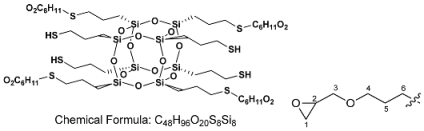
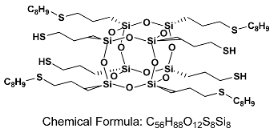
Click Addition Reaction of Urethane–Acrylate Resin Using Octa(3-thiopropyl)silsesquioxane Derivatives as Cross-Linking Agents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Analytical Methods and Methodology
2.3. Synthesis of Precursor Octa(3-thiopropyl)silsesquioxane
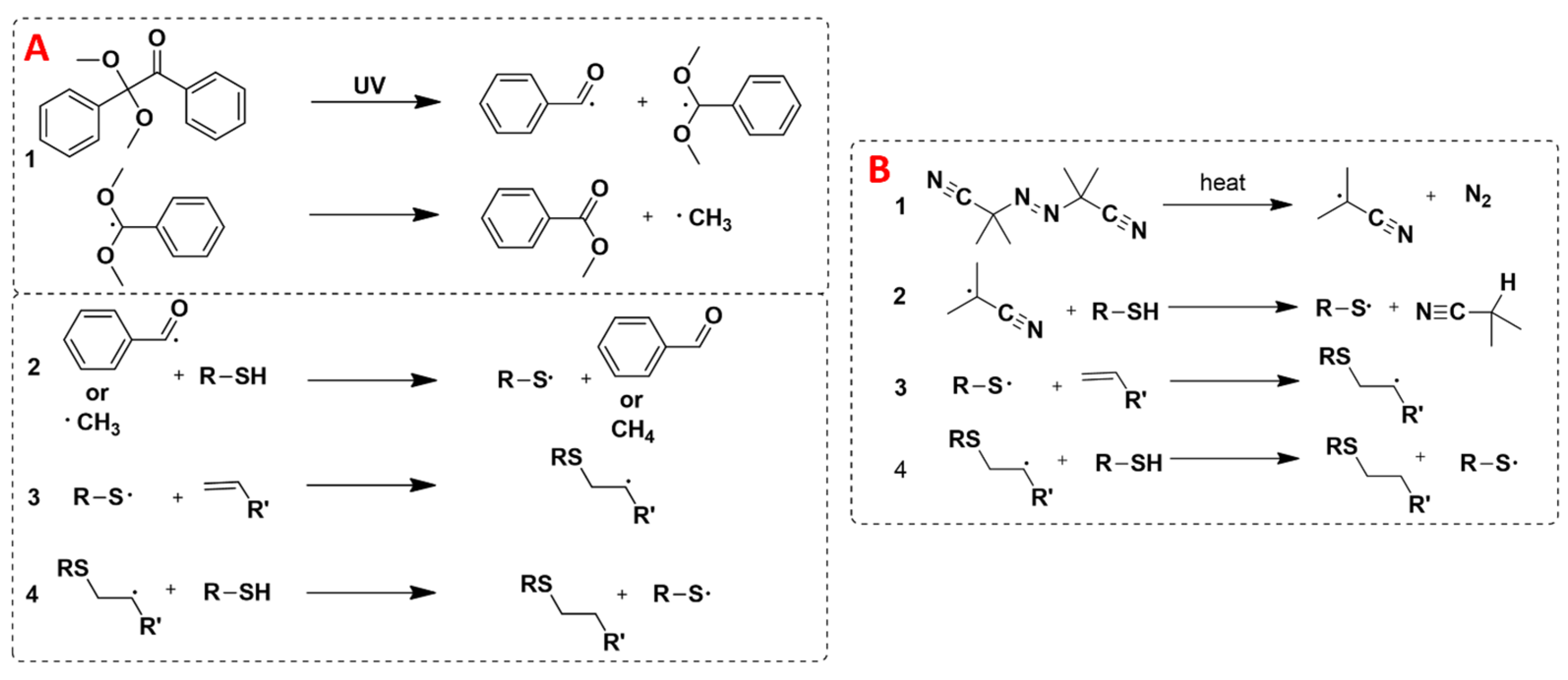
2.4. General Procedure for Thiol-Ene Tests with Octa(3-thiopropyl)silsesquioxane in the Presence of AIBN
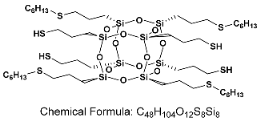
- SSQ-4SH-4HEX
- 1H NMR (400 MHz, CDCl3): δ(ppm) = 2.72–2.62 (m, 4H, alpha product SCH(CH3)CH2(CH2)2CH3), 2.56–2.48 (m, 24H, -CH2SCH2-), 1.73–1.65 (m, 16H, SiCH2CH2CH2S), 1.59–1.53 (m, 8H, SCH2CH2(CH2)3CH3, alpha product SCH(CH3)CH2(CH2)2CH3), 1.40–1.35 (m, 4H, SH), 1.33–1.24 (m, 24H, SCH2CH2(CH2)3CH3, alpha product SCH(CH3)CH2(CH2)2CH3), 0.89–0.86 (m, 12H, chain end -CH3), 0.76–0.73 (m, 16H, SiCH2CH2CH2S)
- 29Si NMR (79,5 MHz, CDCl3): δ(ppm) = −67.05, −67.18 (Si-O-Si)
- MALDI-TOF MS: [M] + Na+: 1375.3333 (measured), 1375.3339 (calculated);
- [M] + H• + Na+: 1376.3370 (measured), 1376.3417 (calculated)
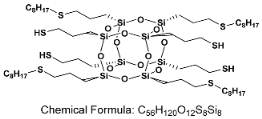
- SSQ-4SH-4OCT
- 1H NMR (400 MHz, CDCl3): δ(ppm) = 2.71–2.64 (m, 4H, alpha product SCH(CH3)CH2(CH2)4CH3), 2.57–2.49 (m, 24H, -CH2SCH2-), 1.74–1.65 (m, 16H, SiCH2CH2CH2S), 1.61–1.53 (m, 8H, SCH2CH2(CH2)5CH3), alpha product SCH(CH3)CH2(CH2)4CH3), 1.39–1.35 (m, 4H, SH), 1.30–1.25 (m, 40H, SCH2CH2(CH2)5CH3, alpha product SCH(CH3)CH2(CH2)4CH3), 0.89–0.86 (m, 12H, chain end -CH3), 0.77–0.73 (m, 16H, SiCH2CH2CH2S)
- 29Si NMR (79,5 MHz, CDCl3): δ(ppm) = −67.06 (cage)
- MALDI-TOF MS: [M] + Na+: 1487.436 (measured), 1487.459 (calculated);
- [M] + H• + Na+: 1488.439 (measured), 1488.467 (calculated)
- SSQ-4SH-4OD
