Significance of Pressure Drop, Changing Molar Flow, and Formation of Steam in the Accurate Modeling of a Multi-Tubular Fischer–Tropsch Reactor with Cobalt as Catalyst
Abstract
:1. Introduction
- (1)
- The velocity of the gas us in the tubes was assumed as constant, which was an (over)simplification.
- (2)
- The pressure drop, which affects the reaction rate and gas velocity (and, thus, residence time), was only calculated separately but not considered in the (as yet) isobaric reactor model.
- (3)
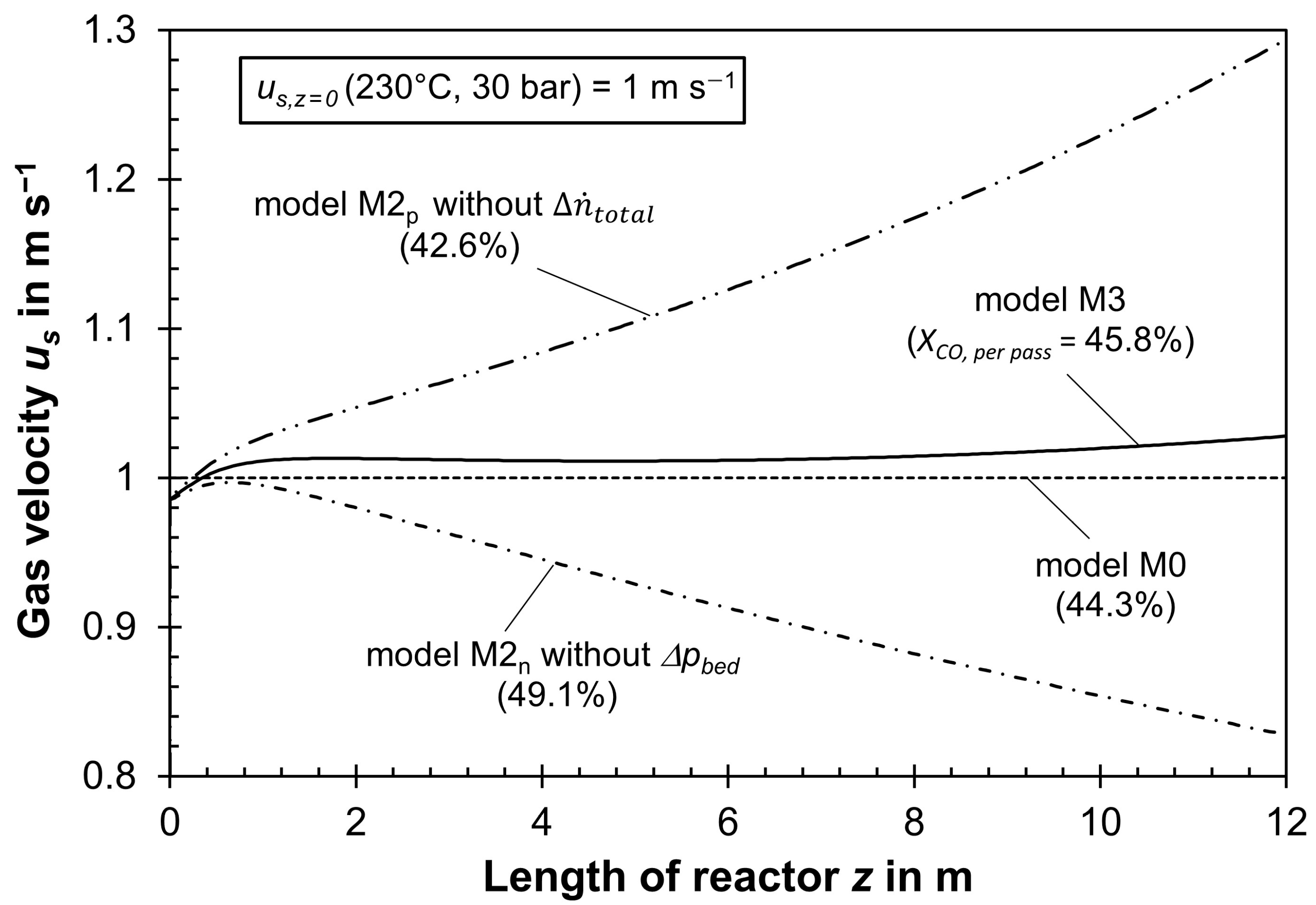
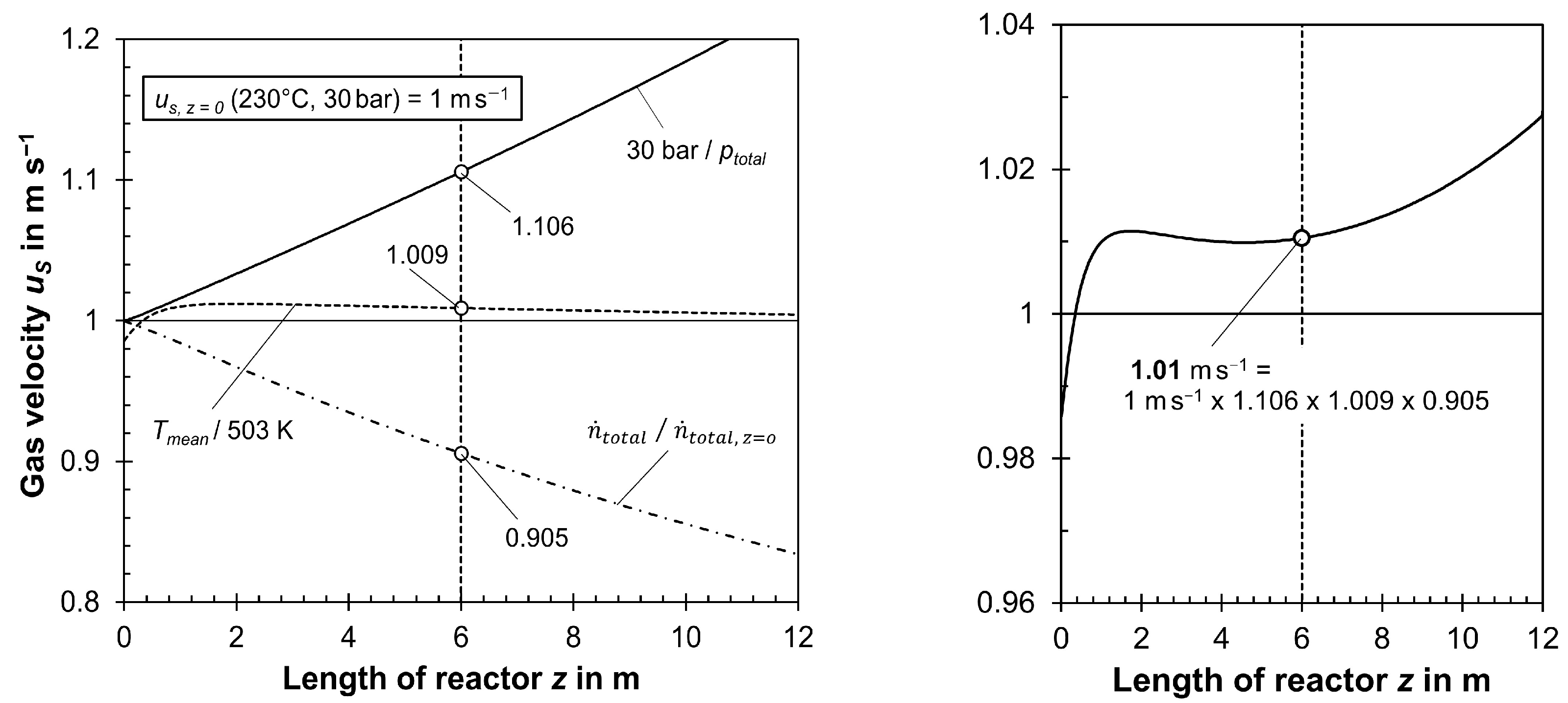
- The decline in the total molar flow in the tubes via FT reaction—about 20% for a CO conversion of 50% (see Section 3.2)—was neglected, although this, accordingly, reduces gas velocity, increases residence time, and, through this—as explained descriptively—the conversion (to be precise, a lower molar flow raises the residual concentrations of CO and H2 and, thus, the reaction rate and conversion compared to disregarding this effect).
