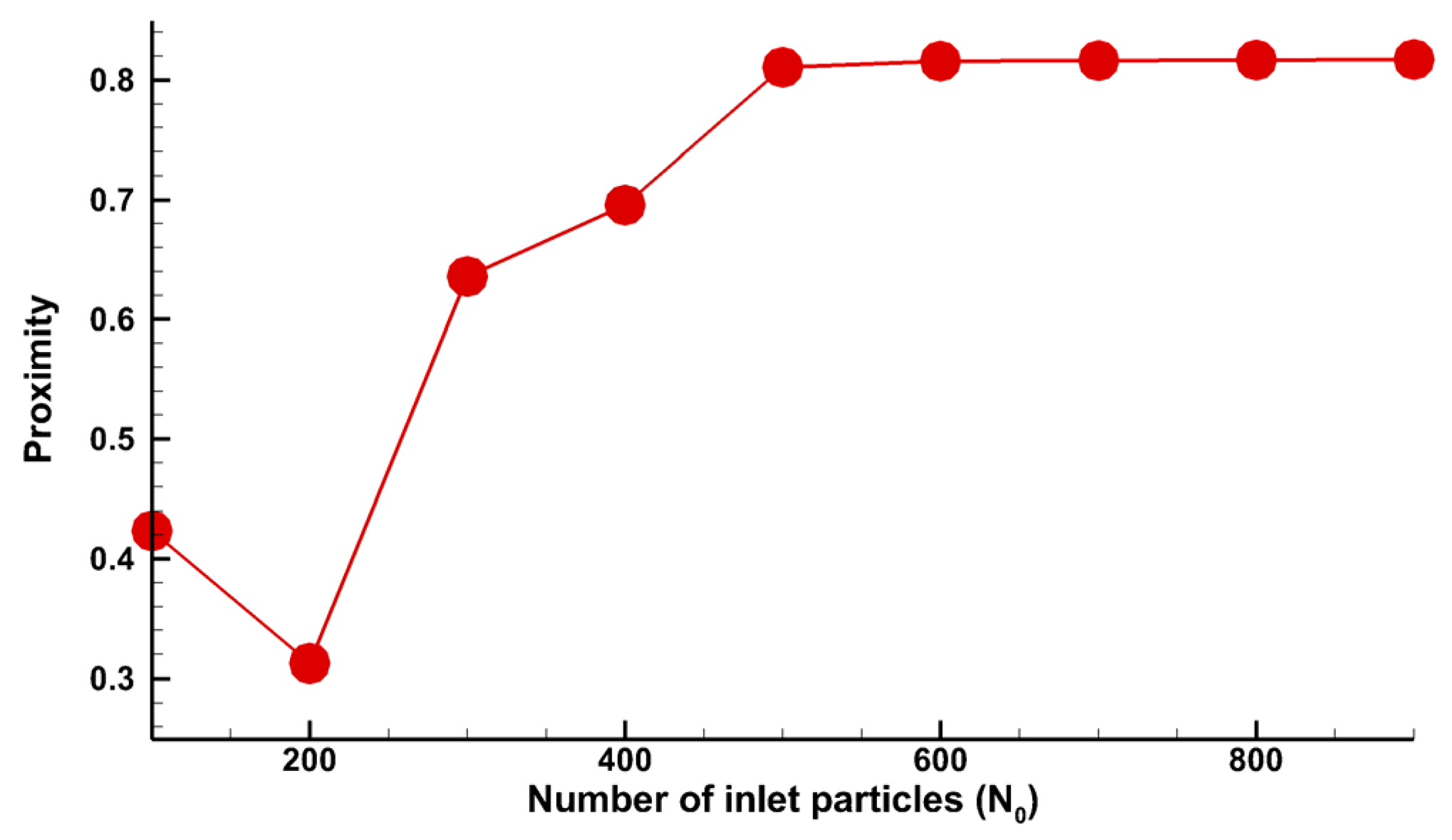
3.3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yusaf, T.; Laimon, M.; Alrefae, W.; Kadirgama, K.; Dhahad, H.A.; Ramasamy, D.; Kamarulzaman, M.K.; Yousif, B. Hydrogen Energy Demand Growth Prediction and Assessment (2021–2050) Using a System Thinking and System Dynamics Approach. Appl. Sci. 2022, 12, 781. [Google Scholar] [CrossRef]
- de Abreu, V.H.S.; Pereira, V.G.F.; Proença, L.F.C.; Toniolo, F.S.; Santos, A.S. A Systematic Study on Techno-Economic Evaluation of Hydrogen Production. Energies 2023, 16, 6542. [Google Scholar] [CrossRef]
- Saeedi, S.; Nguyen, X.T.; Bossola, F.; Evangelisti, C.; Dal Santo, V. Methane Reforming Processes: Advances on Mono- and Bimetallic Ni-Based Catalysts Supported on Mg-Al Mixed Oxides. Catalysts 2023, 13, 379. [Google Scholar] [CrossRef]
- González, J.F.; Álvez-Medina, C.M.; Nogales-Delgado, S. Biogas Steam Reforming in Wastewater Treatment Plants: Opportunities and Challenges. Energies 2023, 16, 6343. [Google Scholar] [CrossRef]
- Yeletsky, A.V. Processes in chemical lasers [Protsessy v khimicheskikh lazerakh]. Adv. Phys. Sci. [Uspekhi Fiz. Nauk.] 1981, 134, 279–314. (In Russian) [Google Scholar]
- Kondratiev, V.N. The Energy of Breaking Chemical Bonds. Ionization Potentials and Electron Affinities: Handbook [Energiya Razryva khimicheskikh Svyazey. Potentsialy Ionizatsii i Srodstvo k elektronu: Spravochnik]; Publishing House of the USSR Academy of Sciences: Moscow, Russia, 1962; 216p. (In Russian) [Google Scholar]
- Okechukwu, C.C.; Alabi, S.B. Profitability Analysis of Selected Steam Methane Reforming Methods for Hydrogen Production. J. Power Energy Eng. 2022, 10, 45–53. [Google Scholar] [CrossRef]
- Yonus, T.; Answer, A.; Asim, Z.; Surahio, M. Production of Hydrogen by Steam Methane Reformation Process. In Proceedings of the 2018 3rd International Conference on Advances on Clean Energy Research (ICACER 2018), Barcelona, Spain, 6–8 April 2018; Volume 51, p. 03003. [Google Scholar] [CrossRef]
- Supat, K.; Chavadej, S.; Lobban, L.L.; Richard, G. Mallinson. Combined Steam Reforming and Partial Oxidation of Methane to Synthesis Gas under Electrical Discharge. Energy Fuels 2003, 17, 474–481. [Google Scholar] [CrossRef]
- Yao, S.L.; Suzuki, E.; Meng, N.; Nakayama, A.A. High-Efficiency Reactor for the Pulsed Plasma Conversion of Methane. Plasma Chem. Plasma Process. 2002, 22, 225–237. [Google Scholar] [CrossRef]
- Daniel, C. Schram. Plasma processing and chemistry. Pure Appl. Chem. 2002, 74, 369–380. [Google Scholar] [CrossRef]