- 1H NMR (400 MHz, CDCl3): δ(ppm) = 2.54–2.47 (m, 24H, -CH2SCH2-), 1.73–1.68 (m, 16H, SiCH2CH2CH2S), 1.60–1.55 (m, 8H, SCH2CH2(CH2)15CH3), 1.40–1.37 (m, 4H, SH), 1.34–1.25 (m, 120H, SCH2CH2(CH2)15CH3, 0.90–0.86 (m, 12H, chain end -CH3), 0.77–0.72 (m, 16H, SiCH2CH2CH2S)
- 29Si NMR (79,5 MHz, CDCl3): δ (ppm) = −67.11 (cage)
- MALDI-TOF MS: [M] + H• + Na+: 2049.099 (measured), 2049.093 (calculated);
- [M] + Na+: 2048.103 (measured), 2048.086 (calculated)
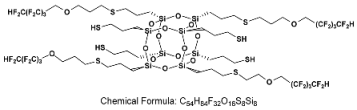
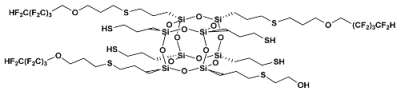
- SSQ-4SH-4OFP
- 1H NMR (400 MHz, CDCl3): δ(ppm) = 6.05 (tt, J1 = 52.0 Hz, J2 = 5.5 Hz, 4H, -CF2H), 3.91 (t, 8H, O-CH2-CF2), 3.67 (t, 8H, O-CH2-CH2-CH2-S), 2.61–2.51 (m, 24H, O-CH2-CH2-CH2-S, S-CH2-CH2-CH2-Si), 1.91–1.82 (m, 8H, O-CH2-CH2-CH2-S), 1.73–1.70 (m, 16H, S-CH2-CH2-CH2-Si), 1.44–1.38 (m, 4H, SH), 0.75 (s, 16H, S-CH2-CH2-CH2-Si)
- 29Si NMR (79,5 MHz, CDCl3): δ (ppm) = −64.56 (Si-OH), −65.86-(−67.14) (Si-O-Si)
- MALDI-TOF-MS
- [M] + Na+: 1885.1563 (measured), 1885.103162 (calculated)
- [M] + H• + Na+: 1886.1454 (measured), 1886.110962 (calculated)
- SSQ-4SH-4TMOS
- 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.57 (s, 36H, OMe), 2.63–2.52 (m, 24H, SiCH2CH2CH2SCH2CH2Si(OMe)3), 1.70–1.63 (m, 16H, SiCH2CH2CH2SCH2CH2Si(OMe)3), 1.40–1.37 (m, 4H, SH), 1.00–0.97 (m, 8H, SiCH2CH2CH2SCH2CH2Si(OMe)3), 0.75–0.72 (m, 16H, SiCH2CH2CH2SCH2CH2Si(OMe)3)
- 29Si NMR (79,5 MHz, CDCl3): δ (ppm) = −44.72 (Si(OMe)3), −67.14 (cage)
- MALDI-TOF-MS: [M] + H• + Na+: 1632.116 (measured), 1632.187 (calculated)
- SSQ-4SH-4AGE
- 1H NMR (400 MHz, CDCl3): δ (ppm) = 3.75–3.71 (m, 4H, position 3), 3.61–3.56 (m, 8H, position 4), 3.39–3.36 (m, 4H, position 3), 3.15–3.12 (m, 4H, position 2), 2.81–2.79 (m, 4H, position 1), 2.61–2.59 (m, 4H, position 1), 2.54–2.49 (m, 24H, -CH2SCH2), 1.89–1.84 (m, 8H, position 5), 1.74–1.66 (m, 24H, SiCH2CH2CH2S, and position 6), 1.39–1.35 (m, 4H, SH), 0.77–0.73 (m, 16H, SiCH2CH2CH2S)
- 29Si NMR (79,5 MHz, CDCl3): δ (ppm) = −67.11 (cage)
- MALDI-TOF-MS: [M] + H• + Na+: 1496.226 (measured), 1496.237 (calculated)
