1. Introduction
Carbon capture is one of the critical paths in the carbon capture, utilization, and storage (CCUS) solution towards a sustainable transition to net zero emissions and to overcome the global warming issue. It is essential to develop a more efficient CO
2 capture process that is at the same time economically feasible to commercialize. In the oil and gas industry, natural gas extracted from the field can often feature elevated CO
2 concentrations. Consequently, it is imperative to manage the surplus CO
2 effectively while safeguarding the natural environment. Acid gases such as CO
2 and H
2S must be removed from natural gas to prevent pipeline corrosion, meet sales gas calorific values, avoid crystallization during liquefaction, and protect humans from exposure to highly toxic H
2S gas [
1,
2,
3,
4]. Several technologies for acid gas removal from natural gas have been developed and commercialized, including membrane separation, cryogenic distillation, and solvent-based absorption processes [
3,
5,
6].
Among these technologies, the acid gas absorption process using an amine-based solvent has been widely used in the oil and gas industries for decades. The chemical absorption technology demonstrates remarkably high separation efficiency (>90%) even at low carbon dioxide (CO2) partial pressure with minimal hydrocarbon loss. The effectiveness of the amine-based absorption process is primarily driven by the chemical reaction occurring between the acid gas and the specific type of solvent employed within the absorber.
Methyldietanolamine (MDEA) is commercially used as the solvent for the CO
2 absorption process due to its promising characteristics such as high absorption capacity, low regeneration energy, low degradation rate, and being less corrosive as compared with primary and secondary amines [
7,
8,
9]. However, the reaction rate between MDEA and acid gas such as CO
2 is slower as compared with the reaction between primary and secondary amines with CO
2, such as monoethanolamine (MEA) and diethanolamine (DEA) [
4,
8,
10,
11]. Due to this, piperazine (PZ), a cyclic diamine, is usually used with MDEA as the activator for CO
2 removal because of its rapid formation to form carbamate and bicarbamate with CO
2 [
5,
12]. The blended MDEA and PZ solvent (known as activated MDEA, or aMDEA) is usually employed to remove both CO
2 and hydrogen sulfide (H
2S) from natural gas during the sweetening process [
9,
13,
14]. The aMDEA solvent was developed in the 1970s and patented by BASF for the CO
2 absorption process, with a removal efficiency higher than the traditional MEA and potassium carbonate solvents. Besides, the solvent requires only one-third of the energy consumption of conventional Benfield technology [
14,
15,
16]. Aqueous aMDEA reacts directly with H
2S to form amine salt (methyldiethanolamine sulphide) as compared with CO
2, and it does not form carbamate [
9,
17]. Therefore, the presence of CO
2 will not affect the absorption of H
2S, even though the partial pressure of H
2S in the feed gas stream is much lower than CO
2.
Although this technology has been applied in acid gas sweetening for several decades, the conventional absorption column suffers several drawbacks, such as a high equipment corrosion rate, low CO
2 loading capacity, causing product off-spec, foaming, flooding, amine degradation, high energy consumption during regeneration, and excessive footprint and tonnage [
12,
18]. Ultrasonic irradiation is an emerging technology that can enhance gas-liquid mass transfer, enabling compact and lightweight designs, especially for offshore operations.
