Effect of Wheat Residue-Derived Biochar on Naphthalene Adsorption in Loess Soil in Northwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Preparation and Characterization
2.2. Soil Collection and Pretreatment
2.3. Chemicals and Reagents
2.4. Experimental Methods
2.4.1. Sorption Kinetics Experiment
2.4.2. Sorption Isotherm Experiment
2.4.3. Experiment on Influencing Factors
2.5. Data Analysis
3. Results and Discussion
3.1. Characterization of Biochar
3.2. Sorption Kinetics
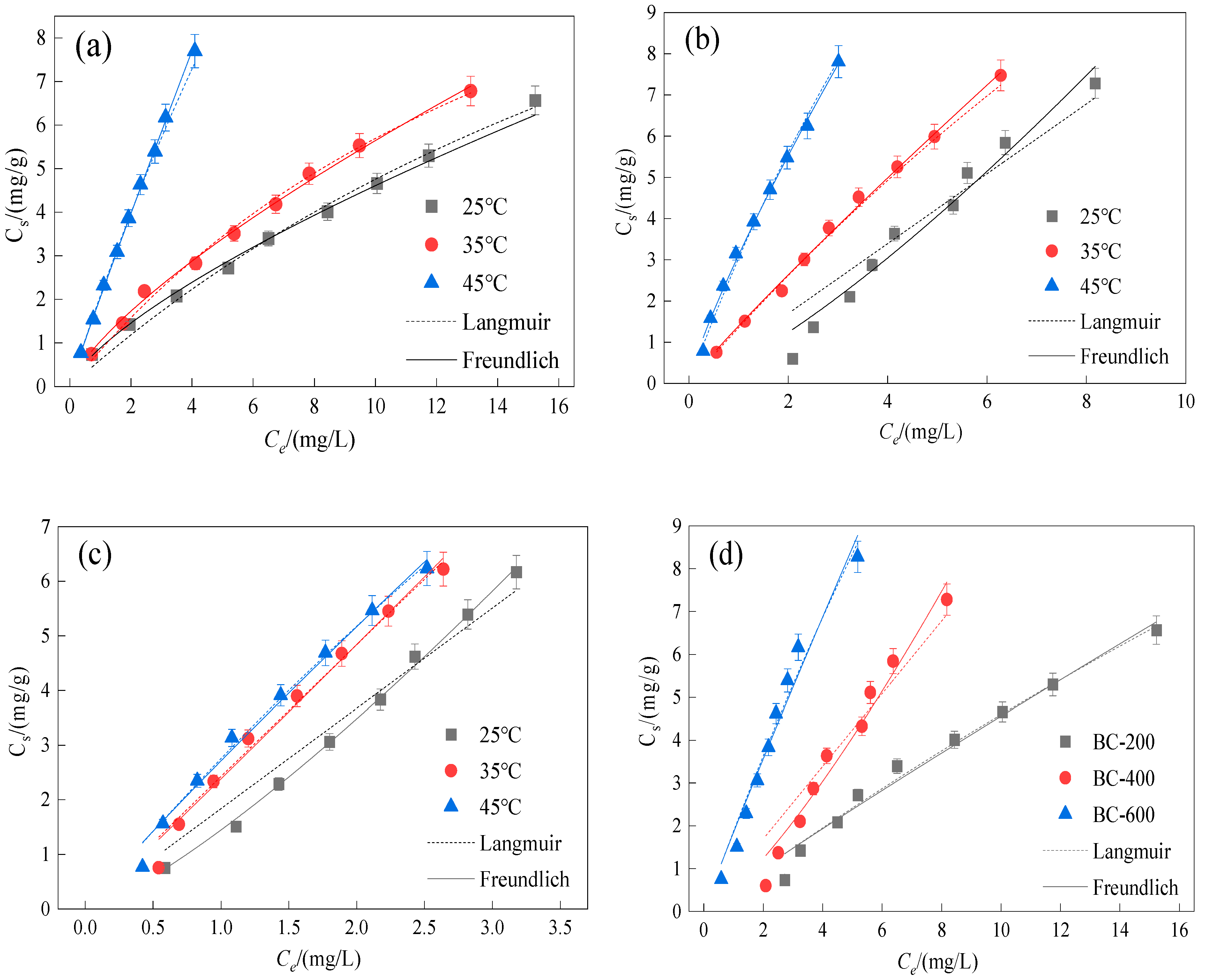
3.3. Sorption Isotherms
3.4. Thermodynamic Parameters
3.5. Factors Influencing NAP Adsorption
3.5.1. Impact of Loess Soil Types
3.5.2. Impact of Initial Concentration of NAP
3.5.3. Impact of Biochar Pyrolysis Temperature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, M.; Yang, Y.; Jiang, L.; Hong, Q.; Zhang, D.; Shen, Z.; Yin, H.; Luo, C. Characterization of the phenanthrene degradation-related genes and degrading ability of a newly isolated copper-tolerant bacterium. Environ. Pollut. 2017, 220, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Dai, Y.; Zhao, W.; Li, H.; Xu, B.; Sun, C. Highly efficient photocatalytic degradation of naphthalene by Co3O4/Bi2O2CO3 under visible light: A novel p–n heterojunction nanocomposite with nanocrystals/lotus-leaf-like nanosheets structure. Appl. Catal. B Environ. 2018, 237, 273–287. [Google Scholar] [CrossRef]
- Shi, C.; Qu, C.; Sun, W.; Zhou, J.; Zhang, J.; Cao, Y.; Zhang, Y.; Guo, J.; Zhang, J.; Qi, S. Multimedia distribution of polycyclic aromatic hydrocarbons in the Wang Lake Wetland, China. Environ. Pollut. 2022, 306, 119358. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Cai, J.; Li, J.; Sun, B.; Wu, F. Effects of different carbon substrates on PAHs fractions and microbial community changes in PAHs-contaminated soils. Environ. Pollut. 2023, 324, 121367. [Google Scholar] [CrossRef] [PubMed]
- Ameur, W.B.; Annabi, A.; Mhadhbi, T.; Hassine, S.B.; Safouen, G.; Megdiche, Y.E.; Khadija, M.; Ennaceur, S.; Trabelsi, S.; Hammami, B.; et al. Polycyclic aromatic hydrocarbons in mullet (Chelon auratus) from two lagoons of great ecological and economic importance in Tunisia: Levels, sources and human health risk implications. J. Sea Res. 2023, 192, 102325. [Google Scholar] [CrossRef]
- Jafarabadi, A.R.; Bakhtiari, A.R.; Yaghoobi, Z.; Yap, C.K.; Maisano, M.; Cappello, T. Distributions and compositional patterns of polycyclic aromatic hydrocarbons (PAHs) and their derivatives in three edible fishes from Kharg coral Island, Persian Gulf, Iran. Chemosphere 2019, 215, 835–845. [Google Scholar] [CrossRef]