- (4)
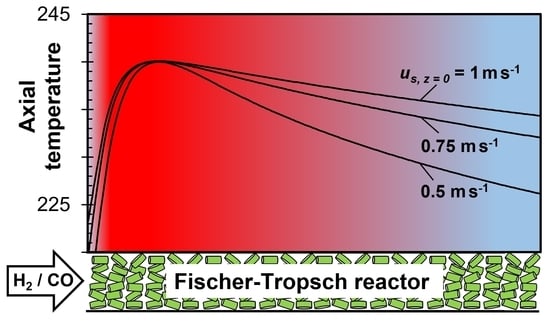
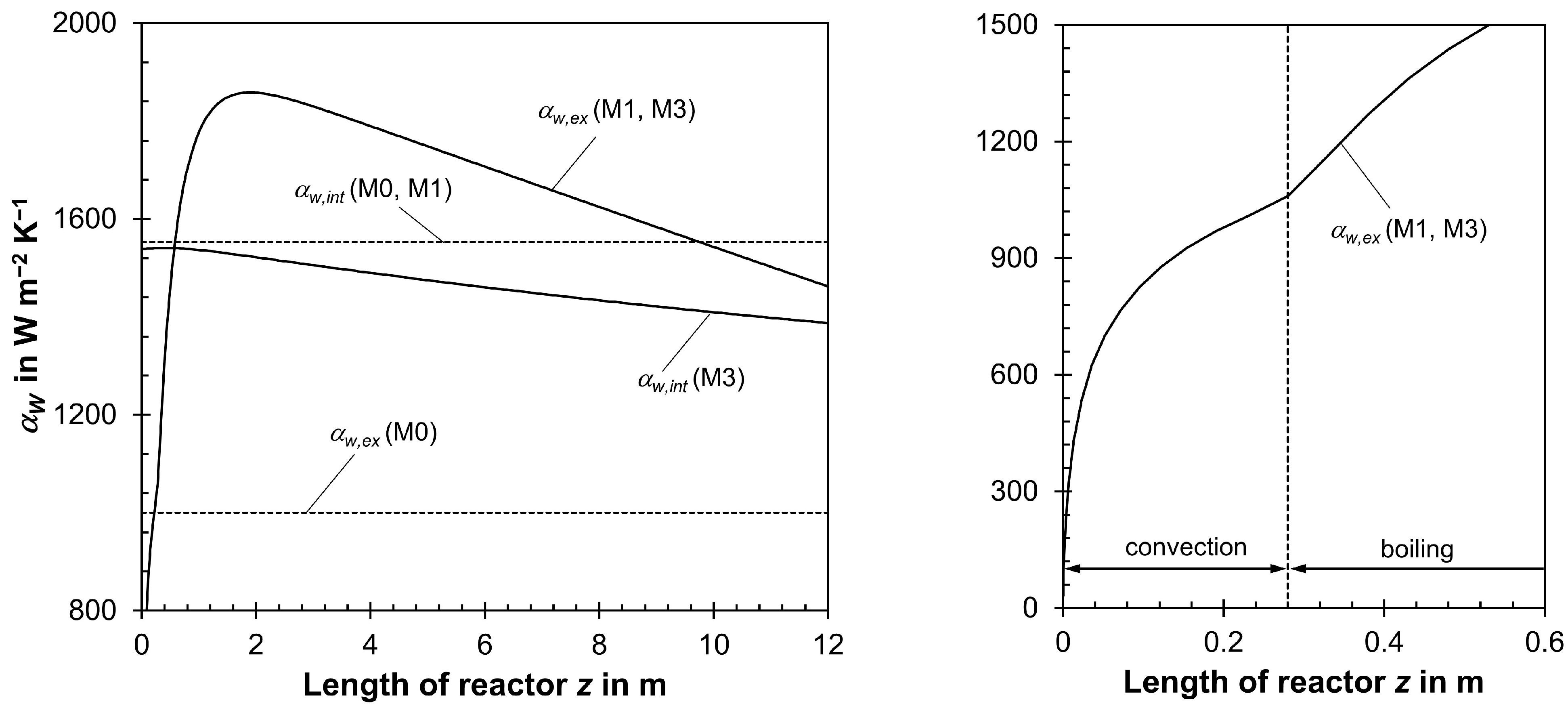
- The heat transfer coefficient αw,ex (external tube wall to boiling water) was assumed to be constant, but this depends on the pressure and temperature of the boiling water and—even more importantly—on the local value of the radial heat flux. Hence, αw,ex varies along the tubes, and a maximum is located at the maximum of the axial temperature.