Ultrasonic irradiation technology has been studied widely to improve mass transfer for a wide variety of processes, such as biotechnology [
19], chemical reactions and processes [
20], wastewater treatment [
19,
21], microfluid systems [
22,
23], food technology [
24], crude oil upgrading [
25], and natural gas purification [
26]. Ultrasonic technology has been widely used to enhance the mass transfer process due to its chemical and physical effects on the liquid medium [
27]. The chemical effect of ultrasonic irradiation is also known as the sonochemistry effect, which is induced by the implosion of cavitation bubbles at high local pressure and temperature, causing the formation of free radicals [
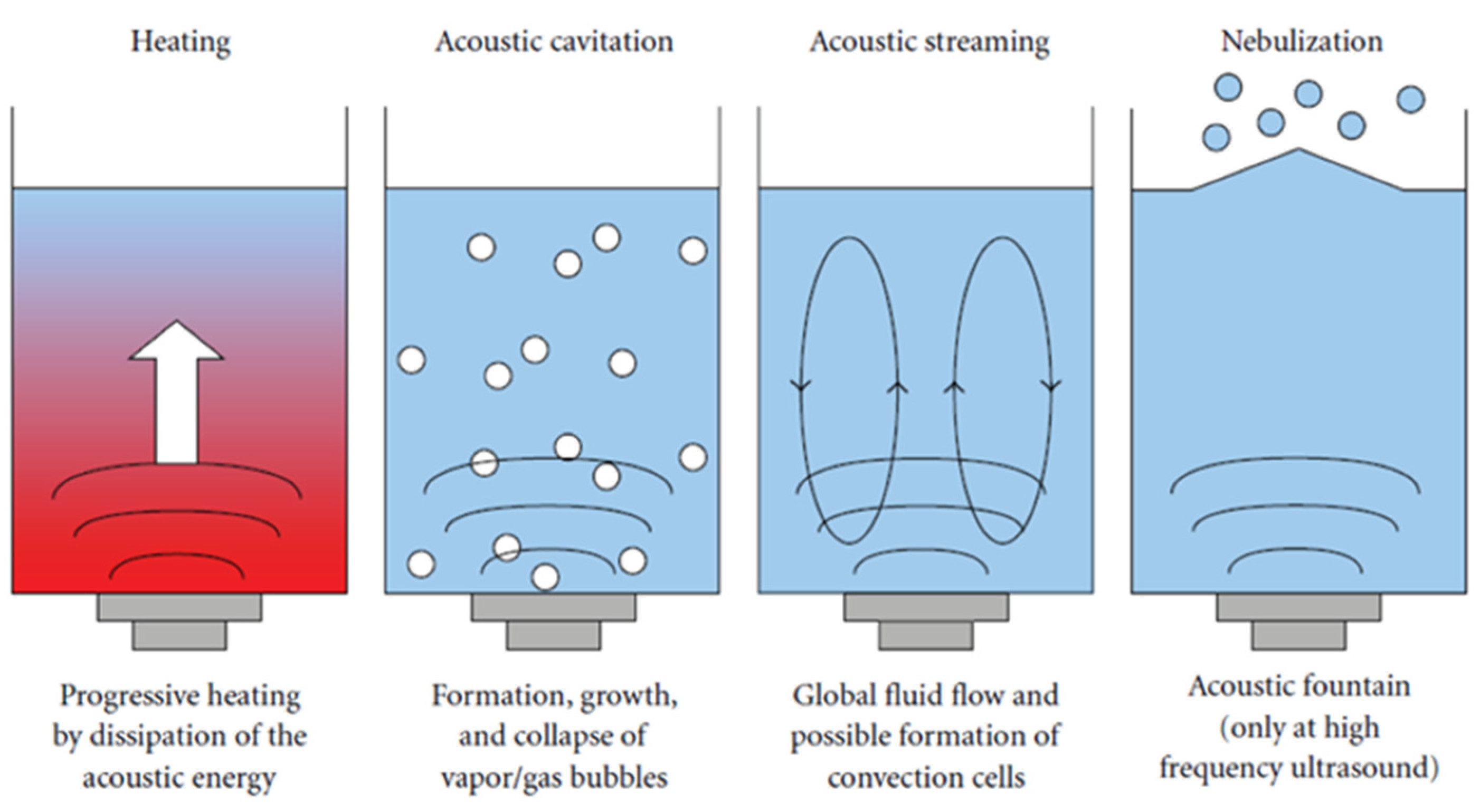
27]. These free radicals may enhance chemical reactions during the gas-liquid mass transfer process. Another phenomenon induced by ultrasonic irradiation in the liquid medium is the sonophysical effects, which refer to the formation of cavitation bubbles, acoustic streaming, an acoustic fountain, and atomization [
27].
Figure 1 shows the schematic diagram of the sonophysical effects of ultrasonic irradiation in the liquid medium. The sonochemistry and sonophysical effects induced by high-frequency ultrasonic waves enhance mixing, stimulate radical formation, and increase the available surface area for mass transfer. This, in turn, results in higher removal efficiency.