- Robin, S.L.; Marchand, C. Polycyclic aromatic hydrocarbons (PAHs) in mangrove ecosystems: A review. Environ. Pollut. 2022, 311, 119959. [Google Scholar] [CrossRef]
- Yu, H.; Li, T.; Liu, Y.; Ma, L. Spatial distribution of polycyclic aromatic hydrocarbon contamination in urban soil of China. Chemosphere 2019, 230, 498–509. [Google Scholar] [CrossRef]
- Wei, Y.P.; Ma, H.Q.; Li, S.; Li, C.; Zhang, L.; Kang, Q. Preparation of sludge-based biochar and its adsorption capacity for naphthalene. New Chem. Mater. 2023, 51, 281–286. [Google Scholar] [CrossRef]
- Gong, X.Y.; Xiong, W.F.; Peng, Z.; Hu, H.Y.; Zhong, P. Adsorption of naphthalene by chicken manure-derived biochar. J. Wuhan Univ. Sci. Technol. 2020, 43, 37–43. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Kalita, M.C.; Deka, S.; Borah, J.M.; Joshi, S.J.; Zhang, M.; Peng, W.; Sharma, G.; Rinklebe, J.; et al. Biodegradation of hazardous naphthalene and cleaner production of rhamnolipids-green approaches of pollution mitigation. Environ. Res. 2022, 209, 112875. [Google Scholar] [CrossRef] [PubMed]
- Bojes, H.K.; Pope, P.G. Characterization of EPA’s 16 priority pollutant polycyclic aromatic hydrocarbons (PAHs) in tank bottom solids and associated contaminated soils at oil exploration and production sites in Texas. Regul. Toxicol. Pharmacol. 2007, 47, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Ji, S.; Liang, W.; Li, C.; Nie, Y.; Dong, J.; Shi, W.; Ai, S. A low-cost and eco-friendly powder catalyst: Iron and copper nanoparticles supported on biochar/geopolymer for activating potassium peroxymonosulfate to degrade naphthalene in water and soil. Chemosphere 2022, 303, 135185. [Google Scholar] [CrossRef]
- Cao, Y.Z.; Han, M.; Xia, F.Y.; Yan, Z.G.; Zhou, Y.Y.; Guo, G.L.; Li, F.S. Regional variance and its influencing factors in deriving standard values of organic pollutants in soil: A case study based on human health risk assessment modeling approach. J. Agro-Environ. Sci. 2010, 29, 270–275. [Google Scholar]
- Yu, L.L.; Li, J.; Liu, G.Q.; Liu, X.; Qi, S.H.; Zhang, G. Polycyclic aromatic hydrocarbons in surface soils of the Pearl River Delta, south China. Ecol. Environ. 2007, 16, 1683–1687. [Google Scholar] [CrossRef]
- Tong, Y.F.; Chen, L.; Liu, Y.; Wang, Y.N.; Tian, S.Y. Distribution, sources and ecological risk assessment of PAHs in surface seawater from coastal Bohai Bay, China. Mar. Pollut. Bull. 2019, 142, 520–524. [Google Scholar] [CrossRef]
- Kaur, V.; Sharma, P. Effect of Prosopis juliflora Biochar on Physico-Chemical Properties of Naphthalene and Phenanthrene Contaminated Soil. Polycycl. Aromat. Compd. 2021, 41, 1406–1417. [Google Scholar] [CrossRef]