- (5)
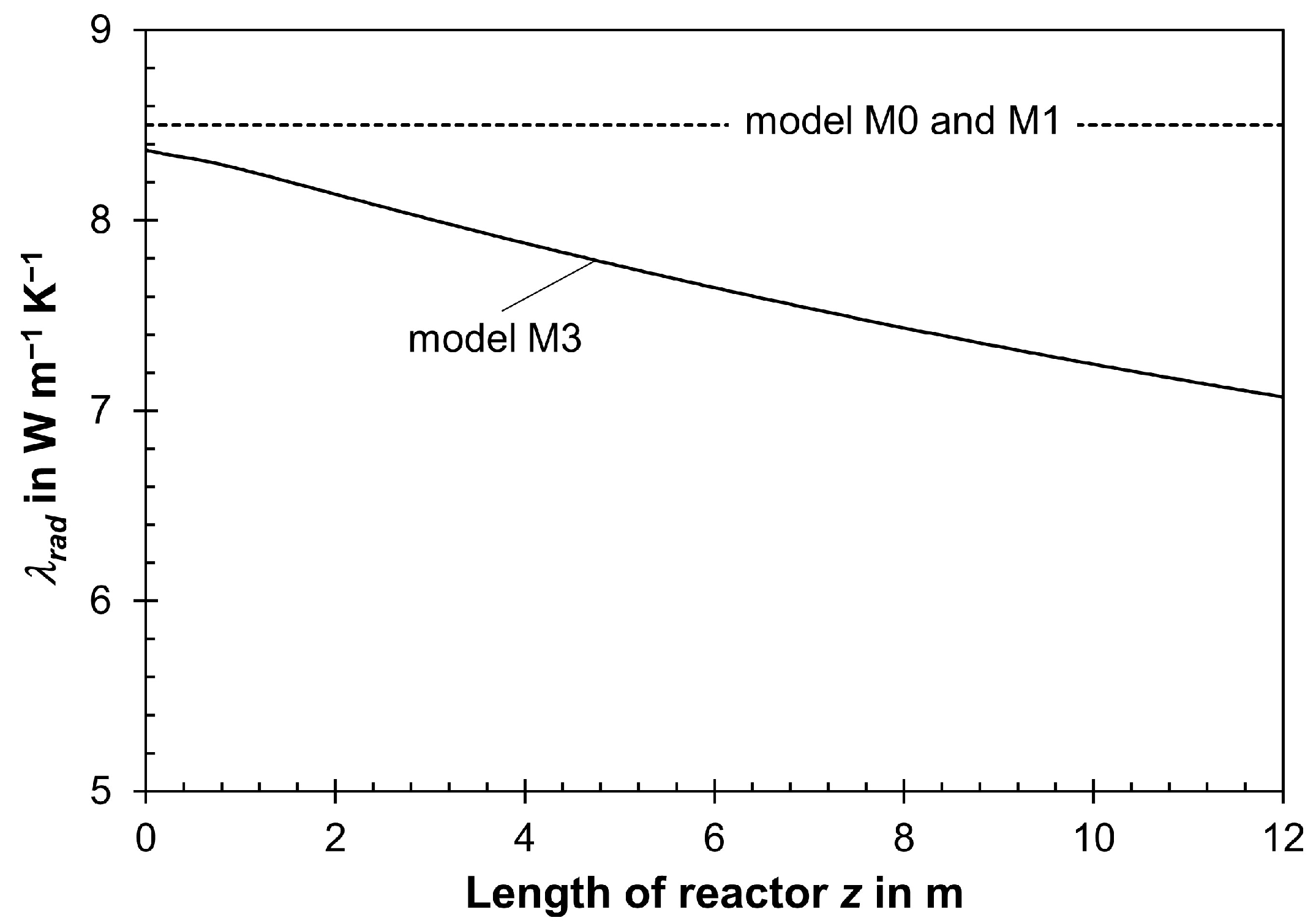
- The effective radial thermal conductivity λrad and, also, the heat transfer coefficient on the internal side of the wall αw,int depend on us, but they were calculated simply based on the initial value of us, which was assumed to be constant and, hence, fixed in each model calculation. In reality, a change in gas velocity in the axial direction, induced by a pressure drop and/or a decrease in total molar flow from the reaction, changes both λrad and αw,in in the axial direction in the tubes.
- (6)
- The kinetic equations for the rate of CO conversion neglected any inhibiting influence of steam on the activity of the co-catalyst, which is not true for a high partial pressure of steam, particularly in the rear part of the tubes. Based on our experience, the influence is almost negligible for pH2O < 5 bar, but it may be relevant if a higher value is reached [3].
2. Objectives and Methodology
2.1. Intrinsic and Effective Reaction Kinetics of Fischer–Tropsch Synthesis (FTS)
2.2. Models (Examined in This Work) of a Multi-Tubular FT Reactor Cooled by Boiling Water
- ○
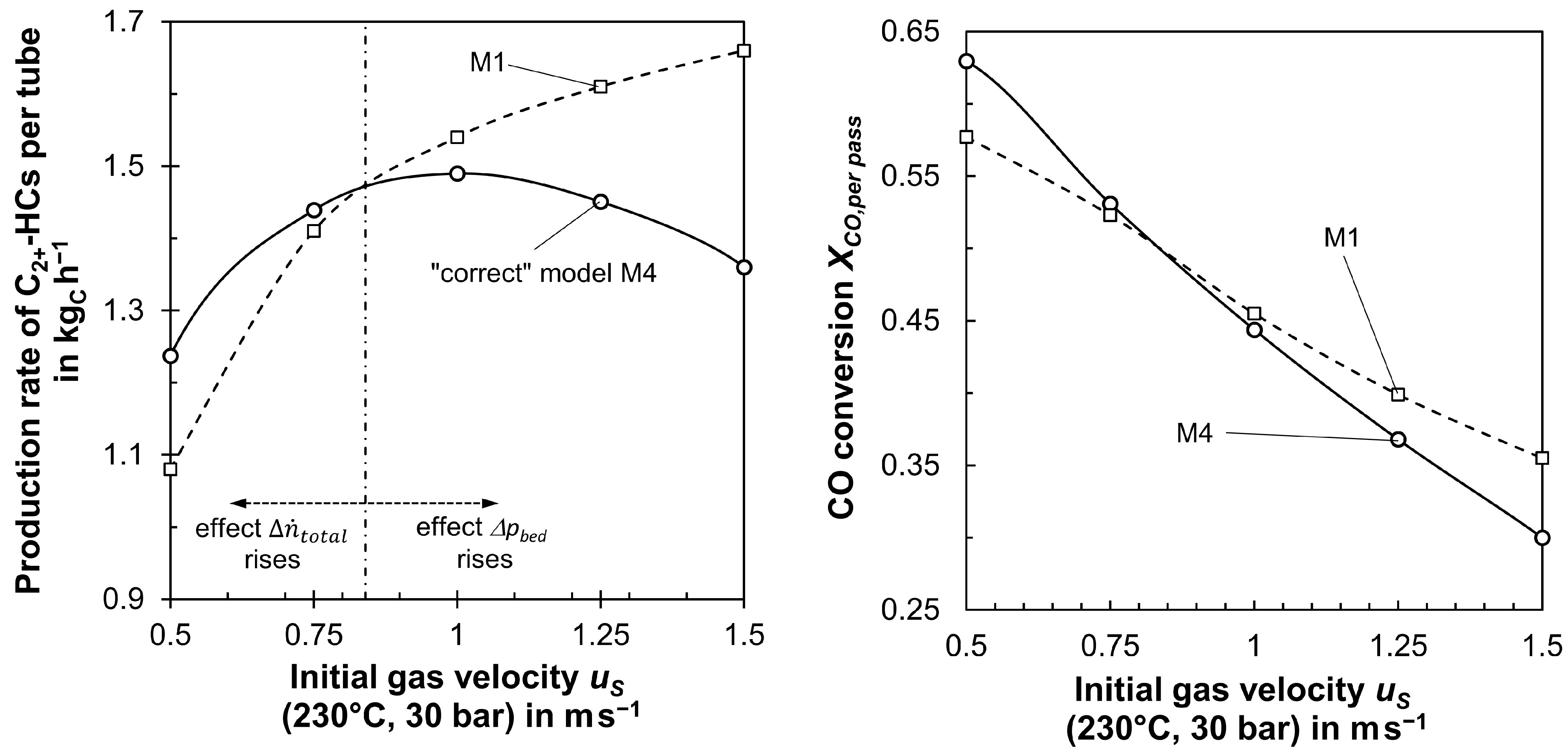
- If ∆pbed is low (low us,z=0), us drops along the tubes and, in return, the residence time (compared to constant us) increases by the then dominating effect of the drop in the total molar flow rate. This, in return, has an influence on the local values (axial direction) of αw,int and λrad. In total, XCO,per pass is then higher compared to a model (as M0 or M1, Table 2) simplified assuming a constant us.
