Pretreatment of Landfill Leachate Using Hydrodynamic Cavitation at Basic pH Condition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
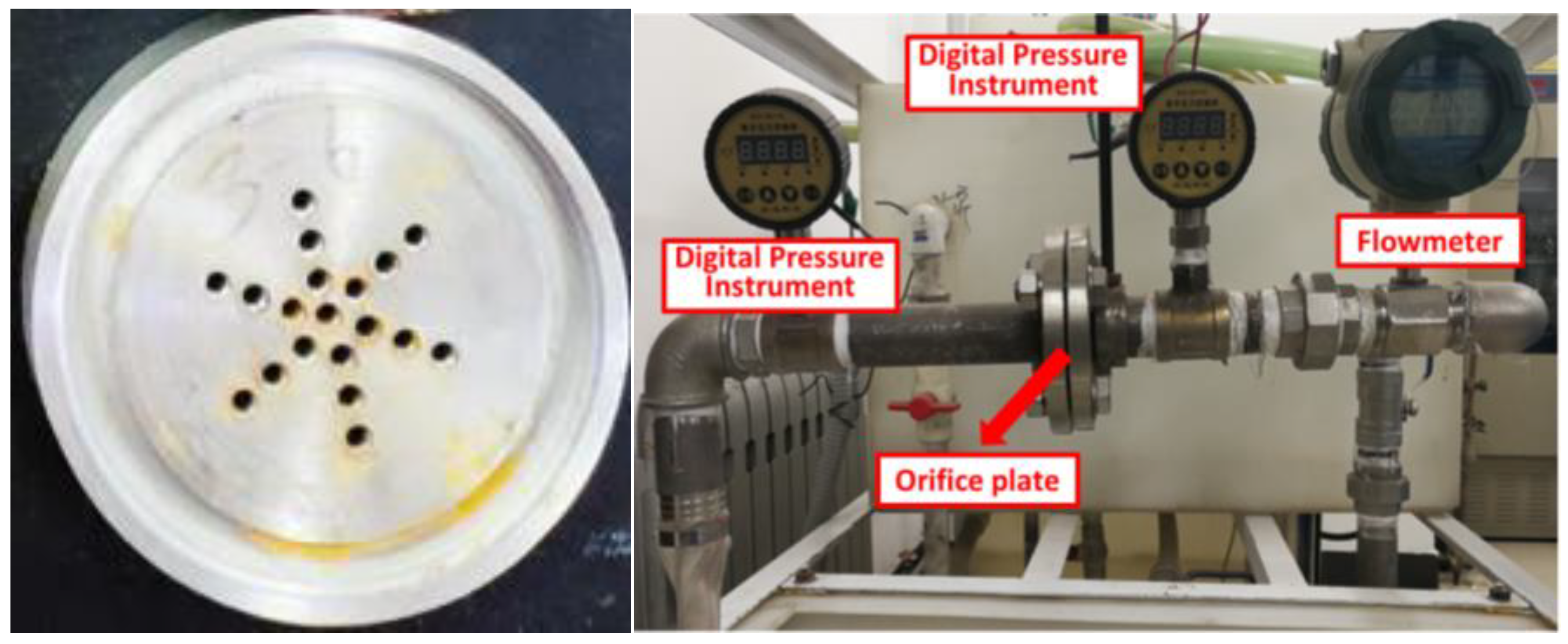
2.2. Experiment Setup
2.3. Analytical Method
2.4. Experimental Method
3. Results
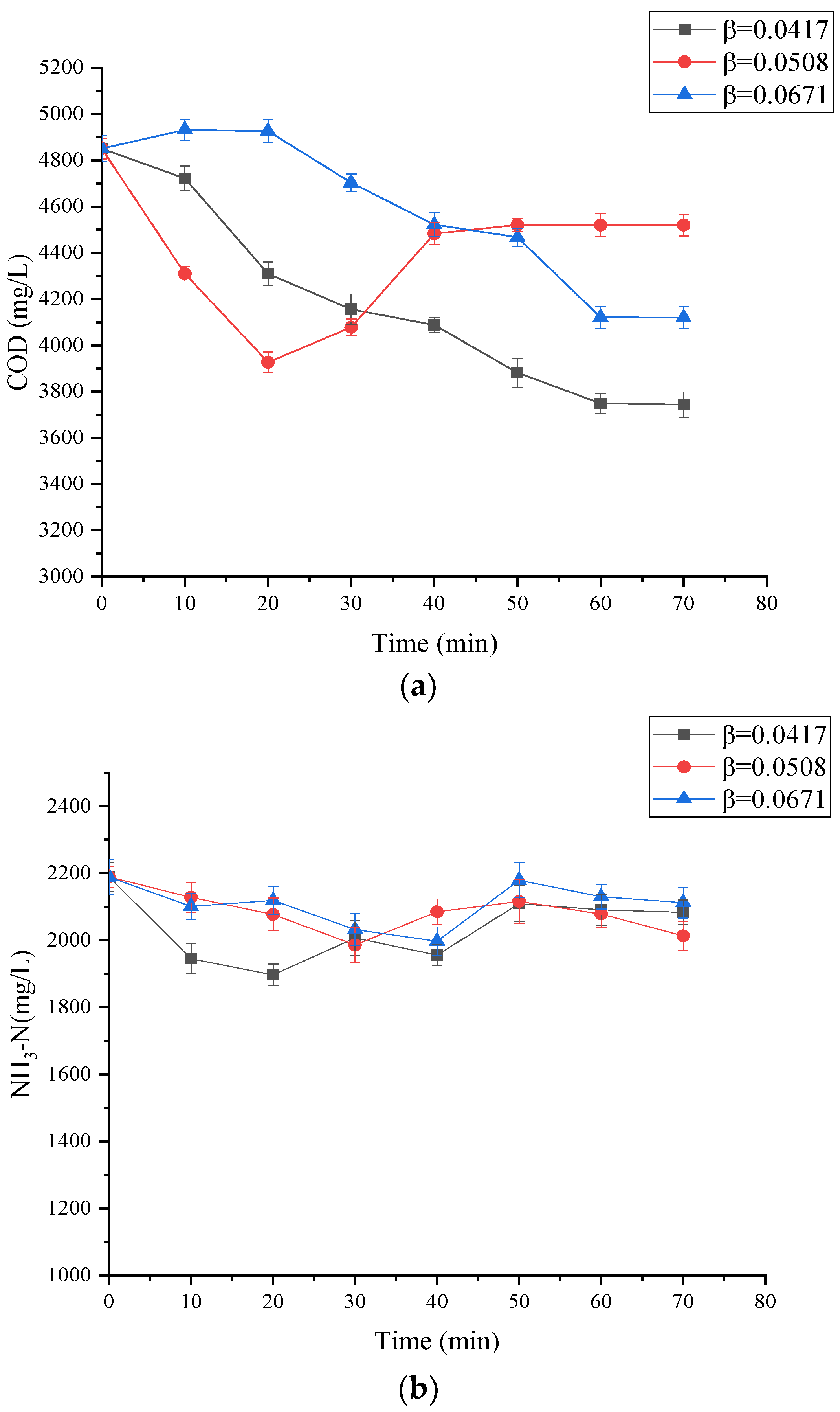
3.1. The Influence of β and Arrangement of Orifices on the LL Treatment
3.2. The Influence of Reaction Time on the LL Treatment
3.3. Changes in the Biodegradability of LL
3.4. Energy Efficiency Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, C.; Chen, W.; Gu, Z.; Li, Q. A review of the characteristics of Fenton and ozonation systems in landfill leachate treatment. Sci. Total Environ. 2020, 762, 143131. [Google Scholar] [CrossRef]
- Teng, C.; Zhou, K.; Peng, C.; Chen, W. Characterization and treatment of landfill leachate: A review. Water Res. 2021, 203, 117525. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.K.; Kumar, M. Suitability of Microwave and Microwave-coupled Systems for Landfill Leachate Treatment: An Overview. J. Environ. Chem. Eng. 2017, 5, 6165–6178. [Google Scholar] [CrossRef]
- GB 16889-2008; Standard for Pollution Control on the Landfill Site of Municipal Solid Waste. General Office of the Ministry of Ecology and Environment, National Standard of the People’s Republic of China: Beijing, China, 2008.