Several experimental studies have been conducted to confirm the effect of ultrasonic irradiation on mass transfer enhancement in the gas-liquid absorption process. Tay et al. (2016) [
29] investigated the effect of ultrasonic power up to 18 W on the CO
2 absorption process. Based on the experimental results, it was observed that the formation of an acoustic fountain and liquid atomization generated from a high ultrasonic power source can amplify the mass transfer absorption process by up to 80 times. Acoustic streaming provides better gas-liquid mixing, while an acoustic fountain increases the gas-liquid interfacial area for mass transfer. M. Yusof et al. (2019) [
27] have conducted a continuous experimental study utilizing 1.7 MHz ultrasonic waves to assist CO
2 absorption in distilled water. The effect of gas flow rate, liquid flow rate, pressure, and voltage on absorption performance has been studied, and numerical optimization using Design Expert software has been conducted. At the optimum condition, the mass transfer coefficient of the continuous ultrasonic contactor system surpasses that of other contacting equipment, including bubble columns, stirred vessels, and counter-current-packed columns.
Akbari et al. (2017) [
30] also conducted an experimental study to investigate the impact of 1.7 MHz ultrasound waves on micro-scale T-shaped glass microreactor gas-liquid contactors. Their research encompassed both physical and chemical absorption of CO
2 into deionized water and sodium hydroxide solutions, respectively. The findings from their study revealed a significant enhancement in mass transfer coefficient when ultrasound waves were utilized as compared with the silent system at various operating conditions.
Numerical simulation has gained prominence in the advancement of ultrasonic-assisted gas-liquid mass transfer technology. This approach offers a comprehensive understanding of the distinctive flow behaviors induced by high-frequency ultrasound waves, such as mixing, cavitation, and heat and mass transfer mechanisms [
31,
32,
33]. For instance, Abolhasani et al. (2012) conducted a numerical study on the effect of high-frequency ultrasonic waves on the heat transfer rate [
34]. The simulation results revealed that the 1.7 MHz ultrasound wave fostered enhanced fluid mixing and the generation of high-velocity flows, resulting in a more uniformly distributed temperature in a vessel. Moreover, the simulation findings were validated against experimental data, illustrating a robust agreement between the CFD simulation and the actual temperature measurements recorded during the experiments.
Another study led by Parvizian et al. (2012) used the CFD model to predict the complex flow pattern induced by the 1.7 MHz transducers in a novel ultrasound reactor [
35]. The outcomes of their CFD simulation have shown the superior performance of the sonoreactor, which exhibited a notably more uniform velocity distribution in contrast to a traditional stirred tank operating at equivalent power consumption. The formation of a jet stream capable of improving liquid mixing can be induced by high-frequency ultrasound waves, even with lower power consumption.
Xu et al. (2016) introduced a numerical model to predict the formation of acoustic fountains by taking into account the influence of surface tension and ultrasound radiation pressure [
36]. With this model, they predicted that the area of the acoustic fountain increased with the increase in ethanol concentration in the liquid medium. This effect stems from ethanol’s lower surface tension, density, and sound speed. Furthermore, numerical modeling has also been developed to determine the mass transfer coefficient for ultrasonic-assisted gas-liquid mass transfer. Researchers frequently employ the dynamic method of gassing-in and the logarithmic expression of the instantaneous pressure method to derive the mass transfer coefficient from experimental data, as previously demonstrated in multiple studies [
29,
37].
Based on previous experimental works, the effectiveness of the ultrasonic-assisted CO2 absorption process has been well established, particularly in batch operations. Yet, for further advancement of this technology toward commercialization, a central challenge lies in cost reduction, encompassing both capital and operational expenses. Therefore, a comprehensive economic analysis is imperative to pinpoint the primary cost components of ultrasonic irradiation technology, enabling further cost optimization. One of the critical criteria for the economic analysis is equipment sizing, which will determine the capital expenditure (CAPEX) of the technology. However, the numerical model to predict the effective volume of the ultrasonic absorber is still lacking in the literature.
To the best of our knowledge, no literature to date has reported on the process simulation and economic analysis for the ultrasonic-assisted CO2 absorption process. This paper presents a numerical model to predict the effective volume and energy consumption of the ultrasonic absorber using data from experimental studies. The predicted effective volume and energy consumption were further employed in process simulation to facilitate the economic comparison analysis between the ultrasonic-assisted CO2 absorption system and conventional packed bed columns. Data from the process simulation was also used to estimate the operating expenditure (OPEX) for 20 years of operation. Finally, the Unit Technical Cost (UTC) was estimated to determine the economic feasibility of the ultrasonic absorber as compared with the conventional packed bed column.