- Peng, Z.; Gong, X.Y.; Xiong, W.F.; Wang, Z.Y.; Ren, D.J. Effect and the mechanism of modified biochar on adsorption of naphthalene. J. Ecol. Rural Environ. 2021, 37, 1080–1088. [Google Scholar] [CrossRef]
- Du, L.; Ahmad, S.; Liu, L.; Wang, L.; Tang, J. A review of antibiotics and antibiotic resistance genes (ARGs) adsorption by biochar and modified biochar in water. Sci. Total Environ. 2022, 858, 159815. [Google Scholar] [CrossRef]
- Shang, Q.; Chi, J. Impact of biochar coexistence with polar/nonpolar microplastics on phenanthrene sorption in soil. J. Hazard. Mater. 2023, 447, 130761. [Google Scholar] [CrossRef]
- Cao, X.; Ma, L.; Liang, Y.; Gao, B.; Harris, W. Simultaneous immobilization of lead and atrazine in contaminated soils using dairy-manure biochar. Environ. Sci. Technol. 2011, 45, 4884–4889. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Zhou, Q.X. Environmental effects and their mechanisms of biochar applied to soils. Environ. Chem. 2013, 32, 768–780. [Google Scholar] [CrossRef]
- Chen, Q.; Chen, L.F.; Wu, D.P.; Wu, M.; Pan, P. Mechanisms of soil minerals enhance biochar stability: A review. J. Agro-Environ. Sci. 2023, 42, 490–499. [Google Scholar]
- Zhang, C.H.; Wang, J.L.; Li, H.X.; Su, H.H.; Sun, Z.; Wang, X.T. Research development of remediation of polycyclic aromatic hydrocarbons contaminated soil by biochar. Appl. Chem. Ind. 2023, 52, 892–897+903. [Google Scholar] [CrossRef]
- Zhou, X.; Shi, L.; Moghaddam, T.B.; Chen, M.; Wu, S.; Yuan, X. Adsorption mechanism of polycyclic aromatic hydrocarbons using wood waste-derived biochar. J. Hazard. Mater. 2022, 425, 128003. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Jiang, Y.F.; Hu, X.F.; Zhan, H.Y.; Yves, U.J. Adsorption and influential factors of carbofuran on the loess soil by adding biochar prepared at different temperatures. Acta Sci. Circumstantiae 2016, 36, 1015–1020. [Google Scholar] [CrossRef]
- Chang, X.L.; Hu, X.F.; Jiang, Y.F.; Sun, H.; Mu, Z.F. Biochar made from wheat straw: Preparation and characterization. J. Environ. Sci. Technol. 2017, 40, 24–29. [Google Scholar]
- Li, C.Y.; Sun, X.T.; Zhu, Y.F.; Liang, W.X.; Nie, Y.X.; Shi, W.J.; Ai, S.Y. Core-shell structural nitrogen-doped carbon foam loaded with nano zero-valent iron for simultaneous remediation of Cd (II) and NAP in water and soil: Kinetics, mechanism, and environmental evaluation. Sci. Total Environ. 2022, 832, 155091. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zeng, J.H. Influence of several surfactants on sorption of toluene and naphthalene in soils. J. Agro-Environ. Sci. 2006, 25, 512–517. [Google Scholar]