- ○
- If ∆pbed is high (high us,z=0), this yields an increase in us in axial direction (decrease in residence time compared to constant us), as the drop in us by the decreasing total molar flow rate is then overcompensated; this again has an impact on the local values of αw,ex and λrad, and, in total, XCO,per pass is then lower compared to a model assuming constant us.
- ○
- For a “medium-sized” ∆pbed (medium-sized us,z=0), we may obtain an almost constant gas velocity along the tubes, i.e., the opposite influence of ∆pbed and drop in total molar flow on us cancel each other out. In this rare case, a model with or without considering both aspects coincidentally yields similar results with regard to XCO,per pass or production rate of C2+-HCs.
3. Results of Simulation of a Single Tube of a Cooled Multi-Tubular FT Reactor
3.1. Influence of External Heat Transfer Coefficient αw,ex on Reactor Modeling
3.2. Influence of Pressure Drop and Change in Total Molar Gas Flow on Reactor Modeling
3.3. Influence of Inhibition of Steam on the Performance of an FT Reactor
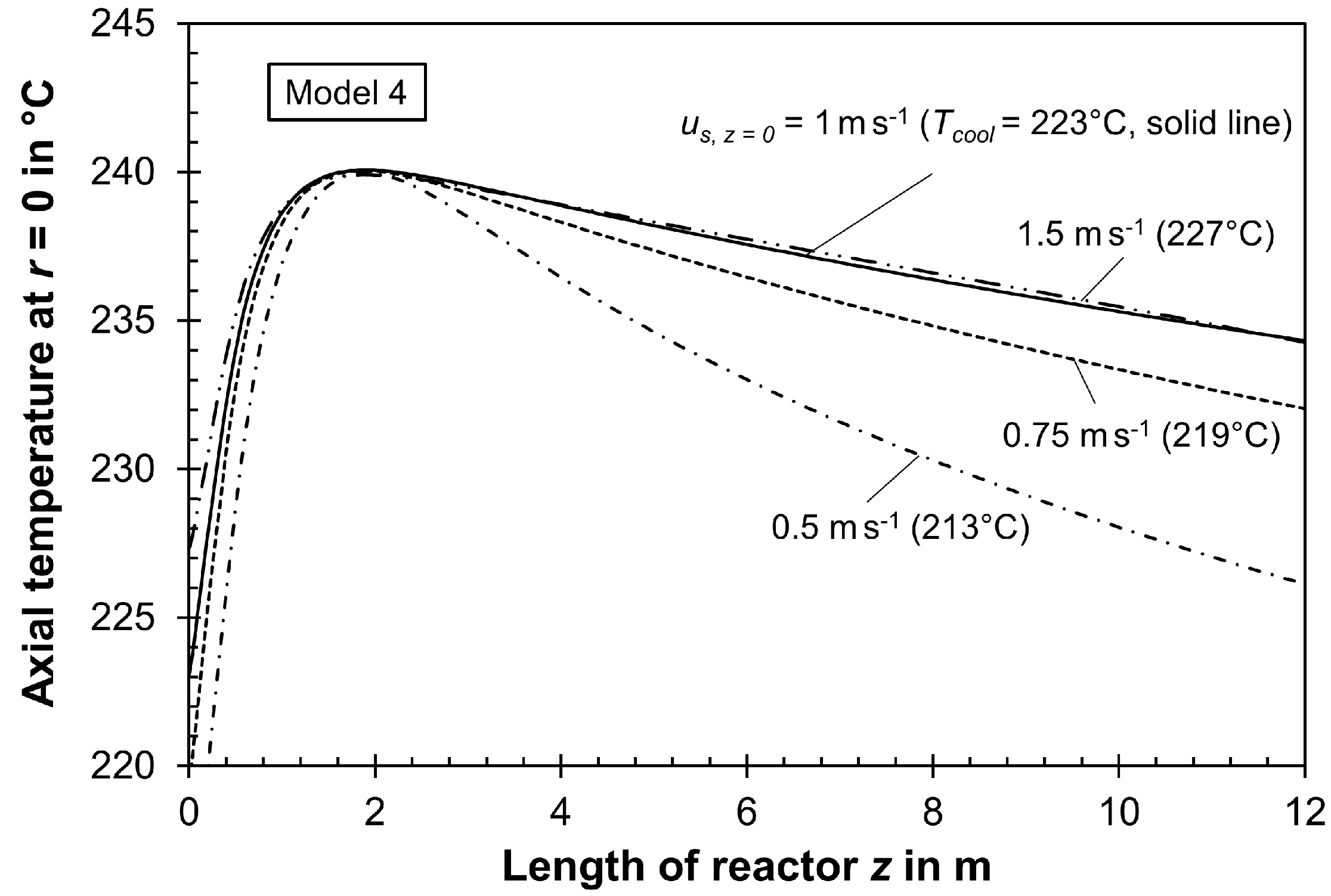
3.4. Impact of Gas Velocity on Modeling an FT Reactor if Pressure Drops, Change in Total Molar Flow Rate, and Inhibition of Reaction Rate by Steam Are Correctly Considered
- ○
- Figure S8 depicts the impact of us,z=0 on the axial profile of the total pressure in the tubes.
- ○
- Figure S9 shows the influence of us on the rate of heat removal from fixed bed to boiling water.
- ○
- ○
- Figure S11 shows the influence of us,z=0 on the axial profile of αw,int, and Figure S12 the corresponding figure for λrad in the bed of the tubes.
- ○
- Figure S13 depicts temperature profiles at different radial positions, and Figure S14 presents a selected radial temperature profile for z = 2 m (location of maximum in temperature).