- SSQ-4SH-4STYR
- 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.28–7.14 (m, 20H, Ph), 2.87–2.83 (m, 8H, SCH2CH2Ar), 2.75–2.70 (m, 8H, SCH2CH2Ar), 2.55–2.48 (m, 16H, SiCH2CH2CH2S), 1.72–1.66 (m, 16H, SiCH2CH2CH2S), 1.40–1.34 (m, 4H, SH), 0.76–0.73 (m, 16H, SiCH2CH2CH2S)
- 29Si NMR (79,5 MHz, CDCl3): δ (ppm) = −67.01 (cage)
- MALDI-TOF-MS: [M] + H• + Na+: 1456.220 (measured), 1456.217 (calculated)
2.5. General Procedure for Thiol-Ene Tests with Octa(3-thiopropyl)silsesquioxane in the Presence of DMPA
2.6. General Procedure for Preparation Composites
3. Results
3.1. Synthesis and Analysis of Derivatives
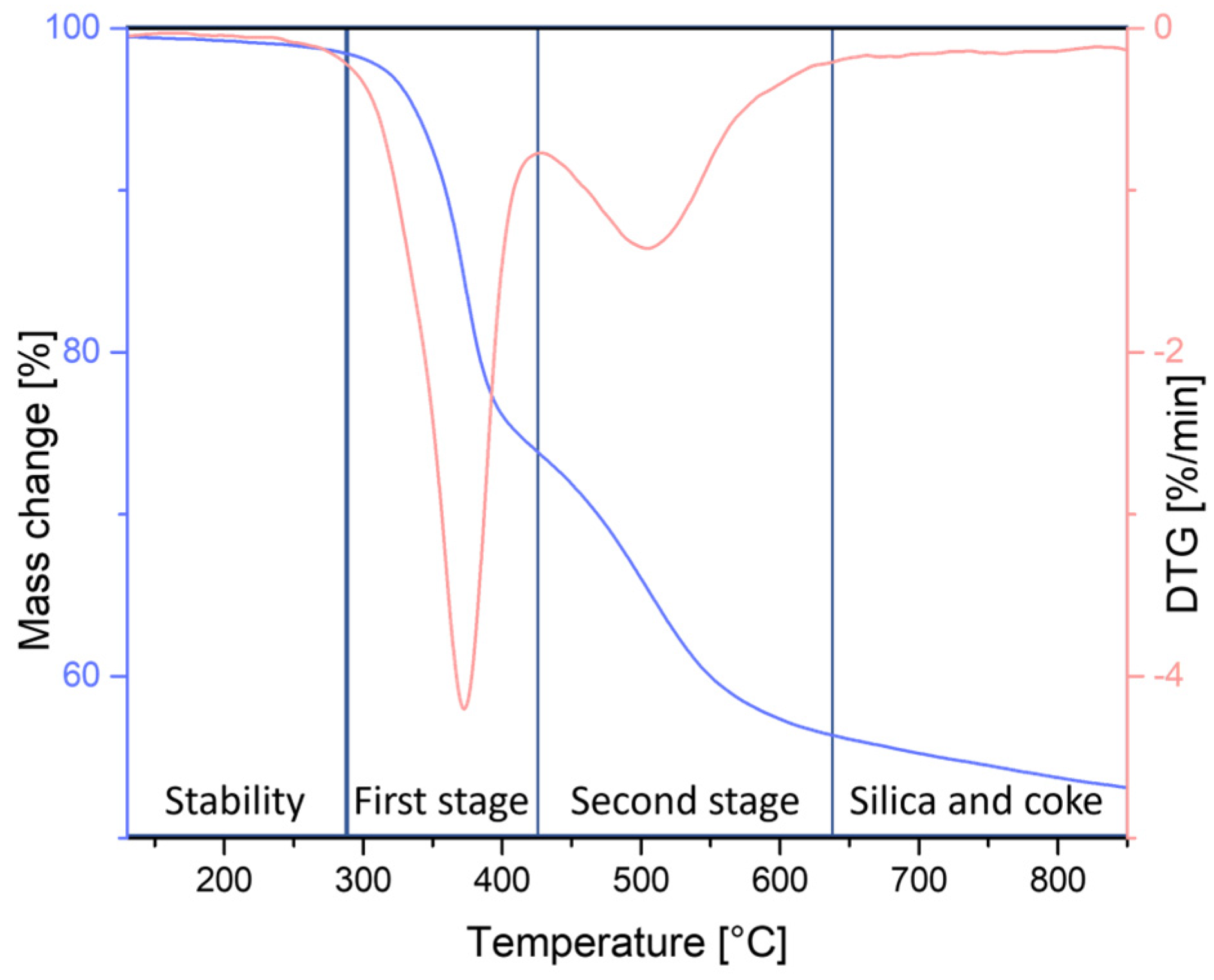
3.2. Thermogravimetric Analysis
3.3. Contact Angle Analysis
3.4. Modification of UV-Curable Resin
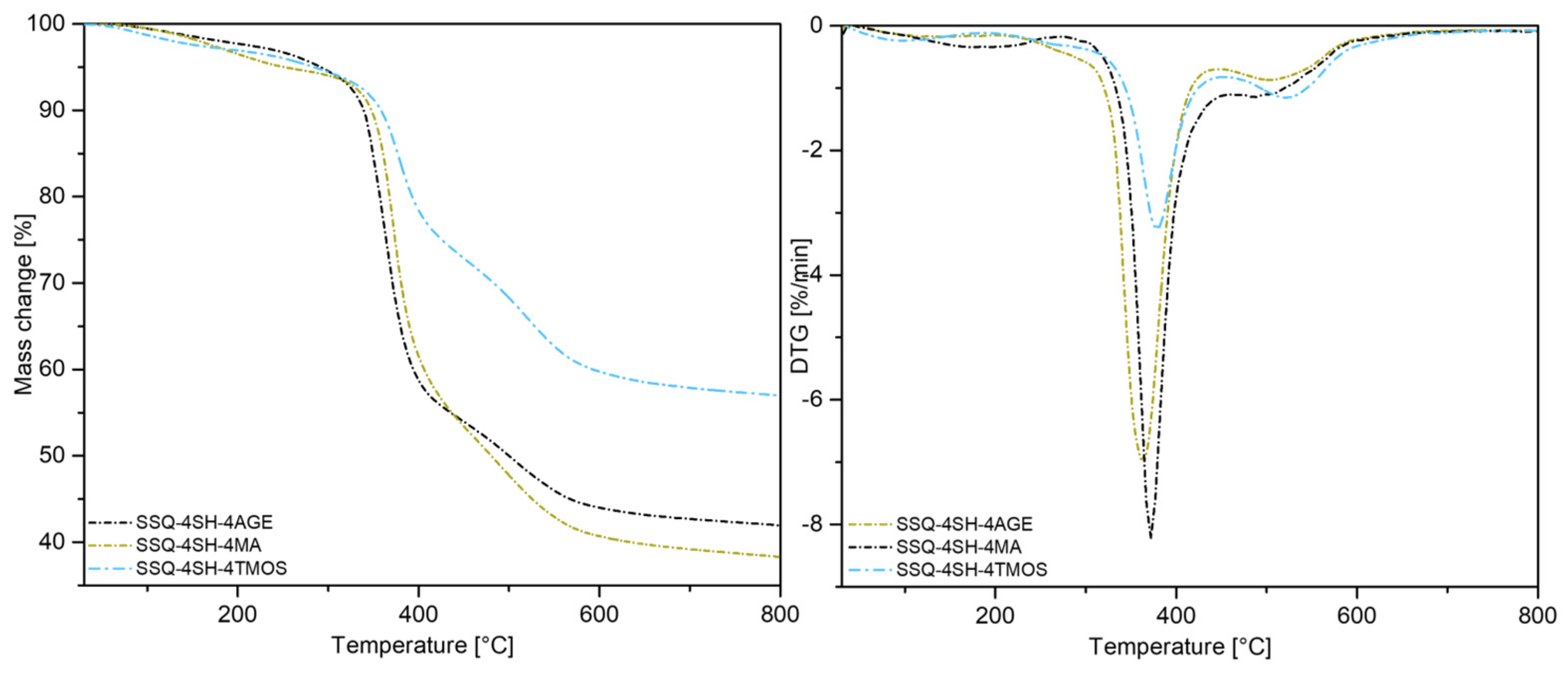
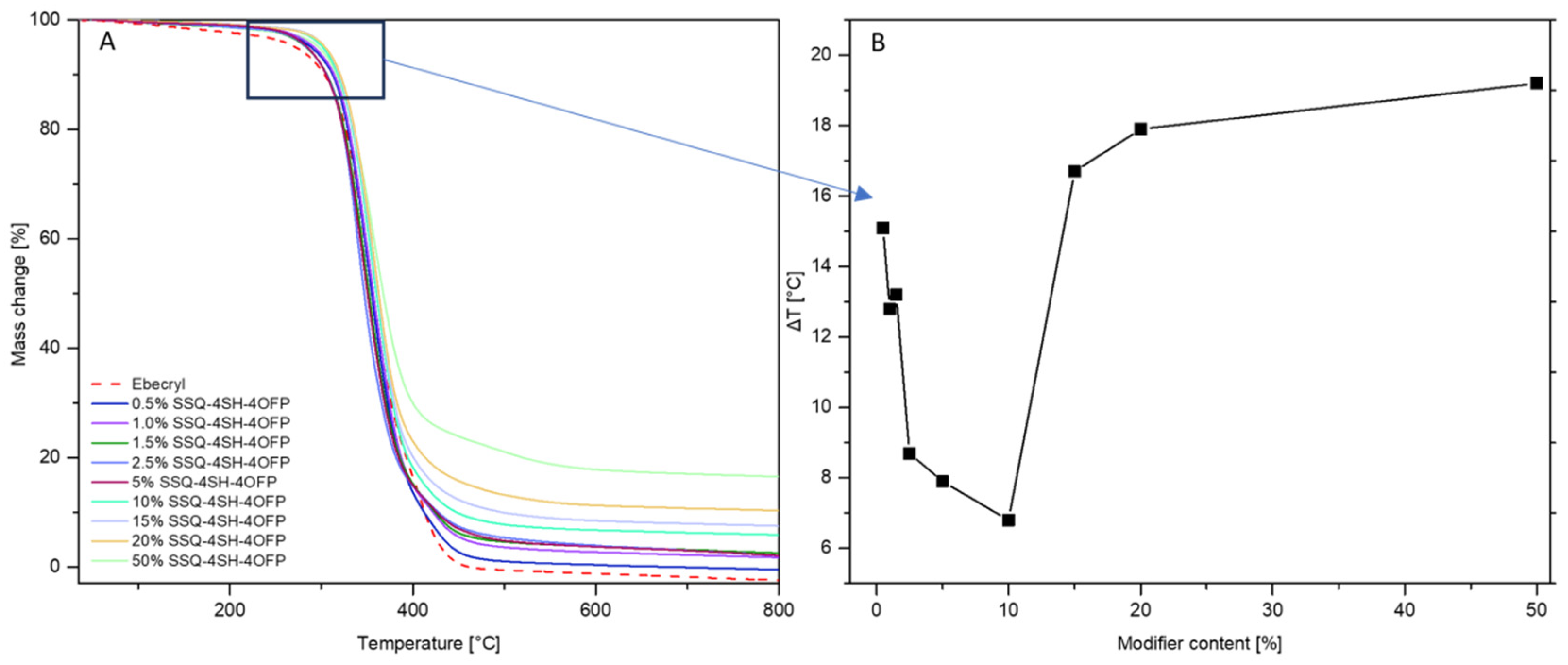
3.4.1. Thermal Analysis (TGA)
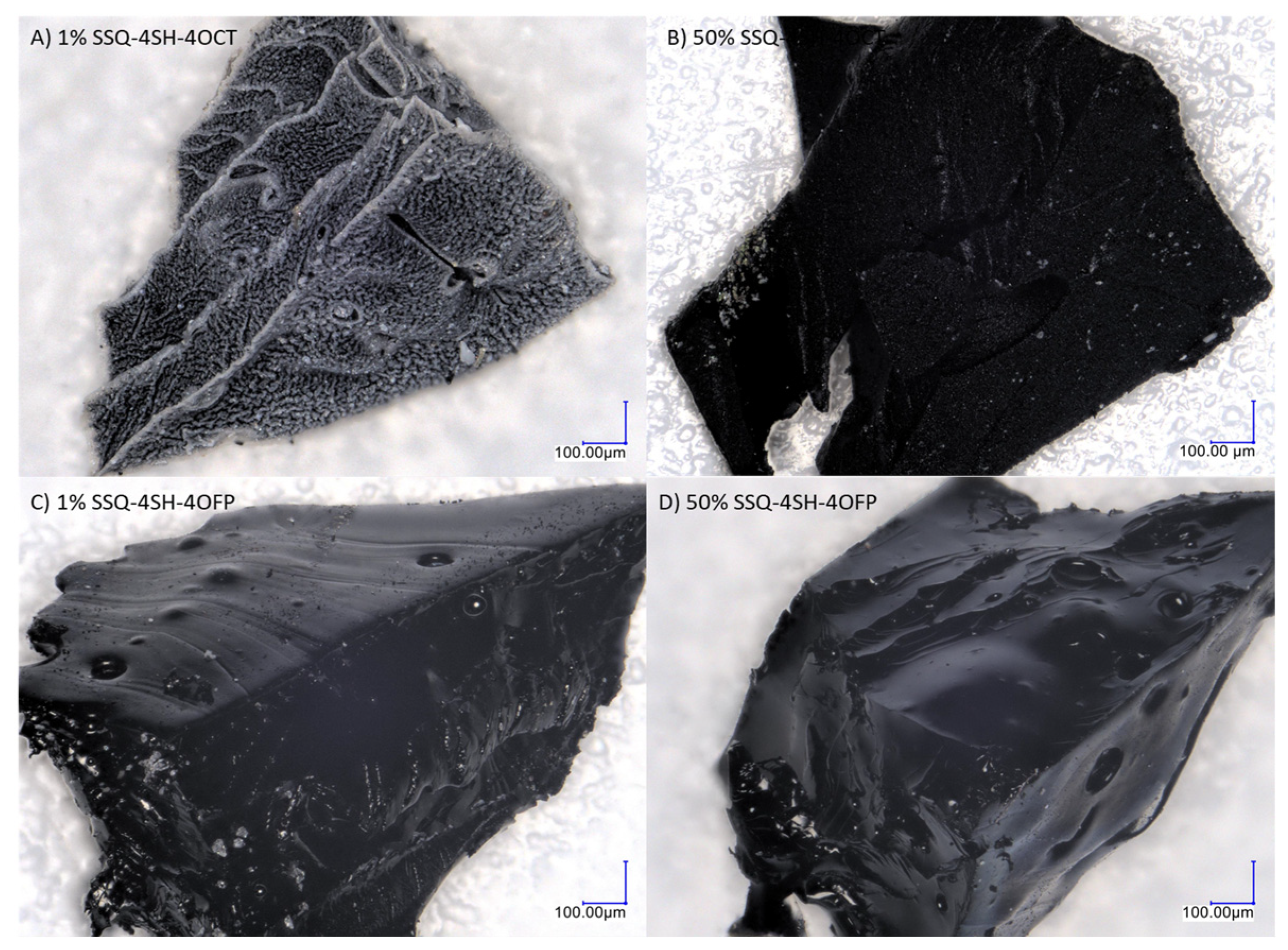
Microscopic Analysis
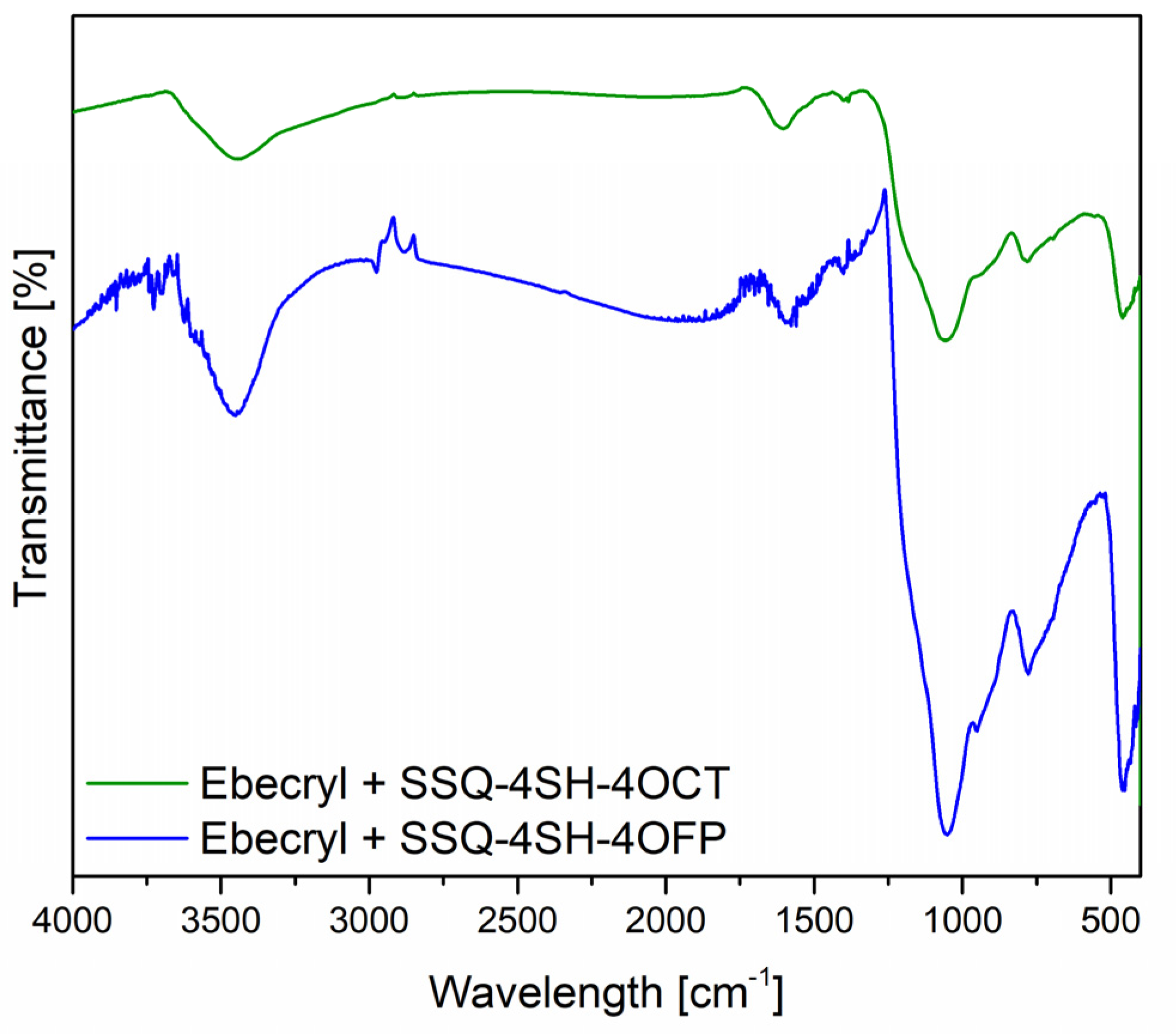
FT-IR Analysis
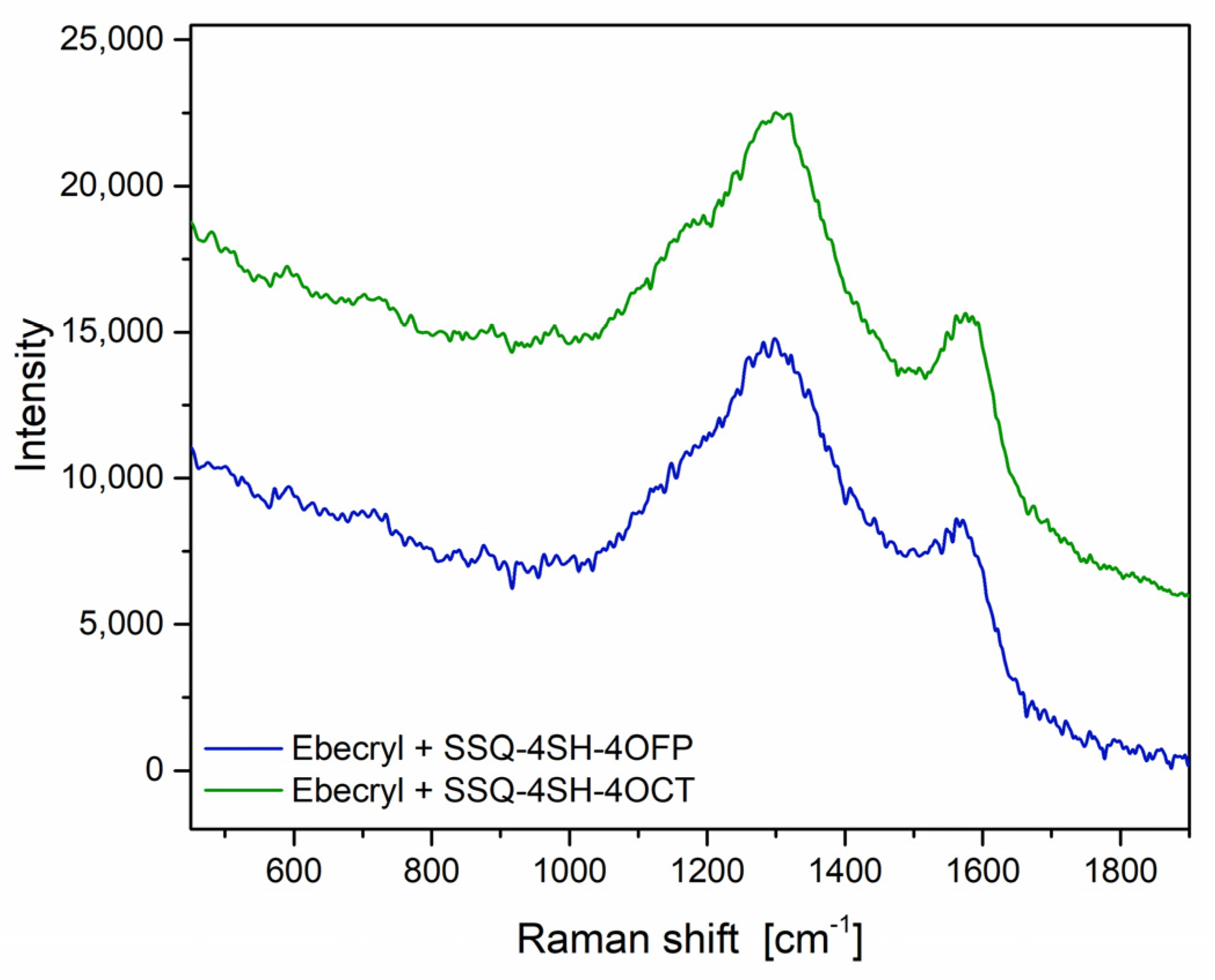
RAMAN Spectroscopy
3.4.2. Contact Angle (WCA)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AIBN | Azobisisobutyronitrile |
| DMPA | 2,2-dimethoxy-2-phenylacetophenone |
| SSQ | Silsesquioxane |
| HEX | Hexene |
| OCT | Octene |
| OD | Octadecene |
| VTMOS | Vinyltrimethoxysilane |
| AGE | Allyl glycidyl ether |
| Allyl-OFP/OFP | Allyl 2,2,3,3,4,4,5,5-octafluoropentylether |
| MA | Allyl methacrylate |
| STYR | Styrene |
| TGA | Thermogravimetric analysis |
| WCA | Water contact angle |
| FT-IR | Fourier-transform infrared spectroscopy |
References
- Posner, T. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber. Dtsch. Chem. Ges. 1905, 38, 646–657. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Przybylak, M.; Przybylska, A.; Szymańska, A.; Maciejewski, H. Superhydrophobization of cotton textiles by difunctional cyclic siloxanes via thiol-ene click reaction. Cellulose 2023, 30, 5419–5436. [Google Scholar] [CrossRef]