- Jiang, Y.F.; Sun, H.; Yves, U.J.; Li, H.; Hu, X.F. Impact of biochar produced from post-harvest residue on the adsorption behavior of diesel oil on loess soil. Environ. Geochem. Health 2016, 38, 243–253. [Google Scholar] [CrossRef]
- Hu, X.F. Effect of Biochar on the Adsorption Behavior of Polycyclic Aromatic Hydrocarbons onto Oil-Contaminated Loess Soil; Lanzhou Jiaotong University: Lanzhou, China, 2015. [Google Scholar]
- OECD. Adsorption-Desorption Using a Batch Equilibrium Method: Technical Guideline 106; Organization for Economic Cooperation and Development: Paris, France, 2000. [Google Scholar]
- Ho, Y.S.; McKay, G. Sorption of dye from aqueous solution by peat. Chem. Eng. J. 1998, 70, 115–124. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Kinetic models for the sorption of dye from aqueous solution by wood. Process Saf. Environ. Prot. 1998, 76, 183–191. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über die adsorption in lösungen (adsorption in solution). Phys. Chem. Period. 1906, 57, 384–470. [Google Scholar]
- Zhong, W.J.; Fu, D.J.; Qi, D.; Liu, R.N.; Lu, X.J. Preparation of Biochar and its Application in Water Treatment. Technol. Water Treat. 2023, 49, 26–30. [Google Scholar] [CrossRef]
- Tang, X.C.; Zhang, M. Review on the Remediation of Contaminated Soil by Biochar Amendment. Environ. Sci. Surv. 2014, 33, 17–26. [Google Scholar] [CrossRef]
- Chen, B.; Chen, Z. Sorption of naphthalene and 1-naphthol by biochars of orange peels with different pyrolytic temperatures. Chemosphere 2009, 76, 127–133. [Google Scholar] [CrossRef]
- Li, F.Y.; Tao, J.G.; Wang, J.F.; Li, X.L. Difference characteristics of biochar derived from peanut shell under different temperatures. Chin. J. Environ. Eng. 2017, 11, 3726–3730. [Google Scholar] [CrossRef]
- Lu, H.N.; Hu, X.Y.; Liu, H.W. Influence of Pyrolysis Conditions on Stability of Biochar. Environ. Sci. Technol. 2013, 36, 11–14. [Google Scholar] [CrossRef]
- Xing, B.S.; Pignatello, J.J. Time-dependent isotherm shape of organic compounds in soil organic matter: Implications for sorption mechanism. Environ. Toxicol. Chem. Int. J. 1996, 15, 1282–1288. [Google Scholar] [CrossRef]
- Karapanagioti, H.K.; Kleineidam, S.; Sabatini, D.A.; Grathwohl, P.; Ligouis, B. Impacts of heterogeneous organic matter on phenanthrene sorption: Equilibrium and kinetic studies with aquifer material. Environ. Sci. Technol. 2000, 34, 406–414. [Google Scholar] [CrossRef]
