Analysis of the Effects of Surfactants on Extracellular Polymeric Substances
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Characterization of EPS
2.1.1. Medium Formulation
2.1.2. Sample Preparation
2.1.3. EPS Yield and Compositional Identification Methods
2.2. Structure and Size Analysis of EPS under SDS Stress
2.2.1. Fourier Transform Infrared Spectral Analysis
2.2.2. Particle Size Analysis
2.2.3. Fluorescence Spectral Analysis
2.2.4. Three-Dimensional Fluorescence Spectral Analysis
3. Results
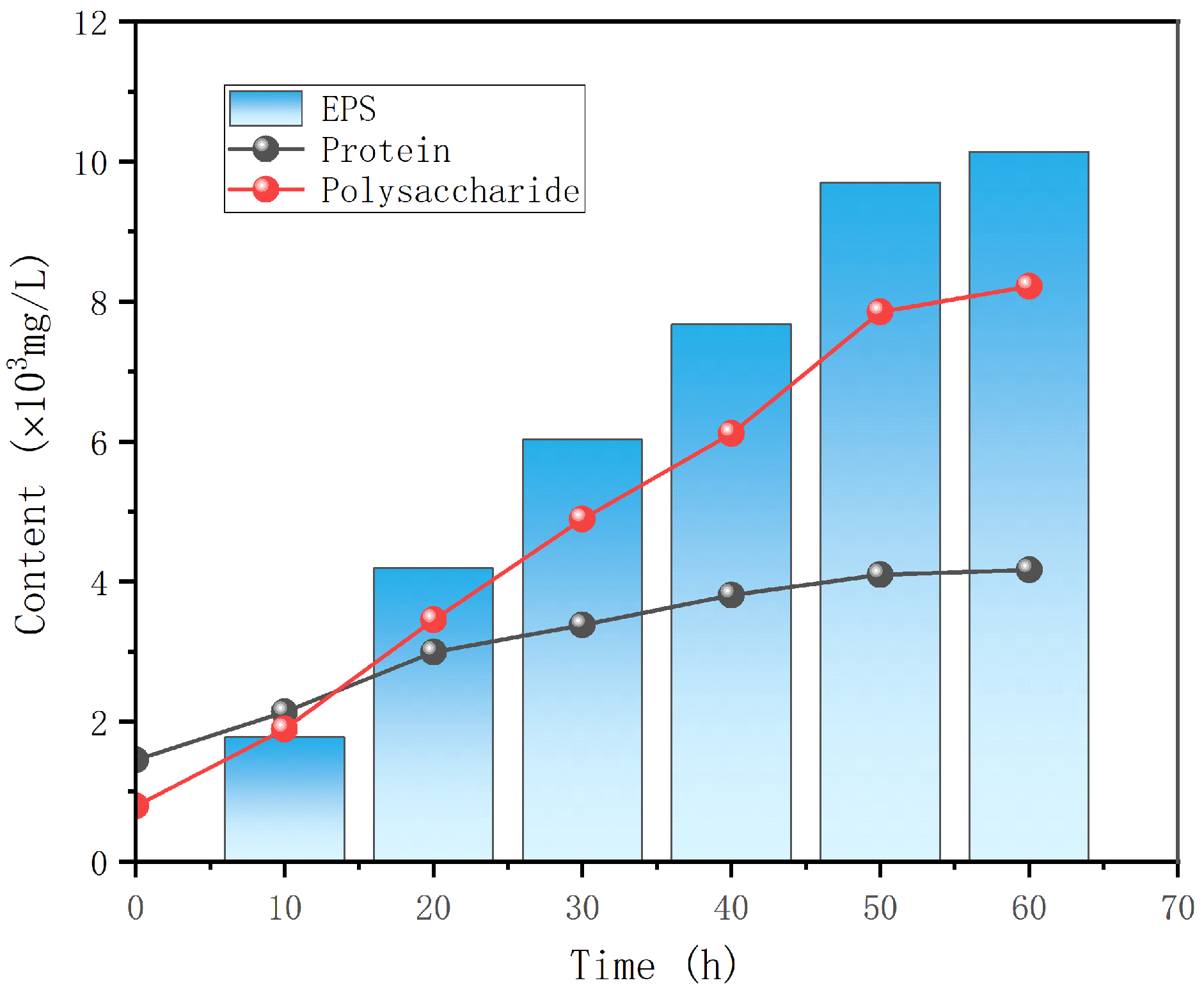
3.1. Composition and Yield
3.1.1. EPS Component Identification
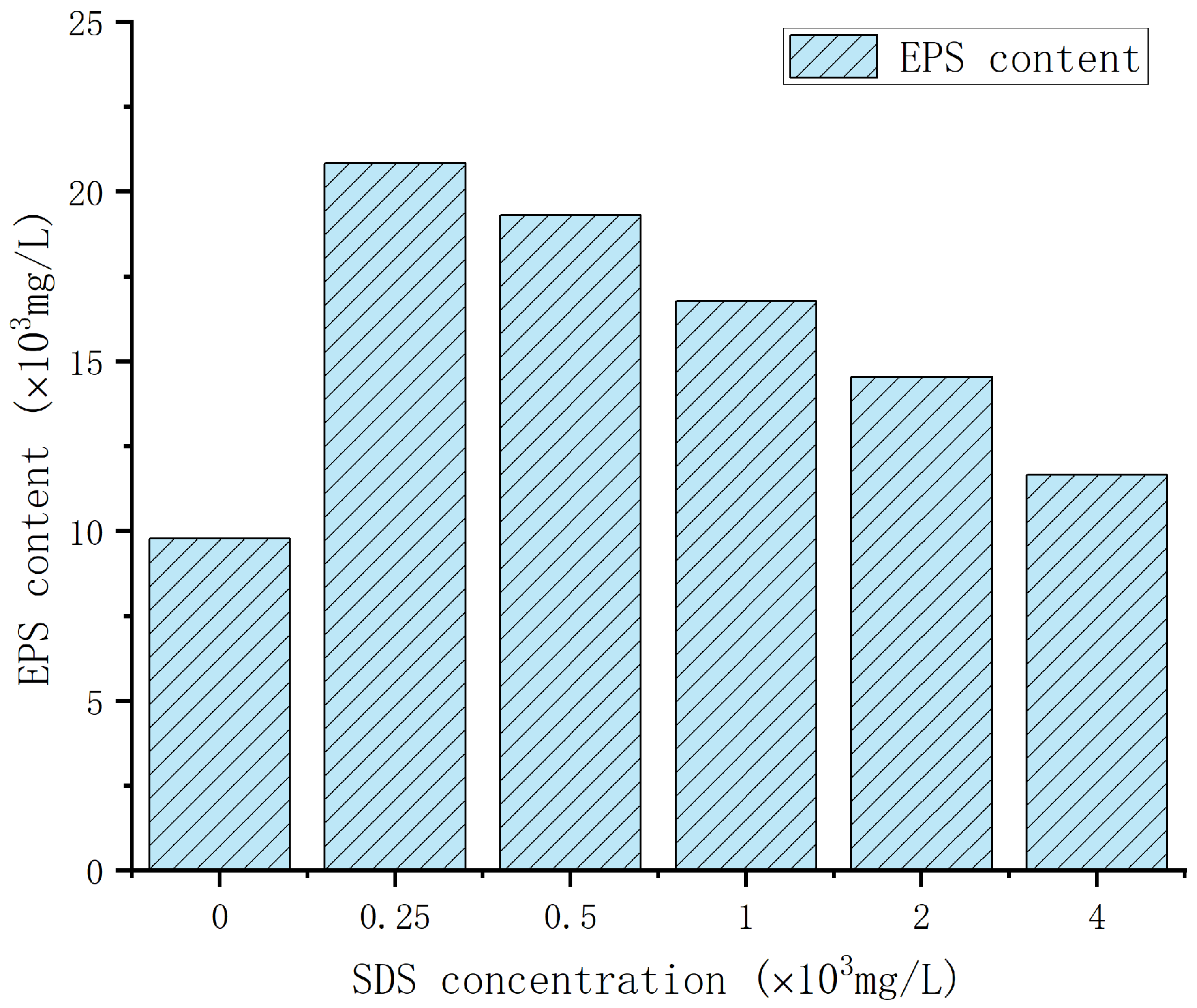