- Nolan, M.D.; Scanlan, E.M. Applications of thiol-ene chemistry for peptide science. Front. Chem. 2020, 8, 583272. [Google Scholar] [CrossRef]
- Rissing, C.; Son, D.Y. Application of thiol-ene chemistry to the preparation of carbosilane−thioether dendrimers. Organometallics 2009, 28, 3167–3172. [Google Scholar] [CrossRef]
- Liang, L.; Tan, W.; Dong, Y.; Gu, F.; Meng, X. Modified cotton fabric based on thiolene click reaction and its oil/water separation application. Environ. Technol. 2022, 43, 4137–4146. [Google Scholar] [CrossRef] [PubMed]
- Przybylak, M.; Szymańska, A.; Maciejewski, H.; Makowska, K. Durable, highly hydrophobic modification of cotton fabric with fluorine-free polysiloxanes obtained via hydrosilylation and hydrothiolation reactions. Cellulose 2020, 27, 8351–8367. [Google Scholar] [CrossRef]
- Matsukawa, K.; Fukuda, T.; Watase, S.; Goda, H. Preparation of photo-curable thiol-ene hybrids and their application for optical materials. J. Photopolym. Sci. Technol. 2010, 23, 115–119. [Google Scholar] [CrossRef]
- Marcinkowska, A.; Gajewski, P.; Szcześniak, K.; Sadej, M.; Lewandowska, A. Ionogels obtained by thiol-ene photopolymerization—physicochemical characterization and application in electrochemical capacitors. Molecules 2021, 26, 758. [Google Scholar] [CrossRef]
- Sawicki, L.A.; Kloxin, A.M. Design of thiol–ene photoclick hydrogels using facile techniques for cell culture applications. Biomater. Sci. 2014, 2, 1612–1626. [Google Scholar] [CrossRef]
- Cabrero-Antonino, J.R.; Leyva-Perez, A.; Corma, A. Iron-Catalysed Markovnikov Hydrothiolation of Styrenes. Adv. Synth. Catal. 2012, 354, 678–687. [Google Scholar] [CrossRef]
- Belley, M.; Zamboni, R. Addition of thiols to styrenes: Formation of benzylic thioethers. J. Org. Chem. 1989, 54, 1230–1232. [Google Scholar] [CrossRef]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B.; Bowman, M. Nucleophile-initiated thiol-Michael reactions: Effect of organocatalyst, thiol, and ene. Macromolecules 2010, 43, 6381–6388. [Google Scholar] [CrossRef]