- Deng, J.C.; Jiang, X.; Hu, W.P.; Lu, X. Effect of sorption reaction time on sorption behavior of herbicide atrazine. Ecol. Environ. Sci. 2007, 2, 402–406. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Chen, T.; Li, W.Y. Adsorption of polycyclic aromatic hydrocarbon naphthalene in water by two kinds of biochar. Appl. Chem. Ind. 2022, 51, 653–657. [Google Scholar] [CrossRef]
- Cabal, B.; Ania, C.O.; Parra, J.B.; Pis, J.J. Kinetics of naphthalene adsorption on an activated carbon: Comparison between aqueous and organic media. Chemosphere 2009, 76, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Lesan, H.M.; Bhandari, A. Contact-time-dependent atrazine residue formation in surface soils. Water Res. 2004, 38, 4435–4445. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z. Mechanism and Model Study on Benzene Sorption and Desorption by Organic Matter in Soil. Ph.D. Thesis, China University of Geosciences, Wuhan, China, 2021. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Q.; Huang, J.; Meng, C.; Lu, J.M. Adsorption equilibrium, kinetics and mechanism studies of copper and nickel on coal-fired fly ash. Coal Process. Compr. Util. 2022, 272, 91–95. [Google Scholar] [CrossRef]
- Yuan, J.; Feng, L.; Wang, J. Rapid adsorption of naphthalene from aqueous solution by naphthylmethyl derived porous carbon materials. J. Mol. Liq. 2020, 304, 112768. [Google Scholar] [CrossRef]
- Chang, M.Y.; Juang, R.S. Adsorption of tannic acid, humic acid, and dyes from water using the composite of chitosan and activated clay. J. Colloid Interface Sci. 2004, 278, 18–25. [Google Scholar] [CrossRef]
- Xiong, H.; Xiao, Y.; Yan, Z. Preparation of activated carbon and its adsorption of naphthalene in liquid paraffin oil. Colloid Interface Sci. Commun. 2021, 43, 100460. [Google Scholar] [CrossRef]
- Chang, C.F.; Chang, C.Y.; Chen, K.H.; Tsai, W.T.; Shie, J.L.; Chen, Y.H. Adsorption of naphthalene on zeolite from aqueous solution. J. Colloid Interface Sci. 2004, 277, 29–34. [Google Scholar] [CrossRef]
- Zhao, E.L.; Yin, A.P.; Zhang, X.L.; Hao, L.Q.; Zhao, S.H. Thermodynamics and kinetics of adsorption of Cr (VI) on sorghum stalk siochar. New Chem. Mater. 2017, 45, 186–188+193. [Google Scholar]
- Zhang, Z.G.; Jiang, Y.F.; Mu, Z.F.; Sun, H.; Zhou, Q.; Zhan, H.Y. Effect of Biochar on Adsorption Behavior of Nonylphenol onto Loess Soil in Northwest China. Environ. Sci. 2016, 37, 4428–4436. [Google Scholar] [CrossRef]
- Pan, Y.F.; Wang, Y.L.; Liu, W.X.; Zhao, B.D.; Chen, B. Adsorption Kinetics and Thermodynamics of ADN on Activated Carbon. Chin. J. Explos. Propellants 2019, 42, 465–472. [Google Scholar] [CrossRef]