3.1.2. Analysis of EPS Yield of Enterobacter Cloacae under Different Concentrations of SDS Stress
3.2. Changes in EPS under the Influence of SDS
3.2.1. EPS Functional Group Changes under the Influence of SDS
3.2.2. Particle Size Analysis of EPS under SDS Stress
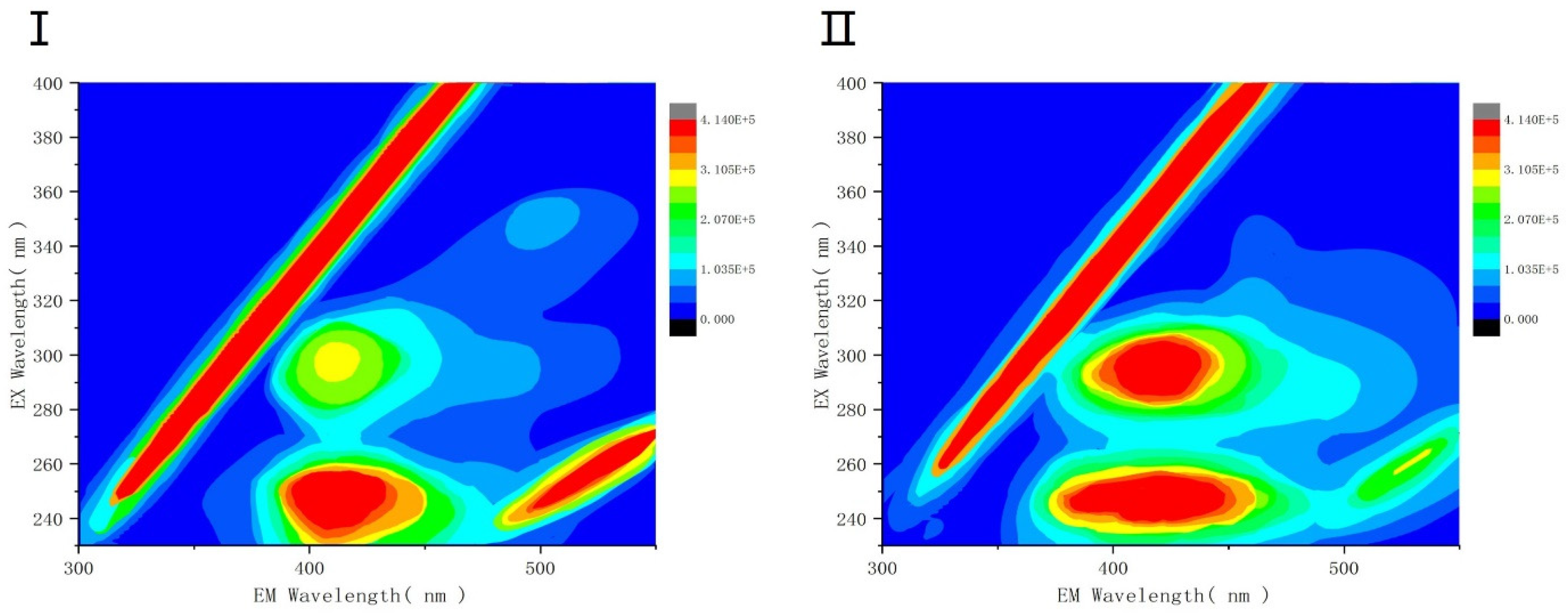
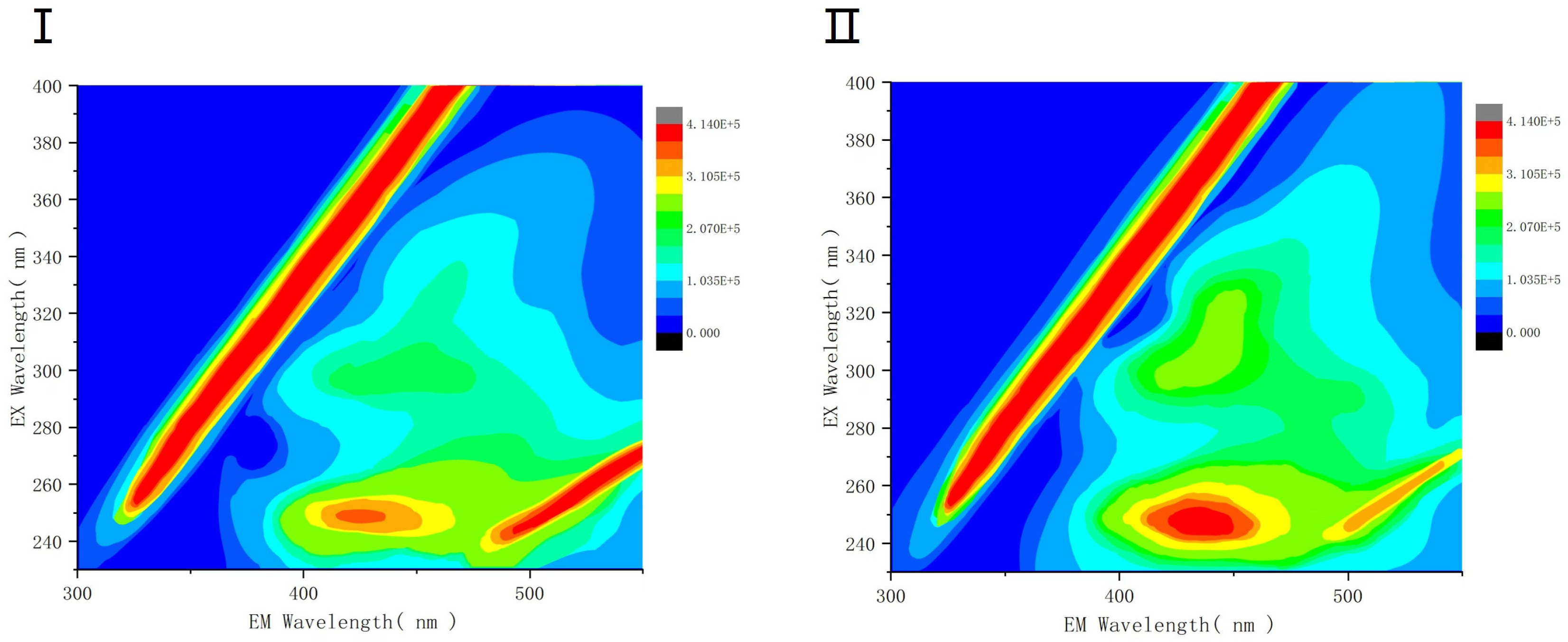
3.2.3. Protein Fluorescence Intensity Analysis of EPS under SDS Stress
3.2.4. Changes in Protein Results of under the Stress of SDS
4. Discussion
4.1. Surfactant Stimulation of Enterobacter cloacae Membrane Mechanism
4.2. Prediction of the Entanglement Pattern between EPS Protein and Surfactant
4.3. Changes in Protein Components in Different Forms of EPS
5. Conclusions
- (1)
- The EPS was used as the research object, and the total production of EPS without surfactant stimulation was the highest 10.14 × 103mg/L, of which the polysaccharide content was 7.42 × 103 mg/L, and the protein content was 2.72 × 103 mg/L. The addition of surfactant could induce the leakage of EPS from the intracellular to the extracellular, and EPS production reached a maximum of 20.83 × 103 mg/L at the concentration of SDS of 2.5 × 103 mg/L.
- (2)
- The particle size of EPS, as well as its functional groups, changed under SDS stimulation conditions. After centrifugation, the laser particle size increased from d (0.5) = 70.912 μm to a particle size of d (0.5) = 117.24 μm. Most of the absorption peaks (3394, 1659, and 1402 cm−1) showed a substantial increase in peak intensity and an increase in the content of functional groups. The increased content of amide and polysaccharide (3394, 1659, 1402 cm−1) indicates that a large number of proteins and polysaccharides were converted from insoluble to soluble state under the action of surfactant. The content of carboxyl groups increased dramatically and significantly and were hydrolyzed to carboxylic acids and carboxylates.