- ○
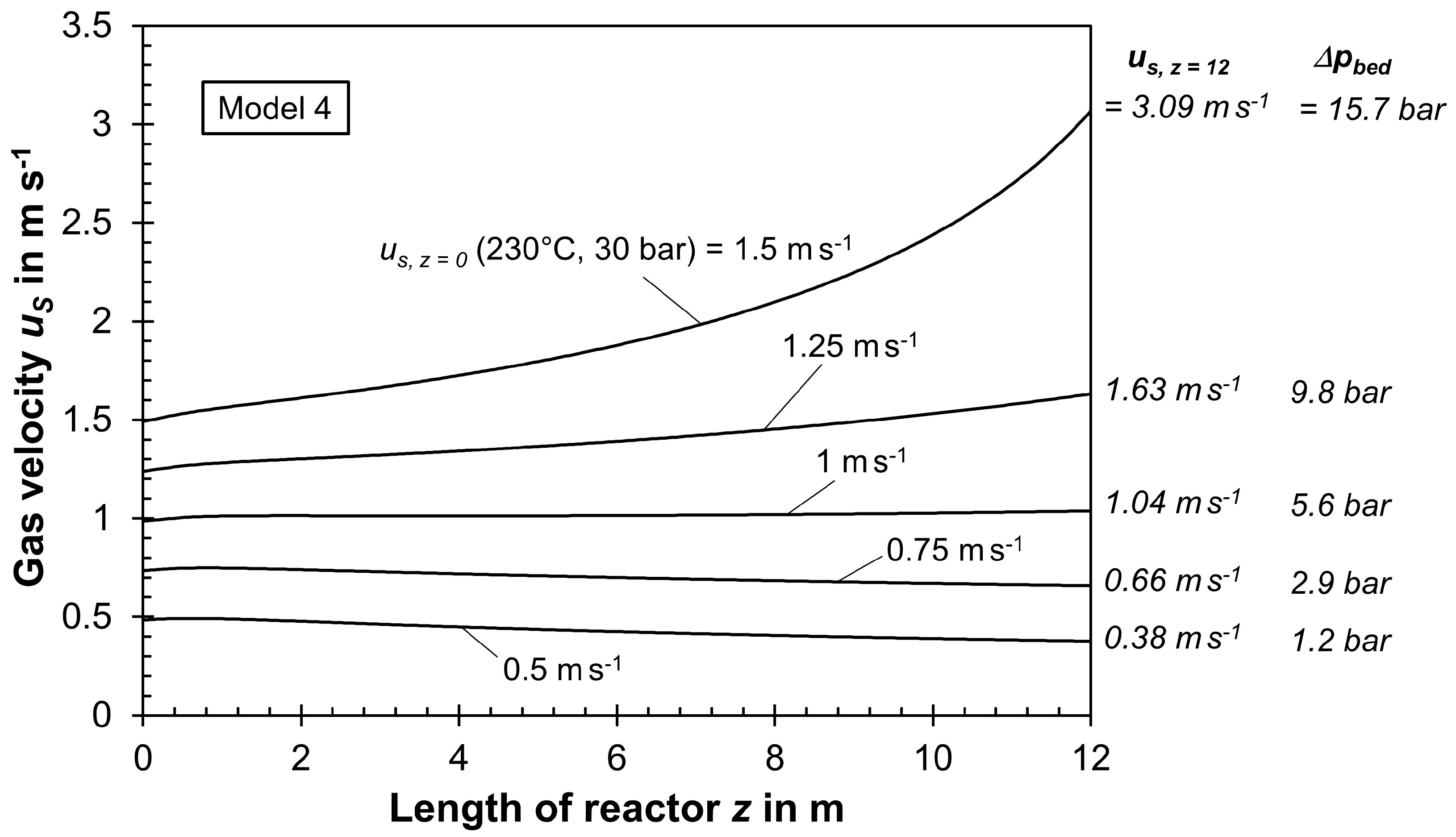
- Figure S15 depicts the influence of us,z=0 on pressure drop and final gas velocity us,z=12m, indicating a strong rise both in ∆pbed and us,z=12m with increasing us,z=0.
- ○
- Axial profiles of the radial heat fluxes in the tubes (heat removal, heat production, and heat flux from/to gas) are given by Figure S16.
- ○
- The parametric sensitivity of the FT reactor with and without influence of pore diffusion is also discussed in the Supporting Information (Figures S17 and S18), which explains in detail that pore diffusion “helps” with regard to thermal stability of a fixed-bed FT reactor.
- ○
- Table S1 compares an FT reactor with constant activity (Ca = 3; simulation by “optimal” model M4) with a two-zone reactor (Ca = 2.5 for z < 6 m and 3.5 for 6 m < z < 12 m) and a reactor with optimal activity distribution (Ca,mean = 3). The data indicate that the output of C2+-HCs can be improved by 4% and 8%, respectively.
- ○
- Axial profiles of ηpore (r = 0) for us (230 °C, 30 bar) of 0.5 and 1 m/s are shown in Figure S19; selected values at different temperatures are listed in Table S2.
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| A | Cross-sectional area of tube (π rt2) | m2 |
| cCO | Concentration of CO | mol m−3 |
| Ca | Coefficient of catalytic activity | |
| cCO | Concentration of CO (gas phase) | mol m−3 |
| cg | Total concentration (molar density) of gas phase | mol m−3 |
| cp | Heat capacity of gas | J mol−1 K−1 |
| Cϕ | Constant factor in Equation (6) (valid for dp = 3 mm) | kg0.5 s0.5m−1.5 |
| dp | Particle diameter | m |
| dt,int | Internal tube diameter | m |
| Deff,CO,liq | Effective diffusion coefficient of CO in liquid filled pore system | m2 s−1 |
| fbed | Friction factor of a packed bed of spherical particles | |
| HCO | Henry coefficient for CO in liquid HCs | J mol−1 |
| Lt | Length of tube (fixed bed) | m |
| Mg | Molar mass of gas mixture | kg mol−1 |
| pfinal | Total pressure at outlet of tubes | Pa |
| ptotal | Total pressure (inlet of tubes) | Pa |
| pH2O | Partial pressure of steam | Pa |
| Pr | Prandtl number (=νg cg cp/λg) | |
| Total molar flux of gas in the tubes | mol s−1 | |
| r | Radial coordinate in fixed bed (radial distance from center of tube) | m |
| rm,CO | Total intrinsic reaction rate of CO, see Equation (3) | molCO kgcat−1 s−1 |
| rm,CO,H2O | Total intrinsic rate of CO, if inhibition by steam is considered | molCO kgcat−1 s−1 |
| rm,CO,CH4 | Intrinsic reaction rate of CO to of methane | molCO kgcat−1 s−1 |
| rm,C2+ | Intrinsic reaction rate of CO to C2+-hydrocarbons | molCO kgcat−1 s−1 |
| rm,CO,eff | Total effective reaction rate of CO | molCO kgcat−1 s−1 |
| rm,CO,H2O,eff | Total effective rate of CO, if steam inhibition is considered | molCO kgcat−1 s−1 |
| rt | Internal radius of tube | m |
| R | Gas constant (8.314) in Equation (6) | J mol−1 K−1 |
| R | Recycle ratio (ratio of recycle gas to fresh syngas) | |
| Rbed | Thermal resistance related to heat conduction (≈0.25 dt,int/λrad) | m2 K W−1 |
| Rep | Reynolds number related to particle diameter (=us dp/νg) | |
| Roverall | Overall thermal resistance (Rbed + Rw,int + Rth,ex,total) | m2 K W−1 |
| Rth,ex.total | Thermal resistance of wall (conduction) and boiling water | m2 K W−1 |
| Rth,H2O | Thermal resistance of boiling water (convection) | m2 K W−1 |
| Rth,wall | Thermal resistance of wall (conduction) | m2 K W−1 |
| Rw,int | Thermal resistance related to heat transfer at internal wall (1/αw,int) | m2 K W−1 |
| swall | Thickness of tube wall | m |
| SCH4 | Selectivity to methane related to carbon (in CO) | |
| T | Temperature of gas and catalyst (pseudo-homogeneous model) | °C, K |
| Tcool | Cooling temperature (constant along the tube) | °C, K |
| Tig | Cooling temperature, where thermal runaway takes place | °C, K |