- Chen, D.; Xing, B.; Xie, W. Sorption of phenanthrene, naphthalene and o-xylene by soil organic matter fractions. Geoderma 2007, 139, 329–335. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Y.X.; Tang, J.C.; Zhu, W.Y. Properties of Maize Stalk Biochar Produced Under Different Pyrolysis Temperatures and Its Sorption Capability to Naphthalene. Environ. Sci. 2014, 35, 1884–1890. [Google Scholar] [CrossRef]
- McCall, P.J.; Laskowski, D.A.; Swann, R.L.; Dishburger, H. Test protocols for environmental fate and movement of toxicants. In Proceedings of the AOAC, Washington, DC, USA, 21–22 October 1980; Association of Official Analytical Chemists: Washington, DC, USA, 1980; pp. 89–109. [Google Scholar]
- Liu, W.; Ma, J.J.; Yao, X.D.; Cheng, L.; Fang, R.N. Adsorption thermodyamics of carbon black to dispersant in isopropanol aqueous. Text. Aux. 2018, 35, 18–22. [Google Scholar]
- Han, J.; Li, J.X.; Cui, H.Y.; LÜ, W.; Bai, S.Q. Kinetic and thermodynamic characteristics of phosphorus adsorption by modified corn straw. J. Agro-Environ. Sci. 2020, 39, 2008–2014. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, F.A.; Shao, J.T.; Xu, J.B.; Yu, C.L. Thermodynamics and kinetics of carmine adsorption on rosin-based aminated polymer microspheres. J. Guilin Univ. Technol. 2019, 39, 439–445. [Google Scholar] [CrossRef]
- Flores, C.; Morgante, V.; González, M.; Navia, R.; Seeger, M. Adsorption studies of the herbicide simazine in agricultural soils of the Aconcagua valley, central Chile. Chemosphere 2009, 74, 1544–1549. [Google Scholar] [CrossRef]
- Zhu, H.J.; Li, J.H. Thermodynamics study on Adsorption-desorption of Pymetrozine in three soils. Guangzhou Chem. Ind. 2022, 50, 78–80+102. [Google Scholar]
- Sun, Y.P. The Properties of Naphthalene Adsorption on Soil and Its Site-Energy Distribution Analysis; Xi’an University of Architecture and Technology: Xi’an, China, 2008. [Google Scholar]
- Wan, J.M.; Liu, X.; Zhang, W.J.; Zhang, M.; Yang, Y.T. Adsorption characteristics of phenanthrene and naphthaleneon on Lou soil. J. Agro-Environ. Sci. 2011, 30, 1991–1997. [Google Scholar]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of copper (II), chromium (III), nickel (II) and lead (II) ions from aqueous solutions by meranti sawdust. J. Hazard. Mater. 2009, 170, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.A.; Huang, Z.; Deng, G.H.; Guo, Y. Adsorption of benzonitrile at the air/water interface studied by sum frequency generation spectroscopy. Chin. Sci. Bull. 2013, 58, 1529–1535. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, D.; Yan, Y.; Wu, Z.; Wu, Z.; Cravotto, G. Preparation of activated carbon from Xinjiang region coal by microwave activation and its application in naphthalene, phenanthrene, and pyrene adsorption. J. Taiwan Inst. Chem. Eng. 2015, 53, 160–167. [Google Scholar] [CrossRef]