- Mojiri, A.; Zhou, J.L.; Ratnaweera, H.; Ohashi, A.; Ozaki, N.; Kindaichi, T.; Asakura, H. Treatment of landfill leachate with different techniques: An overview. Water Reuse 2020, 11, 66–96. [Google Scholar] [CrossRef]
- Lu, W.; Lei, S.; Chen, N.; Feng, C. Research on two-step advanced treatment of old landfill leachate by sequential electrochemical peroxidation-electro-Fenton process. Chem. Eng. J. 2022, 451, 138746. [Google Scholar] [CrossRef]
- Belouhova, M.; Yotinov, I.; Schneider, I.; Dinova, N.; Todorova, Y.; Lyubomirova, V.; Mihaylova, V.; Daskalova, E.; Lincheva, S.; Topalova, Y. Purposely Development of the Adaptive Potential of Activated Sludge from Municipal Wastewater Treatment Plant Focused on the Treatment of Landfill Leachate. Processes 2022, 10, 460. [Google Scholar] [CrossRef]
- Zhang, F.; Peng, Y.; Liu, Z.; Liu, Y.; Zhao, L. Development of a novel partial nitrification, fermentation-based double denitrification bioprocess (PN-F-Double/DN) to simultaneous treatment of mature landfill leachate and waste activated sludge. Water Res. 2021, 203, 117540. [Google Scholar] [CrossRef]
- Hube, S.; Eskafi, M.; Hrafnkelsdóttir, K.F.; Bjarnadóttir, B.; Bjarnadóttir, M.Á.; Axelsdóttir, S.; Wu, B. Direct membrane filtration for wastewater treatment and resource recovery: A review. Sci. Total Environ. 2020, 710, 136375. [Google Scholar] [CrossRef]
- Brennan, R.B.; Clifford, E.; Devroedt, C.; Morrison, L.; Healy, M.G. Treatment of landfill leachate in municipal wastewater treatment plants and impacts on effluent ammonium concentrations. J. Environ. Manag. 2017, 188, 64–72. [Google Scholar] [CrossRef]
- Chin, P.M.; Naim, A.N.; Suja, F.; Ahmad Usul, M.F. Impact of Effluent from the Leachate Treatment Plant of Taman Beringin Solid Waste Transfer Station on the Quality of Jinjang River. Processes 2020, 8, 1553. [Google Scholar] [CrossRef]
- Hu, Y.; Gu, Z.; He, J.; Li, Q. Novel strategy for controlling colloidal instability during the flocculation pretreatment of landfill leachate. Chemosphere 2022, 287, 132051. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Mo, K.; Kim, M.; Cui, F. Development of a Novel Hydrodynamic Sequencing Batch Reactor for Landfill Leachate Treatment by Shortcut Biological Nitrogen Removal. Processes 2023, 11, 1868. [Google Scholar] [CrossRef]
- Shadi, A.M.H.; Kamaruddin, M.A.; Niza, N.M.; Omar, F.M.; Hossain, M.S. Facile isotherms of iron oxide nanoparticles for the effectively removing organic and inorganic pollutants from landfill leachate: Isotherms, kinetics, and thermodynamics modelling. J. Environ. Chem. Eng. 2022, 10, 107753. [Google Scholar] [CrossRef]