- Sharafutdinov, R.G.; Zarvin, A.Y.e.; Madirbayev, V.Z.h.; Gagachev, V.V.; Gartvich, G.G. Production of hydrogen from methane in electron beam plasma [Polucheniye vodoroda iz metana v elektronno-puchkovoy plazme]. Lett. J. Tech. Phys. [Pis’ma V Zhurnal Tekhnicheskoy Fiz.] 2005, 31, 23–28. (In Russian) [Google Scholar]
- Kozlov, K.V.; Michel, P.; Wagner, H.-E. Synthesis of Organic Compounds from Mixtures of Methane with Carbon Dioxide in Dielectric-Barrier Discharges at Atmospheric Pressure. Plasmas Polym. 2000, 5, 129–150. [Google Scholar] [CrossRef]
- Ruzinska, E.; Kurdel, M.; Morvova, M. Influence of DC corona discharge with one electrode deep in water on the mixture of combustion products of natural gas with natural gas. In Proceedings of the 1996 5th International Symposium on High Pressure Low Temperature Plasma Chemistry (HAKONE-5), Milovy, Czech Republic, 2–4 September 1996; Volume 1, pp. 290–294. [Google Scholar]
- Zherlitsyn, A.G.; Korzhenko, D.V.; Shiean, V.P. Hydrogen production from the natural gas in the microwave discharged at atmospheric pressure [Polucheniye vodoroda iz prirodnogo gaza v plazme svch-razryada pri atmosfernom davlenii]. Gas Ind. Sci. Tech. Prod. J. [Gazov. Promyshlennost’ Nauchno-Tekhnicheskiy I Proizv. Zhurnal] 2018, 11, 104–113. (In Russian) [Google Scholar]
- Lemanov, V.; Lukashov, V.; Sharov, K.; Nezavitin, D. Turbulent spots in the flame of a diffusion torch. J. Phys. Conf. Ser. 2019, 1382, 012058. [Google Scholar] [CrossRef]
- Margulis, M.A. Sonochemistry and Cavitation; Gordon&Breach: London, UK, 1995; 545p. [Google Scholar]
- Bagrov, V.V.; Grafov, D.Y.; Desyatov, A.V.; Kruchinina, N.E.; Kuterbekov, K.A.; Nurakhmetov, T.N.; Yakushin, R.V. Ecologically Safe Cavity Way of Hydrogen Generation in Water Flows with Low Ionized Plasma Emergence. Ecol. Saf. 2013, 5, 21–24. [Google Scholar] [CrossRef]
- Pushkarev, A.I.; Novoselov, Y.N.; Remnev, G.E. Chain Processes in Low-Temperature Plasma [Tsepnyye Protsessy v Nizkotemperaturnoy Plazme]; Nauka: Novosibirsk, Russia, 2008; 226p. (In Russian) [Google Scholar]
- Aleksandrov, M.L.; Kusner, Y.S. Gas-Dynamic Molecular, Ion and Clustered Beams [Gazodinamicheskiye Molekulyarnyye, Ionnyye i Klastirovannyye Puchki]; Nauka: Leningrad, Russia, 1989; 271p. (In Russian) [Google Scholar]
- Gartvich, G.G.; Dudnikov, V.G.; Zarvin, A.E. Spatio-temporal characteristics of a supersonic flow formed using a pulsed electromagnetic valve [Prostranstvenno-vremennyye kharakteristiki sverkhzvukovogo potoka, formiruyemogo s pomoshch’yu impul’s¬nogo elektromagnitnogo klapana]. Instrum. Exp. Technol. [Prib. I Tekhnika Eksperimenta] 1977, 2, 134–136. (In Russian) [Google Scholar]