- Yu, X.Y. Impact of Charcoal on the Sorption/Desorption Behavior and Bioavailability of Pesticide in Soil; Northwest A&F University: Xianyang, China, 2007. [Google Scholar]
- Zhao, M.; Chen, B.F.; Feng, M.H.; Chen, K.N.; Pan, J.Z. Spectral characteristics of dissolved organic matter released from biochar at different pyrolysis temperatures. Spectrosc. Spectr. Anal. 2020, 40, 2505–2511. [Google Scholar]





| Soils | pH | TOC | CEC | Clay | Silt | Sand |
|---|---|---|---|---|---|---|
| g/kg | cmol/kg | g/kg | ||||
| S1 | 7.56 | 9.17 | 37.10 | 7.30 | 52.40 | 40.30 |
| S2 | 8.23 | 9.20 | 23.20 | 9.70 | 32.70 | 57.60 |
| S3 | 8.42 | 10.84 | 21.30 | 12.20 | 69.60 | 18.20 |
| Names | Expression Equations | |
|---|---|---|
| Sorption kinetics models | Pseudo-first-order model | |
| Pseudo-second-order model | ||
| Sorption thermodynamics models | Langmuir model | |
| Freundlich model |
| Biochar | T | Yields | C | H | N | O | (O + N)/C | O/C | H/C | Specific Area | Pore Volume | Aperture |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| °C | % | % | % | % | % | - | - | - | m2·g−1 | mL·g−1 | nm | |
| Wheat straw | -- | -- | 43.32 | 5.63 | 0.29 | 43.68 | 0.85 | 0.83 | 0.06 | 4.17 | 0.024 | 22.62 |
| BC-200 | 200 | 83.70 | 63.63 | 5.15 | 0.40 | 30.81 | 0.49 | 0.48 | 0.08 | 1.72 | 0.008 | 18.63 |
| BC-400 | 400 | 23.60 | 74.97 | 3.09 | 1.07 | 20.87 | 0.29 | 0.28 | 0.04 | 304.18 | 0.176 | 2.31 |
| BC-600 | 600 | 21.50 | 79.49 | 2.18 | 0.42 | 17.91 | 0.23 | 0.23 | 0.03 | 521.29 | 0.322 | 2.47 |
| Soils | Adsorbent | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | q1 | R12 | p | k2 | q2 | R22 | p | ||
| min−1 | mg·g−1 | - | - | g·(mg·min)−1 | mg·g−1 | - | - | ||
| S1 | BC-600 + S1 | 9.41 | 2.73 | 0.924 | 1.13 × 10−10 | 0.016 | 2.81 | 1.000 | 0.025 |
| BC-400 + S1 | 13.40 | 2.41 | 0.751 | 1.82 × 10−8 | 0.006 | 2.66 | 0.999 | 0.023 | |
| BC-200 + S1 | 24.80 | 2.16 | 0.811 | 2.62 × 10−8 | 0.005 | 2.45 | 0.999 | 0.023 | |
| S1 | 36.60 | 2.17 | 0.759 | 2.33 × 10−7 | 0.003 | 2.58 | 0.998 | 0.022 | |
| S2 | BC-600 + S2 | 14.20 | 2.68 | 0.852 | 3.66 × 10−9 | 0.016 | 2.79 | 0.999 | 0.026 |
| BC-400 + S2 | 23.20 | 2.53 | 0.898 | 4.81 × 10−8 | 0.008 | 2.71 | 0.998 | 0.023 | |
| BC-200 + S2 | 22.50 | 2.34 | 0.708 | 7.77 × 10−8 | 0.009 | 2.59 | 0.999 | 0.022 | |
| S2 | 30.80 | 2.46 | 0.845 | 1.60 × 10−7 | 0.006 | 2.69 | 0.999 | 0.022 | |