- Chan, J.W.; Yu, B.; Hoyle, C.E.; Lowe, A.B. Convergent synthesis of 3-arm star polymers from RAFT-prepared poly (N,N-diethylacrylamide) via a thiol–ene click reaction. Chem. Comm. 2008, 40, 4959–4961. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B. Sequential phosphine-catalyzed, nucleophilic thiol− ene/radical-mediated thiol− yne reactions and the facile orthogonal synthesis of polyfunctional materials. J. Am. Chem. Soc. 2009, 131, 5751–5753. [Google Scholar] [CrossRef]
- Kakwere, H.; Perrier, S. Orthogonal “relay” reactions for designing functionalized soft nanoparticles. J. Am. Chem. Soc. 2009, 131, 1889–1895. [Google Scholar] [CrossRef]
- Cramer, N.B.; Scott, J.P.; Bowman, C.N. Photopolymerizations of thiol− ene polymers without photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
- Lowe, A.B. Thiol-ene “click” reactions and recent applications in polymer and materials synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Sinha, A.K.; Equbal, D. Thiol− Ene Reaction: Synthetic Aspects and Mechanistic Studies of an Anti-Markovnikov-Selective Hydrothiolation of Olefins. Asian J. Org. Chem. 2019, 8, 32–47. [Google Scholar] [CrossRef]
- Degirmenci, I. Effect of Initiator Structure on Thiol-Ene Polymerization: A DFT Study. Macromol. Theory Simul. 2022, 31, 2100040. [Google Scholar] [CrossRef]
- Mackey, N.M.; Confait, B.S.; Wynne, J.H.; Buchanan, J.P. Preparation of novel hydrolyzing urethane modified thiol-ene networks. Polymers 2011, 3, 1849–1865. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Groch, P.; Dziubek, K.; Czaja, K. Coordination polymerization of alkenylsilsesquioxanes and their copolymerization with olefins or styrene/Polimeryzacja koordynacyjna alkenylosilseskwioksanow i ich kopolimeryzacja z olefinami lub styrenem. Polimery 2015, 60, 28–298. [Google Scholar] [CrossRef]