- (3)
- By protein fluorescence spectroscopy, it was concluded that the fluorescence intensity of EPS under the influence of surfactant SDS at a concentration of 1 × 103 mg/L were lower than that of the blank group, and the process occurred as a fluorescence bursting effect, suggesting that a conjugate was generated, and so the interaction of the three EPS with SDS was further combined with the 3D-EEM technique, which showed that tryptophan-like, humic acid-like, and humic acid-like species were present in the EPS, aromatic compounds, and protein-like proteins. Tryptophan- and protein-like substances were detected in the three EPS components, whereas humic acid-like substances were distributed only in SL-EPS and aromatic-like proteins were present only in LB-EPS and TB-EPS. It was demonstrated that SDS surfactant had a greater effect on SL-EPS than on other types of EPS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patel, J.; Borgohain, S.; Kumar, M.; Rangarajan, V.; Somasundaran, P.; Sen, R. Recent Developments in Microbial Enhanced Oil Recovery. Renew. Sustain. Energy Rev. 2015, 52, 1539–1558. [Google Scholar] [CrossRef]
- Haddad, H.; Khaz’ali, A.R.; Mehrabani-Zeinabad, A.; Jazini, M. Investigating the Potential of Microbial Enhanced Oil Recovery in Carbonate Reservoirs Using Bacillus Persicus. Fuel 2023, 334, 126757. [Google Scholar] [CrossRef]
- Yunita Halim, A.; Marie Nielsen, S.; Eliasson Lantz, A.; Sander Suicmez, V.; Lindeloff, N.; Shapiro, A. Investigation of Spore Forming Bacterial Flooding for Enhanced Oil Recovery in a North Sea Chalk Reservoir. J. Pet. Sci. Eng. 2015, 133, 444–454. [Google Scholar] [CrossRef]
- Xu, W.; Song, X.; Qu, Q.; Gong, Z.; Xiao, W. Synergistic Effects of l-Theanine and Epigallocatechin Gallate in Alleviating Ovalbumin Allergy by Regulating Intestinal Immunity through Inhibition of Mast Cell Degranulation. Food Funct. 2023, 14, 2059–2073. [Google Scholar] [CrossRef]
- Van Hamme, J.D.; Ward, O.P. Physical and Metabolic Interactions of Pseudomonas sp. Strain JA5-B45 and Rhodococcus sp. Strain F9-D79 during Growth on Crude Oil and Effect of a Chemical Surfactant on Them. Appl. Environ. Microbiol. 2001, 67, 4874–4879. [Google Scholar] [CrossRef]
- Li, M.; Yu, J.; Cao, L.; Yin, Y.; Su, Z.; Chen, S.; Li, G.; Ma, T. Facultative Anaerobic Conversion of Lignocellulose Biomass to New Bioemulsifier by Thermophilic Geobacillus Thermodenitrificans NG80-2. J. Hazard. Mater. 2023, 443, 130210. [Google Scholar] [CrossRef]
- Liu, S.; Peng, X.; Yang, H.; Zhang, X.; Qu, Y.G.; Li, J.; Xu, H.; Xie, T. A Novel Iron Biomineralization on Basaltic Rocks from the Challenger Deep, Southern Mariana Trench. Chem. Geol. 2023, 635, 121617. [Google Scholar] [CrossRef]
- Pal, A.; Paul, A.K. Microbial Extracellular Polymeric Substances: Central Elements in Heavy Metal Bioremediation. Indian J. Microbiol. 2008, 48, 49–64. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Hu, G.; Zhang, C.; Shen, T.; Liu, S.; Zhou, Y. Impacts of Surfactant Stress on the Growth of Four Kinds of Bacteria. Environ. Sci. Surv. 2021, 40, 1–4+46. [Google Scholar] [CrossRef]
- Groot, R.D.; Rabone, K.L. Mesoscopic Simulation of Cell Membrane Damage, Morphology Change and Rupture by Nonionic Surfactants. Biophys. J. 2001, 81, 725–736. [Google Scholar] [CrossRef]
- Berlin, E.; Lizano-Fallas, V.; Carrasco Del Amor, A.; Fresnedo, O.; Cristobal, S. Nonionic Surfactants Can Modify the Thermal Stability of Globular and Membrane Proteins Interfering with the Thermal Proteome Profiling Principles to Identify Protein Targets. Anal. Chem. 2023, 95, 4033–4042. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Cheng, J.; Wang, J.; Cui, L.; Zhang, Y.; Liao, W.; Liu, Z.; Zhou, H.; Wu, X.; Li, J.; et al. Comparison of Extraction Methods for Extracellular Polymeric Substances (EPS) and Dynamic Characterization of EPS from Sessile Microorganisms during Pyrite Bioleaching. J. Environ. Chem. Eng. 2022, 10, 107922. [Google Scholar] [CrossRef]