4. Process Simulation and Economic Analysis
Economic analysis was carried out to evaluate the potential of ultrasonic-assisted CO2 absorption technology against conventional packed columns. Process simulation was carried out using Aspen HYSYS V10 software to estimate the CAPEX and OPEX for both technologies. The economic evaluation was conducted by activating the costing engine in the Aspen HYSYS software. By activating the economic module, the process simulation results were automatically transferred to the Aspen Capital Cost Estimator (ACCE) for further mapping of unit operations to equipment and equipment sizing using default-assigned mapping and sizing algorithms.
4.1. Process Simulation
The process simulation for CO
2 absorption was conducted for two (2) cases. The first case uses the conventional packed column as the acid gas removal unit (AGRU), and the second uses the ultrasonic absorber to replace the AGRU. The case study for process simulation was based on the typical offshore natural gas sweetening process facility that contains 19.22 mol% of CO
2 in the feed gas stream with a target removal efficiency of 83.4% for bulk CO
2 removal. Detailed information on this case study is listed in
Table 3.
The same feed gas and product gas specifications are used for the packed column and ultrasonic absorber process simulations. The performance of the AGRU unit was simulated using the Amine property package. The solvent circulation rate was determined based on the L/G. The L/G for the conventional packed column was obtained from the heat and material balance of the case study process plant, which was 193 gal/lbmol CO2 removed. For the ultrasonic absorber, the L/G was determined from the experimental run. In the process simulation, the equipment installed weight and cost were determined, together with the total energy from the pumps, heater, and cooler in the overall acid gas removal process. Other information that was obtained from the process simulation includes the total amount of CO2 captured, the total amount of solvent, and the water top-up in the amine regeneration process. This information would be utilized to estimate the OPEX.
The process flow diagram for the process simulation is shown in
Figure 4. The process consists of three main unit operations, which are the AGRU (packed column or ultrasonic absorber), high-pressure (HP) flash vessel, and low-pressure (LP) flash vessel. The acid gas absorption process occurs at the AGRU to remove the CO
2 in the feed gas from 19.22% to 3.2% and other components as provided in
Table 2. This absorption process occurs at high pressure (59.8 barg), and the pressure drop across the AGRU was assumed to be less than 1 bar. The rich amine solvent leaving the AGRU was sent to the HP flash vessel and Coalescer operating at 8–9 barg to recover the hydrocarbon loss. The rich amine was regenerated by heating the solvent to 90–119 °C to release the acid gas from the amine at the LP Flash vessel operating at 1 barg. The regeneration temperature was adjusted to meet the target regenerated lean amine of 0.2 mol CO
2/mol amine loading. The regenerated amine was cooled down using cooling water in a shell and tube heat exchanger and recycled back to the AGRU. Since the ultrasonic absorber unit was not readily available in the Aspen HYSYS software, the efficiency of the absorption column unit, including the number of trays, was tuned to match the ultrasonic absorber’s experimental performance.
4.2. CAPEX Estimation
The installed cost and weight for all unit operations in the process simulation were based on the Icarus technology, except for the AGRU (packed column and ultrasonic absorber). The size of the conventional packed column was obtained from the absorber column data sheet of the case study.
Table 4 summarizes the details of the packed column.
For the ultrasonic absorber, the effective volume was determined numerically, as explained in
Section 2.2. The total volume was assumed to be 30% more than the calculated effective volume as a result of contingencies. The ultrasonic absorber weight was estimated based on a typical column density of 2.04 tonne/m
3, and the installed weight was assumed to be 30% more than the equipment weight.
The CAPEX for the CO
2 absorption process was the sum of all equipment installed costs, including the structure cost for offshore operations, with a 30% contingency. For the AGRU, the equipment cost was estimated based on a commercial absorber column price in 1999, and the value is updated to the year 2021 using the Chemical Engineering Plant Cost Index (CEPCI), where [
56]:
The cost index for 1999 and 2021 was 390.6 and 750, respectively [
57]. The cost of the AGRU was then calculated using the cost-capacity equation [
58]:
where
was the capacity of the commercial absorber column and
was the capacity of AGRU, for which the cost was to be determined in this study.
defines the relationship between the capacities, where in this study the value was 0.6. This value indicates that larger capacity was less costly due to economies of scale.