- Kickelbick, G. Silsesquioxanes. In Functional Molecular Silicon Compounds I. Structure and Bonding; Scheschkewitz, D., Ed.; Springer: Cham, Switzerland, 2013; Volume 155, pp. 1–28. [Google Scholar]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Seifalian, A.M. Polyhedral oligomeric silsesquioxane nanocomposites: The next generation material for biomedical applications. Acc. Chem. Res. 2005, 38, 879–884. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W. Functional polyimide/polyhedral oligomeric silsesquioxane nanocomposites. Polymers 2018, 11, 26. [Google Scholar] [CrossRef]
- Li, G.Z.; Wang, L.; Toghiani, H.; Daulton, T.L.; Koyama, K.; Pittman, C.U. Viscoelastic and mechanical properties of epoxy/multifunctional polyhedral oligomeric silsesquioxane nanocomposites and epoxy/ladderlike polyphenylsilsesquioxane blends. Macromolecules 2001, 34, 8686–8693. [Google Scholar] [CrossRef]
- Lee, K.S.; Chang, Y.W. Thermal and mechanical properties of poly (ε-caprolactone)/polyhedral oligomeric silsesquioxane nanocomposites. Polym. Int. 2013, 62, 64–70. [Google Scholar] [CrossRef]
- Sztorch, B.; Brząkalski, D.; Głowacka, J.; Pakuła, D.; Frydrych, M.; Przekop, R.E. Trimming flow, plasticity, and mechanical properties by cubic silsesquioxane chemistry. Sci. Rep. 2023, 13, 14156. [Google Scholar] [CrossRef]
- Sztorch, B.; Pakuła, D.; Kustosz, M.; Romanczuk-Ruszuk, E.; Gabriel, E.; Przekop, R.E. The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers 2022, 14, 5493. [Google Scholar] [CrossRef]
- Alves, F.; Nischang, I. Tailor-made hybrid organic–inorganic porous materials based on polyhedral oligomeric silsesquioxanes (POSS) by the step-growth mechanism of thiol-ene “click” chemistry. Chem. Eur. J. 2013, 19, 17310–17313. [Google Scholar] [CrossRef]