- Khmel, S.Y.; Sharafutdinov, R.G. Electron-induced fluorescence of carbon dioxide clusters. 1. Free stream of condensing gas [Elektronno-indutsirovannaya flyuorestsen¬tsiya klasterov dvuokisi ugleroda. 1. Svobodnaya struya kondensiruyushchegosya gaza]. J. Tech. Phys. [Zhurnal Tekhnicheskoy Fiz.] 1997, 67, 43–51. (In Russian) [Google Scholar]
- Vostrikov, A.A.; Gileva, V.P.; Dubov, D.Y. Electron—Induced fluorescence of clusters [Elektron—Indutsirovannaya flyuo-restsentsiya klasterov]. J. Tech. Phys. [Zhurnal Tekhnicheskoy Fiz.] 1992, 62, 60–69. (In Russian) [Google Scholar]
- Zarvin, A.E.; Korobeyshchikov, N.G.; Madirbaev, V.Z.; Sharafutdinov, R.G. The influence of condensation on the parameters of supersonic flows [Vliya¬niye kondensatsii na parametry sverkhzvukovykh potokov]. Lett. J. Tech. Phys. [Pis’ma V Zhurnal Tekhnicheskoy Fiz.] 2000, 26, 21–25. (In Russian) [Google Scholar]
- Anderson, J. Fundamentals of Aerodynamics, 6th ed.; McGraw-Hill Education: New York, NY, USA, 2017; 218p. [Google Scholar]
- Teso-Fz-Betoño, D.; Juica, M.; Portal-Porras, K.; Fernandez-Gamiz, U.; Zulueta, E. Estimating the Reattachment Length by Realizing a Comparison between URANS k-Omega SST and LES WALE Models on a Symmetric Geometry. Symmetry 2021, 13, 1555. [Google Scholar] [CrossRef]
- Han, M.; Sane, S.; Johnson, C.R. Exploratory lagrangian-based particle tracing using deep learning. J. Flow Vis. Image Process. 2022, 29, 73–96. [Google Scholar] [CrossRef]
- Dixon, P.M. Ripley’s K function. In Encyclopedia of Environmetrics; John Wiley & Sons: Chinchester, UK, 2002; Volume 3, pp. 1796–1803. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mamytbekov, G.; Shayakhmetov, N.; Aizhulov, D.; Kurmanseiit, M.; Tungatarova, M. Transport of Steam-Gas Mixture in Hydrodynamic Devices: A Numerical Study of Steam Reforming of Methane. Processes 2023, 11, 2991. https://doi.org/10.3390/pr11102991
Mamytbekov G, Shayakhmetov N, Aizhulov D, Kurmanseiit M, Tungatarova M. Transport of Steam-Gas Mixture in Hydrodynamic Devices: A Numerical Study of Steam Reforming of Methane. Processes. 2023; 11(10):2991. https://doi.org/10.3390/pr11102991
Chicago/Turabian StyleMamytbekov, Galymzhan, Nurlan Shayakhmetov, Daniar Aizhulov, Maksat Kurmanseiit, and Madina Tungatarova. 2023. "Transport of Steam-Gas Mixture in Hydrodynamic Devices: A Numerical Study of Steam Reforming of Methane" Processes 11, no. 10: 2991. https://doi.org/10.3390/pr11102991