- Mahata, C.; Dhar, S.; Ray, S.; Das, D. Flocculation Characteristics of Anaerobic Sludge Driven-Extracellular Polymeric Substance (EPS) Extracted by Different Methods on Microalgae Harvesting for Lipid Utilization. Biochem. Eng. J. 2021, 167, 107898. [Google Scholar] [CrossRef]
- Han, J.; Li, H.; Liu, Y.; Liu, P.; Song, Y.; Wang, Y.; Zhang, L.; Wang, W. Extraction of Extracellular Polymeric Substances (EPS) from Indigenous Bacteria of Rare Earth Tailings and Application to Removal of Thorium Ions (Th4+). Water Sci. Technol. 2023, 87, 83–98. [Google Scholar] [CrossRef]
- Wu, L.; Gao, Y.; Ren, W.; Su, Y.; Li, J.; Du, Y.; Wang, Q.; Kuang, H. Rapid Determination and Origin Identification of Total Polysaccharides Contents in Schisandra Chinensis by Near-Infrared Spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 264, 120327. [Google Scholar] [CrossRef]
- Kielkopf, C.L.; Bauer, W.; Urbatsch, I.L. Bradford Assay for Determining Protein Concentration. Cold Spring Harb. Protoc. 2020, 2020, 102269. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, Y.; Xiao, K.-Q.; Gao, C.-H.; Wang, S.; Zhu, D.; Wu, Y.; Huang, Q.; Cai, P. Characterising Soil Extracellular Polymeric Substances (EPS) by Application of Spectral-Chemometrics and Deconstruction of the Extraction Process. Chem. Geol. 2023, 618, 121271. [Google Scholar] [CrossRef]
- Azadi, G.; Chauhan, A.; Tripathi, A. Dilution of Protein-surfactant Complexes: A Fluorescence Study. Protein Sci. 2013, 22, 1258–1265. [Google Scholar] [CrossRef]
- Huang, H.; Pan, J.; Spielberg, D.R.; Hanchard, N.A.; Scott, D.A.; Burrage, L.C.; Dai, H.; Murdock, D.; Rosenfeld, J.A.; Mohammad, A.; et al. A Dominant Negative Variant of RAB5B Disrupts Maturation of Surfactant Protein B and Surfactant Protein C. Proc. Natl. Acad. Sci. USA 2022, 119, e2105228119. [Google Scholar] [CrossRef]
- Chen, W.; Westerhoff, P.; Leenheer, J.A.; Booksh, K. Fluorescence Excitation−Emission Matrix Regional Integration to Quantify Spectra for Dissolved Organic Matter. Environ. Sci. Technol. 2003, 37, 5701–5710. [Google Scholar] [CrossRef]
- Bevia, V.; Calatayud, J.; Cortés, J.-C.; Jornet, M. On the Generalized Logistic Random Differential Equation: Theoretical Analysis and Numerical Simulations with Real-World Data. Commun. Nonlinear Sci. Numer. Simul. 2023, 116, 106832. [Google Scholar] [CrossRef]
- Palomares-Navarro, J.J.; Bernal-Mercado, A.T.; González-Aguilar, G.A.; Ortega-Ramirez, L.A.; Martínez-Téllez, M.A.; Ayala-Zavala, J.F. Antibiofilm Action of Plant Terpenes in Salmonella Strains: Potential Inhibitors of the Synthesis of Extracellular Polymeric Substances. Pathogens 2022, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, C.; Zhou, G.; Che, G.; You, J.; Suo, Y. Determination of the Carbohydrates from Notopterygium Forbesii Boiss by HPLC with Fluorescence Detection. Carbohydr. Polym. 2013, 97, 794–799. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Xiao, M.; Li, S.; Chen, R.; Li, Y.; Yang, Y. Systematic Analysis and Case Series of the Diagnosis and Management of Trichilemmal Carcinoma. Front. Oncol. 2023, 12, 1078272. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.D.; Subramanian, S. Thermodynamics of Protein Association Reactions: Forces Contributing to Stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Anamul Hoque, M.; Abdul Rub, M.; Majibur Rahman, M.; Abdullah Khan, M.; Kumar, D.; Asiri, A.M. Micellization, Interaction and Thermodynamics Behavior of BSA + SDS Mixture in Aqua-Organic Mixed Solvent: Influences of Temperature and Solvent Composition. J. Mol. Liq. 2021, 344, 117770. [Google Scholar] [CrossRef]