- Daga, V.K.; Anderson, E.R.; Gido, S.P.; Watkins, J.J. Hydrogen bond assisted assembly of well-ordered polyhedral oligomeric silsesquioxane–block copolymer composites. Macromolecules 2011, 44, 6793–6799. [Google Scholar] [CrossRef]
- Hartmann-Thompson, C. Polyhedral oligomeric silsesquioxanes in electronics and energy applications. In Applications of Polyhedral Oligomeric Silsesquioxanes; Springer: Dordrecht, The Netherlands, 2010; pp. 247–325. [Google Scholar]
- Bivona, L.A.; Fichera, O.; Fusaro, L.; Giacalone, F.; Buaki-Sogo, M.; Gruttadauria, M.; Aprile, C. A polyhedral oligomeric silsesquioxane-based catalyst for the efficient synthesis of cyclic carbonates. Catal. Sci. Technol. 2015, 5, 5000–5007. [Google Scholar] [CrossRef]
- Li, X.; Du, Y.; Dai, J.; Wang, X.; Yang, P. Metal nanoparticles stabilized by cubic silsesquioxanes for catalytic hydrogenations. Catal. Lett. 2007, 118, 151–158. [Google Scholar] [CrossRef]
- Jash, P.; Wilkie, C.A. Effects of surfactants on the thermal and fire properties of poly (methyl methacrylate)/clay nanocomposites. Polym. Degrad. Stab. 2005, 88, 401–406. [Google Scholar] [CrossRef]
- Cazacu, M.; Bargan, A.; Balan, M.; Vornicu, N.; Varganici, C.D.; Shova, S. Full functionalized silica nanostructure with well-defined size and functionality: Octakis (3-mercaptopropyl) octasilsesquioxane. J. Organomet. Chem. 2015, 799, 195–200. [Google Scholar]
- Sierke, J.K.; Ellis, A.V. High purity synthesis of a polyhedral oligomeric silsesquioxane modified with an antibacterial. Inorg. Chem. Commun. 2015, 60, 41–43. [Google Scholar] [CrossRef]
- Dai, J.; Zhang, T.; Zhao, H.; Fei, T. Preparation of organic-inorganic hybrid polymers and their humidity sensing properties. SensActuators B Chem. 2017, 242, 1108–1114. [Google Scholar] [CrossRef]
- Gan, Y.; Jiang, X.; Yin, J. Self-wrinkling patterned surface of photocuring coating induced by the fluorinated POSS containing thiol groups (F-POSS-SH) as the reactive nanoadditive. Macromolecules 2012, 45, 7520–7526. [Google Scholar] [CrossRef]