| S3 | BC-600 + S3 | 12.00 | 2.75 | 0.922 | 2.68 × 10−10 | 0.012 | 2.85 | 0.999 | 0.025 |
| BC-400 + S3 | 21.10 | 2.58 | 0.945 | 1.45 × 10−8 | 0.008 | 2.71 | 0.998 | 0.023 | |
| BC-200 + S3 | 34.60 | 2.44 | 0.940 | 2.72 × 10−7 | 0.006 | 2.64 | 0.998 | 0.023 | |
| S3 | 32.80 | 2.50 | 0.895 | 5.41 × 10−7 | 0.007 | 2.69 | 0.999 | 0.021 | |
| Adsorbent | T | Langmuir Adsorption Model | Freundlich Adsorption Model | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm | KL | RL2 | p | n | KF | RF2 | p | ||
| °C | mg·g−1 | L·g−1 | - | - | - | L·g−1 | - | - | |
| Soil | 25 | 24.40 | 0.05 | 0.999 | 0.001 | 1.12 | 1.18 | 0.996 | 0.001 |
| 35 | 12.60 | 0.12 | 0.990 | 0.001 | 1.15 | 1.29 | 0.998 | 0.002 | |
| 45 | 65.80 | 0.01 | 0.981 | 0.004 | 0.58 | 0.25 | 0.933 | 0.007 | |
| Soil + BC-200 | 25 | 1.56 | 1.61 | 0.997 | 0.001 | 1.39 | 0.88 | 0.997 | 0.001 |
| 35 | 1.30 | 1.57 | 0.988 | 0.001 | 1.31 | 0.99 | 0.996 | 0.001 | |
| 45 | 52.60 | 0.04 | 0.999 | 0.003 | 1.04 | 2.04 | 0.999 | 0.008 | |
| Soil + BC-400 | 25 | 52.10 | 0.02 | 0.999 | 0.001 | 1.04 | 1.32 | 0.997 | 0.001 |
| 35 | −65.40 | −0.04 | 0.967 | 0.001 | 1.09 | 2.99 | 0.981 | 0.001 | |
| 45 | −1.98 | −0.13 | 0.827 | 0.012 | 0.58 | 0.25 | 0.933 | 0.021 | |
| Soil + BC-600 | 25 | −8.45 | −0.14 | 0.996 | 0.001 | 0.79 | 1.43 | 0.997 | 0.002 |
| 35 | −6.21 | −0.23 | 0.895 | 0.002 | 0.84 | 2.14 | 0.957 | 0.004 | |
| 45 | −10.40 | −0.18 | 0.927 | 0.004 | 0.94 | 2.51 | 0.968 | 0.009 | |
| T (°C) | ΔGθ (kJ/mol) | ΔHθ (kJ/mol) | ΔSθ (J/K × mol) |
|---|---|---|---|
| 25 | −1.86 | ||
| 35 | −2.30 | 5.84 | 0.02 |
| 45 | −2.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Yu, R.; Jiang, Y.; Liu, Z.; Wu, J.; Zhang, X.; Wang, G. Effect of Wheat Residue-Derived Biochar on Naphthalene Adsorption in Loess Soil in Northwest China. Processes 2023, 11, 3085. https://doi.org/10.3390/pr11113085
He R, Yu R, Jiang Y, Liu Z, Wu J, Zhang X, Wang G. Effect of Wheat Residue-Derived Biochar on Naphthalene Adsorption in Loess Soil in Northwest China. Processes. 2023; 11(11):3085. https://doi.org/10.3390/pr11113085
Chicago/Turabian StyleHe, Rui, Ruirui Yu, Yufeng Jiang, Zhewei Liu, Jiali Wu, Xiaozhen Zhang, and Gang Wang. 2023. "Effect of Wheat Residue-Derived Biochar on Naphthalene Adsorption in Loess Soil in Northwest China" Processes 11, no. 11: 3085. https://doi.org/10.3390/pr11113085
APA StyleHe, R., Yu, R., Jiang, Y., Liu, Z., Wu, J., Zhang, X., & Wang, G. (2023). Effect of Wheat Residue-Derived Biochar on Naphthalene Adsorption in Loess Soil in Northwest China. Processes, 11(11), 3085. https://doi.org/10.3390/pr11113085