- Ravi, S.G.; Shadizadeh, S.R.; Moghaddasi, J. Core Flooding Tests to Investigate the Effects of IFT Reduction and Wettability Alteration on Oil Recovery: Using Mulberry Leaf Extract. Pet. Sci. Technol. 2015, 33, 257–264. [Google Scholar] [CrossRef]
- Meng, F.; Lin, W. Application of three-dimensional fluorescence technique in the detection of sarcosine oxidase of active protein. Chin. J. Anal. Lab. 2021, 40, 1257–1264. [Google Scholar] [CrossRef]
- Zhu, L.; Qi, H.; Lv, M.; Kong, Y.; Yu, Y.; Xu, X. Component Analysis of Extracellular Polymeric Substances (EPS) during Aerobic Sludge Granulation Using FTIR and 3D-EEM Technologies. Bioresour. Technol. 2012, 124, 455–459. [Google Scholar] [CrossRef]
- Peng, J.; Wen, K.; Liu, W.; Yue, X.; Wang, A.; Zhou, A. EPS Solubilization and Waste Activated Sludge Acidification Enhanced by Alkaline-Assisted Bi-Frequency Ultrasonic Pretreatment Revealed by 3D-EEM Fluorescence. RSC Adv. 2016, 6, 80493–80500. [Google Scholar] [CrossRef]
- Tatsumi, T.; Imai, Y.; Kawaguchi, K.; Miyano, N.; Ikeda, I. Antimicrobial Activity of Cationic Gemini Surfactant Containing an Oxycarbonyl Group in the Lipophilic Portion against Gram-Positive and Gram-Negative Microorganisms. J. Oleo Sci. 2014, 63, 137–140. [Google Scholar] [CrossRef] [PubMed]
- Salama, Y.; Chennaoui, M.; Sylla, A.; Mountadar, M.; Rihani, M.; Assobhei, O. Characterization, Structure, and Function of Extracellular Polymeric Substances (EPS) of Microbial Biofilm in Biological Wastewater Treatment Systems: A Review. Desalination Water Treat. 2016, 57, 16220–16237. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, D.; Yang, T.; Zhang, Z.; Xu, W.; Liu, H.; Li, W. Head-to-Head Comparison of High-Performance Liquid Chromatography versus Nuclear Magnetic Resonance for the Quantitative Analysis of Carbohydrates in Yiqi Fumai Lyophilized Injection. Molecules 2023, 28, 765. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Lau, D. Molecular Dynamics Study on Stiffness and Ductility in Chitin-Protein Composite. J. Mater. Sci. 2015, 50, 7149–7157. [Google Scholar] [CrossRef]








| Time (min) | A% | B% |
|---|---|---|
| 1 | 4 | 96 |
| 14 | 35 | 65 |
| 16 | 60 | 40 |
| 112 | 60 | 40 |
| 112.1 | 5 | 95 |
| SDS (mM) | Micelle | Fl (Calculated Value) | FI (Experimental Value) | % Error |
|---|---|---|---|---|
| 15 | 1.13 × 1020 | 1.67 × 106 | 1.67 × 106 | — |
| 10 | 7.53 × 1019 | 1.11 × 106 | 1.13 × 106 | 1.12 |
| 12 | 6.02 × 1019 | 12.92 × 105 | 12.122 × 105 | 1.1 |
| 6 | 4.52 × 1019 | 6.69 × 10⁵ | 6.60 × 105 | 1.4 |
| Metabolite | Ninhydrin Color Development | Phenol-Sulfuric Acid Color Development |
|---|---|---|
| supernatant | no color change | reddish brown |
| biosurfactant | no color change | reddish brown |
| Peak Position cm−1 | Functional Group |
|---|---|
| 1402.27 | δ(C-H), δ(OH) |
| 1265.32 | δ(C-O-C) |
| 1153.45 | υ(C=O) |
| 2164.168 | υ(C-H) |
| 2990.68 | υ(C=O) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zheng, X.; Lai, D. Analysis of the Effects of Surfactants on Extracellular Polymeric Substances. Processes 2023, 11, 3212. https://doi.org/10.3390/pr11113212
Zhang H, Zheng X, Lai D. Analysis of the Effects of Surfactants on Extracellular Polymeric Substances. Processes. 2023; 11(11):3212. https://doi.org/10.3390/pr11113212
Chicago/Turabian StyleZhang, Hongyu, Xuecheng Zheng, and Dongmin Lai. 2023. "Analysis of the Effects of Surfactants on Extracellular Polymeric Substances" Processes 11, no. 11: 3212. https://doi.org/10.3390/pr11113212
APA StyleZhang, H., Zheng, X., & Lai, D. (2023). Analysis of the Effects of Surfactants on Extracellular Polymeric Substances. Processes, 11(11), 3212. https://doi.org/10.3390/pr11113212