| Tmax | Maximum axial temperature at r = 0 (center of tubes) | °C, K |
| Tmean,bed | Mean temperature of fixed bed in radial direction (≈T at r = 0.7 rt) | °C, K |
| Trt,bed | Temperature of fixed bed directly at inner wall of tube, where a jump (related to αw,int) from Trt,bed to Tw,int is assumed | °C, K |
| Tw,ex | Temperature at external wall of the tube | °C, K |
| Tw,int | Temperature at internal wall of the tube | °C, K |
| us | Superficial gas velocity (initial/final value: index z = 0 or z = 12 m) | m s−1 |
| XCO,per pass | Conversion of CO per pass through a single tube | |
| XCO,total | Total conversion of CO reached in the reactor including the gas recycle | |
| yCH4,in | Molar content of CH4 at reactor inlet | |
| yCH4,recycle | Molar content of CH4 in recycle and purge gas stream | |
| yCO,in | Molar content of CO at reactor inlet | |
| yH2,in | Molar content of H2 at reactor inlet | |
| z | Axial coordinate in fixed bed | m |
| Greek letters | ||
| αH2O | Heat transfer coefficient (external area of tube to boiling water) | W m−2 s−1 |
| αw,ex | Heat transfer coefficient (tube to boiling water incl. heat transfer by conduction through wall, see Equation (S18)) | W m−2 s−1 |
| αw,int | Heat transfer coefficient (bed to internal tube wall) | W m−2 s−1 |
| ∆RHi | Enthalpy of reaction (i = reaction of CO to methane or to C2+-HCs) | J molCO−1 |
| ∆pbed | Pressure drop of fixed bed (tube) | Pa |
| εbed | Porosity of fixed bed Thiele modulus (defined by Equation (6)) | |
| ηpore | Pore effectiveness factor (defined by Equation (5)) | |
| λg | Thermal conductivity of gas mixture | W m−1 K−1 |
| λrad | Effective radial thermal conductivity in fixed bed | W m−1 K−1 |
| λwall | Thermal conductivity of wall material (steel) | W m−1 K−1 |
| νg | Kinematic viscosity of gas (mixture) | mol m−3 |
| Stoichiometric coefficient of component i (i = CO, H2, CH2, CH4, or H2O) in reaction n (n = 1 for methane formation and 2 for formation of C2+-HCs) | ||
| ρbed | Bulk density of fixed bed | kg m−3 |
| Abbreviations | ||
| C2+ | Hydrocarbons with two and more carbon atoms (all HCs without CH4) | |
| FT(S) | Fischer–Tropsch (synthesis) | |
| HCs | Hydrocarbons | |
References
- Kern, C.; Jess, A. Performance of a multi-tubular Fischer-Tropsch reactor with two catalytic zones of different intrinsic chemical activity. Catal. Sci. Technol. 2023, 13, 516–527. [Google Scholar] [CrossRef]
- Kern, C.; Jess, A. Improvement of a multi-tubular Fischer-Tropsch reactor with gas recycle by appropriate combination of axial activity distribution and gas velocity. Catal. Sci. Technol. 2023, 13, 2212–2222. [Google Scholar] [CrossRef]
- Poehlmann, F. Zusammenspiel von Chemischer Reaktion und Porendiffusion bei der Kobaltkatalysierten Fischer-Tropsch-Synthese unter Einsatz von CO2-Haltigem Synthesegas. Ph.D. Thesis, University Bayreuth, Bayreuth, Germany, 2017. [Google Scholar]
- Dry, M.E. FT Catalysts; Fischer-Tropsch Technology, Studies in Surface Science and Catalysis; Steynberg, A.P., Dry, M.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2004; Volume 152, pp. 533–600. [Google Scholar]
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes; Chapter 6.5, Fischer-Tropsch Synthesis; Wiley Interscience: Hoboken, NJ, USA, 2006; pp. 398–464. [Google Scholar]
- Gholami, Z.; Tisler, Z.; Rubas, V. Recent advances in Fischer-Tropsch synthesis using cobalt-based catalysts: A review on supports, promoters, and reactors. Catal. Rev. 2021, 63, 512–595. [Google Scholar] [CrossRef]
- Fox, J.M. Fischer-Tropsch reactor selection. Catal. Lett. 1990, 7, 281–292. [Google Scholar] [CrossRef]
- Jess, A.; Wasserscheid, P. Chemical Technology: From Principles to Processes, 2nd ed.; Wiley VCH: Weinheim, Germany, 2020. [Google Scholar]