- Chen, W.; Gu, Z.; Ran, G.; Li, Q. Application of membrane separation technology in the treatment of leachate in China: A review. Waste Manag. 2021, 121, 127–140. [Google Scholar] [CrossRef]
- Kamal, A.; Makhatova, A.; Yergali, B.; Baidullayeva, A.; Satayeva, A.; Kim, J.; Inglezakis, V.J.; Poulopoulos, S.G.; Arkhangelsky, E. Biological Treatment, Advanced oxidation and membrane separation for landfill leachate treatment: A review. Sustainability 2022, 14, 14427. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, X.; Chen, S.; Huang, X.; Zhang, X. Hydrogen peroxide and peroxymonosulfate intensifying Fe−doped NiC−Al2O3−framework−based catalytic ozonation for advanced treatment of landfill leachate: Performance and mechanisms. Sci. Total Environ. 2022, 843, 156904. [Google Scholar] [CrossRef]
- Roudi, A.M.; Salem, S.; Abedini, M.; Maslahati, A.; Imran, M. Response surface methodology (RSM)-based prediction and optimization of the Fenton process in landfill leachate decolorization. Processes 2021, 9, 2284. [Google Scholar] [CrossRef]
- Hussain, S.; Aneggi, E.; Comuzzi, C. Abatement of the ecotoxicological risk of landfill leachate by heterogeneous Fenton-like oxidation. Environ. Sci. Pollut. Res. 2023, 30, 21025–21032. [Google Scholar] [CrossRef] [PubMed]
- Parthenidis, P.; Evgenidou, E.; Lambropoulou, D. Wet and supercritical oxidation for landfill leachate treatment: A short review. J. Environ. Chem. Eng. 2022, 10, 107837. [Google Scholar] [CrossRef]
- Jegadeesan, C.; Somanathan, A.; Jeyakumar, R.B. Sanitary landfill leachate treatment by aerated electrochemical Fenton process. J. Environ. Manag. 2023, 337, 117698. [Google Scholar] [CrossRef] [PubMed]
- Montusiewicz, A.; Bis, M.; Pasieczna-Patkowska, S.; Majerek, D. Mature landfill leachate utilization using a cost-effective hybrid method. Waste Manag. 2018, 76, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.-D. Study on the Degradation of Phenol by Hydrodynamic Cavitation and Chlorine Dioxide. Master’s Thesis, North University of China, Taiyuan, China, 2017. [Google Scholar]
- Wang, B.; Su, H.; Zhang, B. Hydrodynamic cavitation as a promising route for wastewater treatment: A review. Chem. Eng. J. 2021, 412, 128685. [Google Scholar] [CrossRef]
- Bis, M.; Montusiewicz, A.; Ozonek, J.; Pasieczna-Patkowska, S. Application of hydrodynamic cavitation to improve the biodegradability of mature landfill leachate. Ultrason. Sonochem. 2015, 26, 378–387. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Wastewater treatment using hybrid treatment schemes based on cavitation and Fenton chemistry: A review. Ultrason. Sonochem. 2014, 21, 1–14. [Google Scholar] [CrossRef]