For the ultrasonic absorber equipment cost, the cost of the ultrasonic transducers was added to the cost of the AGRU column. Due to confidentiality, the number and unit price of the ultrasonic transducers will not be disclosed. The installed cost for the AGRU was assumed to be 30% higher than the equipment cost due to additional engineering, insurance, and administration costs [
56].
The structure cost for the offshore operation was estimated based on the total equipment installed weight, including the piping, instruments, and solvent weight. Piping and instrument weight were assumed to be 50% of the equipment installed weight. The total solvent weight was estimated by assuming that 50% of the vessel’s volume was filled with liquid. An additional 10% of the solvent weight was also included for the solvent weight in the piping during operation. The structure cost was calculated based on a typical commercial cost for a jacketed leg platform, which was USD 36 k/tonne.
4.3. OPEX Estimation
The OPEX for the CO
2 absorption process, which consists of electricity cost, initial solvent cost, solvent top-up, water top-up, maintenance cost, and transducer change-out cost (only for ultrasonic absorbers), was calculated yearly. The OPEX was estimated for the total lifetime of the plant, which was assumed to be 20 years, and the total annual hours of operation for the plant in this study was 8000 h/year [
11,
56]. The maintenance cost was assumed to be 5% of the total equipment installed cost.
The electricity cost was estimated from the total energy consumption for the CO2 absorption process obtained from the Aspen HYSYS process simulation. The electricity generated by the gas turbine was assumed to power the pump, heater, and ultrasonic absorber. The energy required for the plant operation was converted to power consumption costs by multiplying the fuel gas consumption rate by the sales gas price of 3 USD/MMBTU.
Another important expenditure for the plant is the initial cost of the solvent and the annual solvent top-up cost. The initial solvent cost was estimated by multiplying the total solvent weight with the commercial solvent price of 4350 USD/tonne. Over the course of continuous operation, the removal efficiency of the plant will decrease due to solvent degradation, the formation of heat-stable salts, and the accumulation of contaminants. Yearly amine change-out at a rate of 4% from its initial cost was recommended to maintain the CO2 removal performance. Besides, water top-up was also required to maintain the solvent concentration and viscosity at the optimum condition. Some of the water might evaporate during the solvent regeneration process and need to be replaced. The rate of water top-up was determined from the HYSIS process simulation, and the yearly cost was estimated based on the commercial demineralized water cost of RM10.10/m3.
For the ultrasonic absorber, there was another additional cost to be considered, which was the transducer change-out cost. Over time, some transducers, springs, and o-rings might wear and tear. Therefore, scheduled maintenance and replacements must be conducted to maintain the transducer’s performance. Due to this, a yearly change-out at the rate of 7.5% from the initial cost of the transducer was proposed starting in the 2nd year of the operation.
4.4. Unit Technical Cost (UTC) Calculation
Unit technical cost (UTC) was defined as the cost incurred for removing a ton of CO
2 based on 20 years of operation. The amount of CO
2 removed was determined from the process simulation. The total cost was inclusive of CAPEX and OPEX. The UTC can be calculated as follows [
51,
59]:
6. Conclusions
In this work, a numerical model was developed to predict the effective volume and energy consumption of the ultrasonic absorber based on experimental studies at various operating conditions. The effects of ultrasonic power and liquid and gas flow rates were investigated. Findings from the experimental studies and numerical modeling confirmed that Run 4, which operated at 25 V ultrasonic power, 0.2 LPM liquid flow rate, and 17 SLPM gas flow rate, is the most optimum condition for the ultrasonic-assisted CO2 absorption process because the energy consumption at this condition was the lowest, which was 26.04 MW with an effective volume of 222.66 m3. The economic analysis was conducted to compare the economic feasibility of this technology against the conventional packed bed column.
This technology demonstrates a potential 34% reduction in CAPEX compared with the conventional packed bed column. This is attributed to its ingeniously compact design, resulting in decreased equipment weight and lower structural costs, which is particularly advantageous for offshore operations. While the ultrasonic transducers impose an additional electricity demand, leading to an 11% increase in OPEX relative to the packed column, the overall UTC for the ultrasonic-assisted CO2 absorption process remains 3% below that of the conventional packed column.
Therefore, it is concluded that there is a potential cost savings in implementing ultrasonic irradiation technology to improve the existing packed column for acid gas removal. To further maximize the economic advantages of this technology, energy optimization, such as heat integration, water and solvent recovery systems, and transducer power system optimization, is recommended for future work.