- Verein Deutscher Ingenieure (Ed.) VDI-Waermeatlas: Berechnungsblaetter für den Waermeuebergang, 9th ed.; Springer: Berlin/Heidelberg, Germany, 2002. [Google Scholar]
- Schluender, E.-U.; Tsotsas, E. Waermeuebertragung in Festbetten, Durchmischten Schuettguetern und Wirbelschichten; Georg Thieme Verlag: Stuttgart, Germany, 1988. [Google Scholar]
- Nilles, M. Waermeübertragung an der Wand Durchstroemter Schuettungsrohre. Ph.D. Thesis, University Karlsruhe, Karlsruhe, Germany, 1991. [Google Scholar]
- Schluender, E.-U. Einfuehrung in die Waermeuebertragung; Vieweg, Braunschweig: Wiesbaden, Germany, 1986. [Google Scholar]
- Stephan, K. Mechanismus und Modellgesetz des Waermeuebergangs bei der Blasenverdampfung. Chem. Ing. Techn. 1963, 35, 775–784. [Google Scholar] [CrossRef]
- Stephan, K. Waermeuebergang Beim Kondensieren und Beim Sieden; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; London, UK; Paris, France; Tokyo, Japan, 1988. [Google Scholar]
- Ergun, S. Fluid Flow through Packed Columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Fritz, W. Grundlagen der Waermeuebertragung beim Verdampfen von Fluessigkeiten. Chem. Ing. Tech. 1963, 35, 753–764. [Google Scholar] [CrossRef]

| Constant Parameters (230 °C, 30 bar) Not Varied during Modeling | Value | |
|---|---|---|
| Length of reactor (single tube) Lt | 12 m a | |
| Internal tube diameter dt,int | 3 cm | |
| Thickness of tube wall swall | 0.3 cm | |
| Content of CO (in fresh syngas) yCO,fresh,SG | 0.3125 | |
| Content of H2 (in fresh syngas) yH2,fresh,SG = 1 − yCO,fresh,SG | 0.6875 | |
| Total pressure ptotal | 30 bar | |
| Diameter of spherical catalyst particles dp | 3 mm | |
| Bulk density of bed/catalyst ρbed | 960 kg m−3 | |
| Porosity of fixed bed εbed | 0.4 | |
| Heat capacity of gas mixture cp | 29 J mol−1 K−1 | |
| Thermal conductivity of gas mixture λg | 0.016 W m−1 K−1 | |
| Kinematic viscosity of gas mixture νg | 2.3 × 10−6 m2 s−1 | |
| Thermal conductivity of wall material (steel) λwall | 15 W m−1 K−1 | |
| Parameters varied during modeling | Comment | Typical value |
| Content of CO (inlet of reactor) yCO,reactor,in | depends on XCO and corresponding recycle ratio R | 0.19 b |
| Content of H2 (inlet of reactor) yH2,reactor,in | 0.42 b | |
| Content of CH4 (inlet of reactor) yCH4,reactor,in c | 0.39 a | |
| Pressure drop ∆pbed | depends on us | 5.6 bar b |
| Initial superficial gas velocity us,z=0 (230 °C, 30 bar) | varied | 1 m s−1 |
| Heat transfer coefficient (wall to boiling water) αw,ex | depends on us d | 1850 W m−2 K−1 |
| Effective radial thermal conductivity λrad | 8.4 W m−1 K−1 | |
| Heat transfer coefficient (bed to internal tube wall) αw,int | 1540 W m−2 K−1 | |
| Label of Model | Parameter Considered (+)or Neglected (−) | Comment | ||
|---|---|---|---|---|
| pbed | Impact of H2O | |||
| M0 | − | − | − | “Simple” model used in previous publications [1,2] , and αw,ex (1 kW m−2 K−1) |
| M1 | − | − | − | As model M0, but accurate calculation of αw,ex (for details see Section 3.1) |
| M2n | + | − | − | (and of corresponding axial drop of us), i.e., neglecting pbed |
| M2p | − | + | − | |
| M3 | + | + | − | Accurate model, if inhibition by steam is negligible |
| M4 | + | + | + | As M3, but also considering inhibition by steam |
| Tcool in °C | XCO,per pass in % | yCH4,reactor,in in % | R | Prod. of C2+-HCs per Tube in kgC per h | Reactor Model and Parameter Considered (+) or Neglected (−) | |||
|---|---|---|---|---|---|---|---|---|
| Model | αw,ex = f(z) | pbed | ||||||
| 219.9 | 44.3 a | 38.7 | 2.50 | 1.48 | M0 | − | − | − |
| 223.0 | 45.5 | 38.1 | 2.38 | 1.54 | M1 | + (see Figure 1) | − | − |
| Tcool in °C | XCO,per pass in % | yCH4,reactor,in in % | R | Prod. of C2+-HCs per Tube in kgC per h | Reactor Model and Parameters Considered (+) or Neglected (−) | |||
|---|---|---|---|---|---|---|---|---|
| Model | pbed | Impact of H2O | ||||||