- Gągol, M.; Przyjazny, A.; Boczkaj, G. Wastewater treatment by means of advanced oxidation processes based on cavitation: A Review. Chem. Eng. J. 2018, 338, 599–627. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, N.; Hu, W.; Wang, H.; Kuang, P.; Chen, F.; Feng, C. Treatment of old landfill leachate by persulfate enhanced electro-coagulation system: Improving organic matters removal and precipitates settling performance. Chem. Eng. J. 2021, 424, 130262. [Google Scholar] [CrossRef]
- Shi, S.-T. Study on Methyl Orange Degradation by Hydrodynamic Cavitation with Chlorine Dioxide. Master’s Thesis, North University of China, Taiyuan, China, 2016. [Google Scholar]
- Sunita, R.-J.; Badve, M.P.; Pinjari, D.V.; Saini, D.D.R.; Sonawane, S.H.; Pandit, A.B. Treatment of the pesticide industry effluent using hydrodynamic cavitation and its combination with process intensifying additives (H2O2 and ozone). Chem. Eng. J. 2016, 259, 326–335. [Google Scholar]
- Cai, M.-Q.; Su, J.; Zhu, Y.-Z.; Wei, X.-Q.; Jin, M.-C.; Zhang, H.-J.; Dong, C.-Y.; Wei, Z.-S. Decolorization of azo dyes orange g using hydrodynamic cavitation coupled with heterogeneous Fenton process. Ultrason. Sonochem. 2016, 28, 302–310. [Google Scholar] [CrossRef]
- Gore, M.M.; Saharan, V.K.; Pinjari, D.V.; Chavan, P.V.; Pandit, A.B. Degradation of reactive orange 4 dye using hydrodynamic cavitation based hybrid techniques. Ultrason. Sonochem. 2014, 21, 1075–1082. [Google Scholar] [CrossRef]
- Pradhan, A.A.; Gogate, P.R. Removal of p-nitrophenol using hydrodynamic cavitation and Fenton chemistry at pilot scale operation. Chem. Eng. J. 2010, 156, 77–82. [Google Scholar] [CrossRef]
- Barik, A.J.; Gogate, P.R. Degradation of 4-chloro 2-aminophenol using a novel combined process based on hydrodynamic cavitation, UV photolysis and ozone. Ultrason. Sonochem. 2015, 30, 70–78. [Google Scholar] [CrossRef]
- Wang, C.-Q.; Jin, R.-Y.; He, Z.-D.; Qiao, Y.-N.; Wang, Y.; Wang, K.; Lu, Y.-R.; Wang, X.-J.; Liu, D.-D. A new water treatment technology for degradation of B[a]A by hydrodynamic cavitation and chlorine dioxide oxidation. Ultrason. Sonochem. 2020, 61, 104834. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, K.; Dinesh, K.; Sun, X.; Soltani, R.D.C.; Wang, Z.; Sonawane, S.; Boczkaj, G. Synergistic effects of hybrid advanced oxidation processes (AOPs) based on hydrodynamic cavitation phenomenon—A review. Chem. Eng. J. 2022, 432, 134191. [Google Scholar] [CrossRef]