| 223.0 | 45.5 | 38.1 | 2.38 | 1.54 | M1 | − | − | − |
| 222.5 | 49.1 | 36.2 | 2.05 | 1.72 | M2n | + | − | − |
| 223.5 | 42.6 | 39.6 | 2.69 | 1.41 | M2p | − | + | − |
| 222.8 | 45.8 | 37.9 | 2.35 | 1.56 | M3 | + | + | − |
| Tcool in °C | XCO,per pass in % | yCH4,reactor,in in % | R | Prod. of C2+-HCs per Tube in kgC per h | Reactor Model and Parameters Considered (+) or Neglected (−) | |||
|---|---|---|---|---|---|---|---|---|
| Model | pbed | Impact of H2O | ||||||
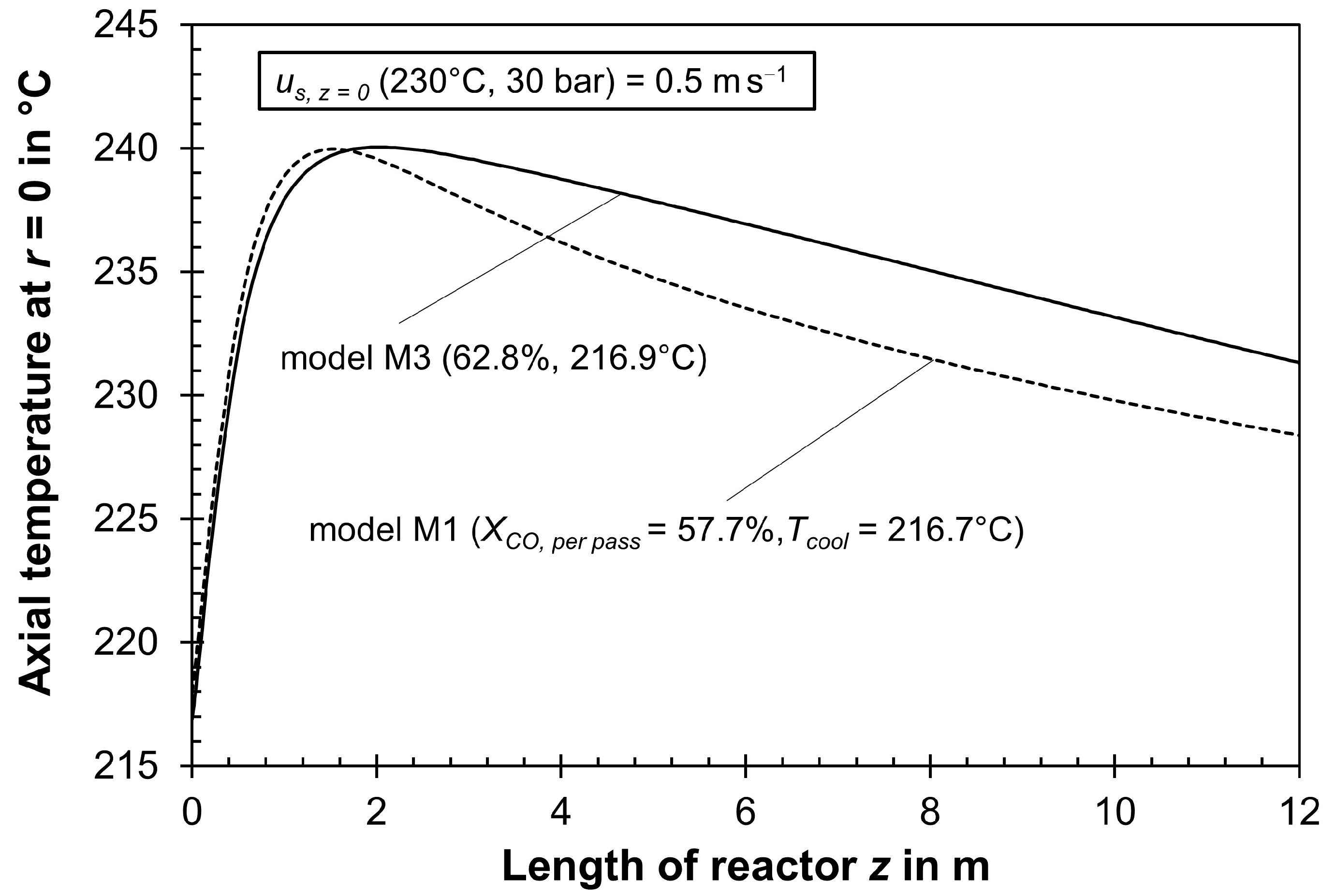
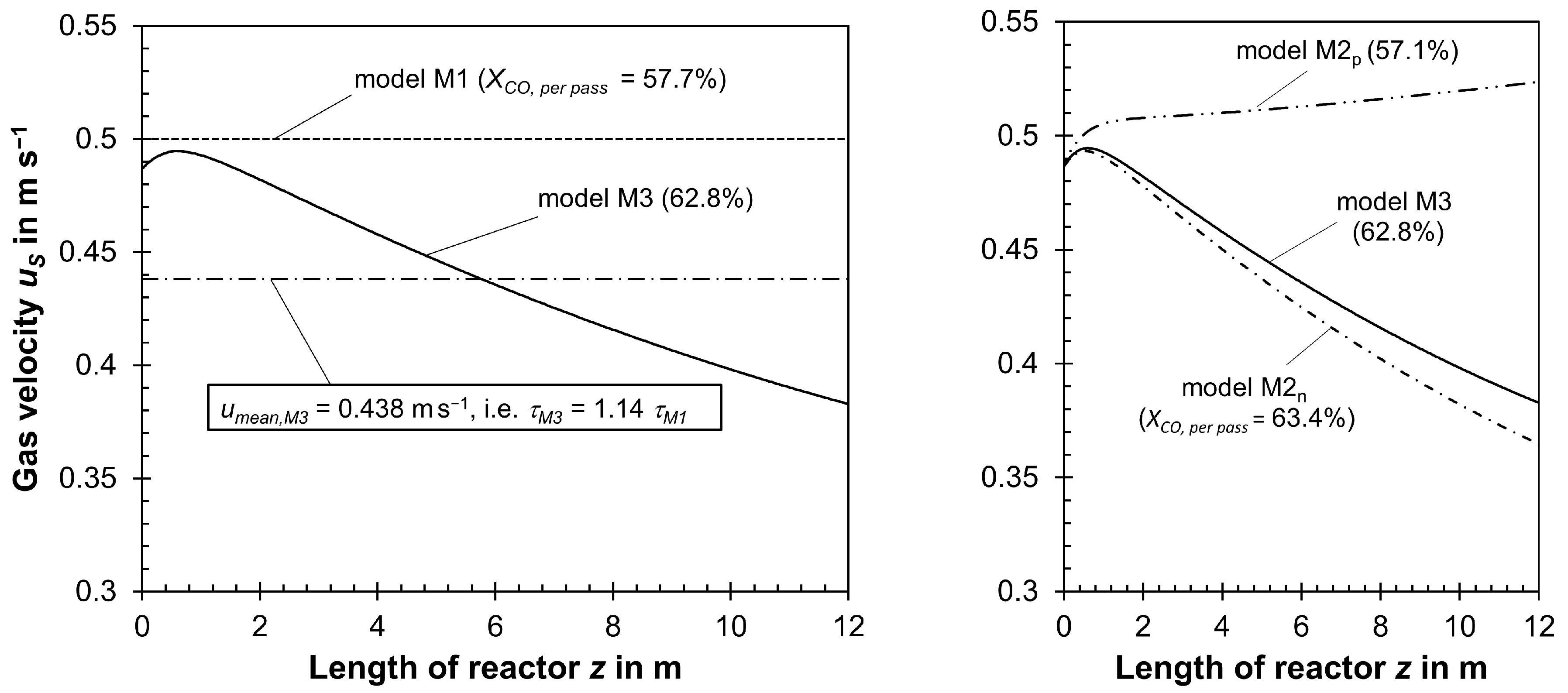
| 218.0 | 57.7 | 31.4 | 1.41 | 1.08 | M1 | − | − | − |
| 216.6 | 63.4 | 27.9 | 1.09 | 1.25 | M2n | + | − | − |
| 218.2 | 57.1 | 31.7 | 1.45 | 1.07 | M2p | − | + | |
| 216.9 | 62.8 | 28.3 | 1.12 | 1.23 | M3 | + | + | − |
| Tcool in °C | XCO,per pass in % | yCH4,reactor,in in % | R | Production of C2+-HCs per Tube in kgC per h | Reactor Model and Parameters Considered (+) or Neglected (−) | |||
|---|---|---|---|---|---|---|---|---|
| Model | pbed | Impact of H2O | ||||||
| us,z=0 = 1 m/s (230 °C, 30 bar); Ca = 3 | ||||||||
| 222.8 | 45.8 | 37.9 | 2.35 | 1.56 | M3 | + | + | − |
| 223.1 | 44.4 a | 38.7 | 2.49 | 1.49 | M4 | + | + | + |
| us,z=0 = 0.5 m/s (230 °C, 30 bar); Ca = 2 | ||||||||
| 216.9 | 62.8 | 28.3 | 1.12 | 1.23 | M3 | + | + | − |
| 217.6 | 59.0 b | 30.6 | 1.34 | 1.12 | M4 | + | + | + |
| CO Conversion XCO at Axial Position z | pH2O (yH2O) at z = 12 m | us at z = 12 m | Reactor Model and Parameters Considered (+) or Neglected (−) | |||||
|---|---|---|---|---|---|---|---|---|
| Model | pbed | Inhibition by H2O | ||||||
| 3 m | 6 m | 12 m | ||||||
| 30.8% | 57.7% | 92.0% | 18.4 bar (63%) | 0.24 m/s | M3 | + | + | − |
| 29.5% | 53.1% | 81.4% | 14.3 bar a (49%) | 0.27 m/s | M4 | + | + | + |
| us, in m/s | pbed in bar | Tcool in °C | pcool in bar | XCO,per pass in % | yCH4,reactor,in in % | R | C2+-HCs/Tube in kgC per h | |
|---|---|---|---|---|---|---|---|---|
| z = 0 a | z = 12 m | |||||||
| 0.23 | 0.17 | 0.3 | 199.6 b | 15.2 | 66.1 | 26.5 | 1.04 | 0.64 |
| 0.48 | 0.38 | 1.2 | 213.3 c | 20.1 | 63.2 | 28.4 | 1.10 | 1.24 |
| 0.73 | 0.66 | 2.9 | 219.4 | 22.9 | 53.1 | 34.0 | 1.73 | 1.44 |
| 0.99 | 1.04 | 5.6 | 223.1 | 24.6 | 44.4 | 38.7 | 2.49 | 1.49 (best case) |
| 1.24 | 1.63 | 9.8 | 225.5 | 25.7 | 36.8 | 42.4 | 3.46 | 1.45 |
| 1.49 | 3.09 | 16.7 | 227.4 | 26.6 | 30.0 | 44.8 | 4.74 | 1.36 |
| 1.59 | 5.19 | 21.5 d | 228.0 | 26.9 | 26.4 | 46.8 | 5.69 | 1.23 |
| For comparison and illustration: Hypothetic cases for absence of mass transfer resistance by pore diffusion (ηpore = 1) | ||||||||
| 0.46 | 0.44 | 1.4 | 191 e | 12.9 | 28.9 | 45.8 | 5.01 | 0.43 |
| 0.94 | 1.07 | 5.7 | 198 f | 14.9 | 28.4 | 46.0 | 5.13 | 0.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jess, A.; Kern, C. Significance of Pressure Drop, Changing Molar Flow, and Formation of Steam in the Accurate Modeling of a Multi-Tubular Fischer–Tropsch Reactor with Cobalt as Catalyst. Processes 2023, 11, 3281. https://doi.org/10.3390/pr11123281
Jess A, Kern C. Significance of Pressure Drop, Changing Molar Flow, and Formation of Steam in the Accurate Modeling of a Multi-Tubular Fischer–Tropsch Reactor with Cobalt as Catalyst. Processes. 2023; 11(12):3281. https://doi.org/10.3390/pr11123281
Chicago/Turabian StyleJess, Andreas, and Christoph Kern. 2023. "Significance of Pressure Drop, Changing Molar Flow, and Formation of Steam in the Accurate Modeling of a Multi-Tubular Fischer–Tropsch Reactor with Cobalt as Catalyst" Processes 11, no. 12: 3281. https://doi.org/10.3390/pr11123281
APA StyleJess, A., & Kern, C. (2023). Significance of Pressure Drop, Changing Molar Flow, and Formation of Steam in the Accurate Modeling of a Multi-Tubular Fischer–Tropsch Reactor with Cobalt as Catalyst. Processes, 11(12), 3281. https://doi.org/10.3390/pr11123281