- Yang, S.-J. The Study on the Mechanism of Breaking Methyl Orange and Benzo[a]pyrene by ClO2 Enhanced with Hydrodynamic Cavitation. Ph.D. Thesis, North University of China, Taiyuan, China, 2018. [Google Scholar]
- Soyama, H.; Hoshino, J. Enhancing the aggressive intensity of hydrodynamic cavitation through a venturi tube by increasing the pressure in the region where the bubbles collapse. AIP Adv. 2016, 6, 045113. [Google Scholar] [CrossRef]
- Petkovšek, M.; Mlakar, M.; Levstek, M.; Strazar, M.; Širok, B.; Dular, M. A novel rotation generator of hydrodynamic cavitation for waste-activated sludge disintegration. Ultrason. Sonochem. 2015, 26, 408–414. [Google Scholar] [CrossRef]
- Kovačič, A.; Škufca, D.; Zupanc, M.; Gostiša, J.; Bizjan, B.; Krištofelc, N.; Dolenc, M.S.; Heath, E. The removal of bisphenols and other contaminants of emerging concern by hydrodynamic cavitation: From lab-scale to pilot-scale. Sci. Total Environ. 2020, 743, 140724. [Google Scholar] [CrossRef]
- Wang, K.; Jin, R.; Qiao, Y.; He, Z.; Wang, X.; Wang, C.; Lu, Y. 2,4,6-Triamino-1,3,5-Trinitrobenzene explosive wastewater treatment by hydrodynamic cavitation combined with chlorine dioxide. Propellants Explos. Pyrotech. 2019, 45, 1243–1249. [Google Scholar] [CrossRef]
- Li, H.; Li, H.; Huang, X.; Han, Q.; Yuan, Y.; Qi, B. Numerical and experimental study on the internal flow of the venturi injector. Processes 2020, 8, 64. [Google Scholar] [CrossRef]
- Scandelai, A.P.J.; Rigobello, E.S.; Oliveira, B.L.C.D.; Tavares, C.R.G. Identification of organic compounds in landfill leachate treated by advanced oxidation processes. Environ. Sci. Technol. 2017, 27, 1–51. [Google Scholar] [CrossRef]
- Banch, T.J.H.; Hanafiah, M.M.; Alkarkhi, A.F.M.; Amr, S.S.A.; Nizam, N.U.M. Evaluation of different treatment processes for landfill leachate using low-cost agro-industrial materials. Processes 2020, 8, 111. [Google Scholar] [CrossRef]
- Boczkaj, G.; Gągol, M.; Klein, M.; Przyjazny, A. Effective method of treatment of effluents from production of bitumens under basic pH conditions using hydrodynamic cavitation aided by external oxidants. Ultrason. Sonochem. 2018, 40, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Tejera, J.; Gascó, A.; Hermosilla, D.; Alonso-Gomez, V.; Negro, C.; Blanco, Á. UVA-LED technology’s treatment efficiency and cost in a competitive trial applied to the photo-Fenton treatment of landfill leachate. Processes 2021, 9, 1026. [Google Scholar] [CrossRef]




| Parameter | Value |
|---|---|
| COD (mg/L) | 4844.2 |
| BOD5 (mg/L) | 535 |
| BOD5/COD | 0.11 |
| pH | 7.18 |
| NH3-N (mg/L) | 2178.82 |
| Turbidity (NTU) | 10,688.9 |
| Total suspended solids (mg/L) | 57 |
| UV254 | 1.72 |
| Device Composition | Model and Manufacturer |
|---|---|
| Booster pump | Model: CDLF-100 (Shanghai Yuquan Pump Co., Ltd., Wenzhou, China) |
| Water tank | Made of PVC material, dimensions: 500 mm × 500 mm × 600 mm, thickness: 5 mm, capacity: 150 L |
| Condensing tube | Made with stainless steel pipe |
| Valve | Stainless steel, DN40 ball valve |
| Pipeline | The main pipeline is made of stainless steel. The side piping is made of PVC, and the pipe diameter is DN40 |
| Flowmeter | Model: LWSY-25, measuring range: 1–10 m3 h−1 (Dongtai Dongxing Instrument Factory, Donxing, China) |
| Pressure gauge | Model: BD-801 K, range: 0–1.0 MPa (Shanghai Kaixun Technology Co., Ltd., Shanghai, China) |
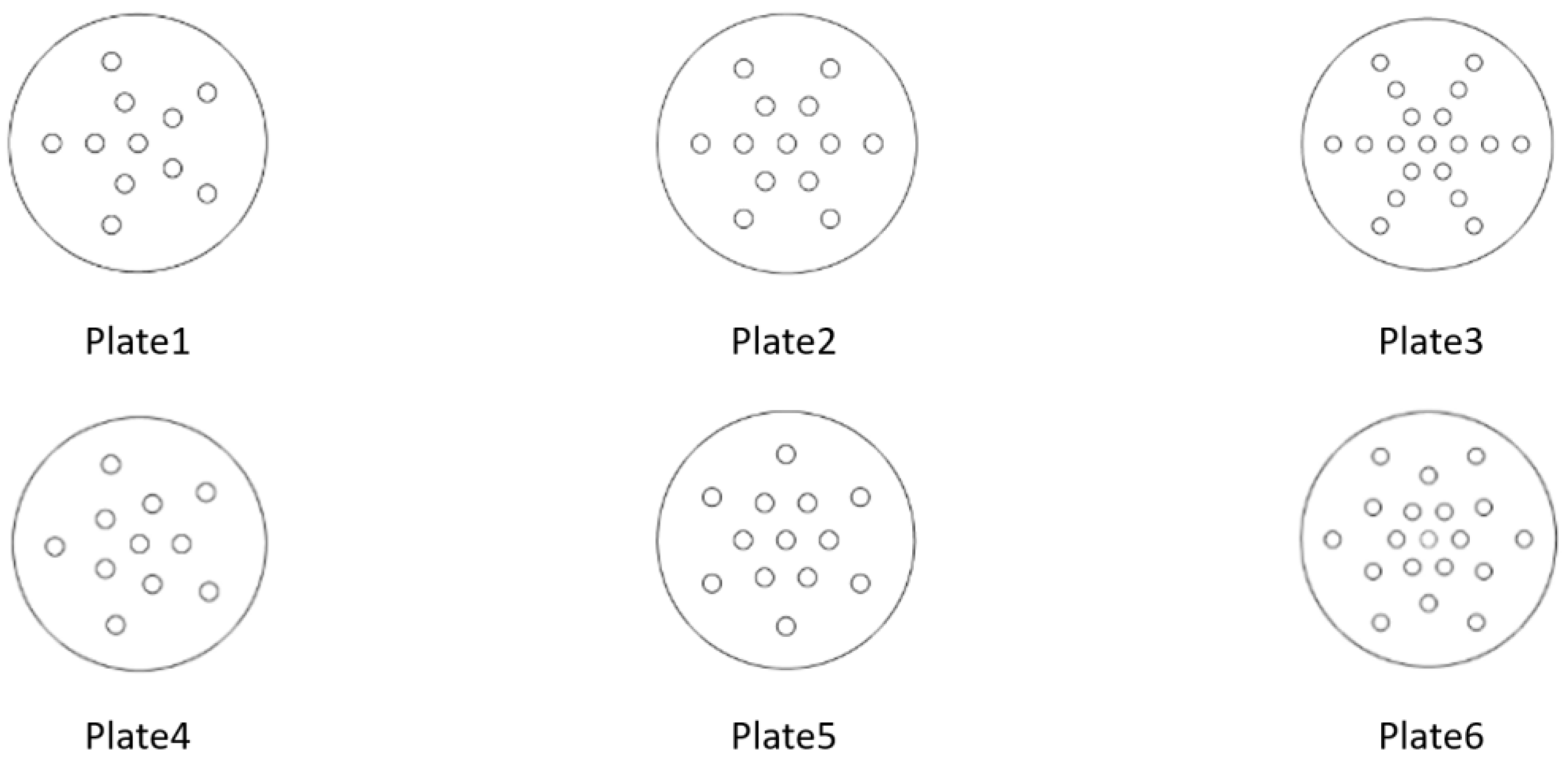
| Orifice Plate Number | The Diameter of the Hole, d/mm | Number of Holes, N | Area Occupied by the Holes, mm2 | Orifice Opening Rate, β | Arrangement of Orifices |
|---|---|---|---|---|---|
| 1 | 2.5 | 11 | 4.9086 | 0.0671 | Radiation distribution |
| 2 | 2 | 13 | 3.1415 | 0.0508 | Radiation distribution |
| 3 | 1.5 | 19 | 1.7671 | 0.0417 | Radiation distribution |
| 4 | 2.5 | 11 | 4.9086 | 0.0671 | Annular distribution |
| 5 | 2 | 13 | 3.1415 | 0.0508 | Annular distribution |
| 6 | 1.5 | 19 | 1.7671 | 0.0417 | Annular distribution |
| Process | COD | BOD5 | BOD5/COD | NH3-N |
|---|---|---|---|---|
| Before HC treatment (mg/L) | 4844.20 | 535 | 0.110 | 2178.82 |
| After HC treatment (mg/L) | 3748.16 | 648 | 0.173 | 2129.10 |
| Changes | 22.63% reduction | 21.12% increase | 57.27% increase | 2.28% increase |
| Orifice Plate Number | COD Reduction (mg/L) | COD Removal Rate (%) | Energy Efficiency ×10−3 mg COD/J |
|---|---|---|---|
| 1 | 424.20 | 8.76 | 3.19 |
| 2 | 352.20 | 7.27 | 2.65 |
| 3 | 742.44 | 15.33 | 5.58 |
| 4 | 707.08 | 14.60 | 5.31 |
| 5 | 313.96 | 6.48 | 2.49 |
| 6 | 1096.04 | 22.63 | 8.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Wang, C.; Jiang, Y.; Feng, X.; Wang, K.; Xiong, J.; Jia, M.; Jin, R. Pretreatment of Landfill Leachate Using Hydrodynamic Cavitation at Basic pH Condition. Processes 2023, 11, 3014. https://doi.org/10.3390/pr11103014
Qiao Y, Wang C, Jiang Y, Feng X, Wang K, Xiong J, Jia M, Jin R. Pretreatment of Landfill Leachate Using Hydrodynamic Cavitation at Basic pH Condition. Processes. 2023; 11(10):3014. https://doi.org/10.3390/pr11103014
Chicago/Turabian StyleQiao, Yina, Chaoqi Wang, Yu Jiang, Xingqiao Feng, Kun Wang, Jian Xiong, Mengye Jia, and Riya Jin. 2023. "Pretreatment of Landfill Leachate Using Hydrodynamic Cavitation at Basic pH Condition" Processes 11, no. 10: 3014. https://doi.org/10.3390/pr11103014