- Lee, A.S.; Jo, Y.Y.; Choi, S.S.; Baek, K.Y.; Hwang, S.S. Thermal, Mechanical, and Photophysical Properties of Carbazole-Substituted POSS and Ladder Polysilsesquioxane. J. Nanosci. Nanotechnol. 2017, 17, 5562–5565. [Google Scholar] [CrossRef]
- Li, W.; Feng, S. New sensors for the detection of picric acid: Ionic liquids based on polyhedral oligomeric silsesquioxanes prepared via a thiol-ene click reaction. J. Mol. Liq. 2018, 265, 269–275. [Google Scholar] [CrossRef]
- Liu, L.; Lee, S.J.; Lee, M.E.; Kang, P.; Choi, M.G. Syntheses of phosphorylcholine-substituted silsesquioxanes via thiol-ene ‘click’reaction. Tetrahedron Lett. 2015, 56, 1562–1565. [Google Scholar] [CrossRef]
- Pakuła, D.; Przekop, R.E.; Brząkalski, D.; Frydrych, M.; Sztorch, B.; Marciniec, B. Sulfur-Containing Silsesquioxane Derivatives Obtained by the Thiol-ene Reaction: Synthesis and Thermal Degradation. ChemPlusChem 2022, 87, e202200099. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, A.; Przybylak, M.; Maciejewski, H.; Przybylska, A. Thiol-ene chemistry as an effective tool for hydrophobization of cotton fabrics. Cellulose 2022, 29, 1231–1247. [Google Scholar] [CrossRef]
- Maciejewski, H.; Karasiewicz, J.; Marciniec, B. Efektywna synteza fluorofunkcyjnych (poli)siloksanów. Polimery 2012, 57, 449–456. [Google Scholar] [CrossRef]
- Feher, F.J.; Wyndham, K.D.; Soulivong, D.; Nguyen, F. Syntheses of highly functionalized cube-octameric polyhedral oligosilsesquioxanes (R8Si8O12). J. Chem. Soc. Dalton Trans. 1999, 19, 1491–1498. [Google Scholar] [CrossRef]
- Roper, T.M.; Chandler, C.M.; Guymon, C.A.; Hoyle, C.E.; Jönsson, E.S. Structural influence of model ene reactivity in photoinduced thiol-ene polymerization of multifunctional alkenes. In Proceedings of the Technical Conference Proceedings-UV & EB Technology Expo & Conference, Charlotte, NC, USA, 2–4 May 2004; pp. 242–251. [Google Scholar]
- Cramer, N.B.; Bowman, C.N. Kinetics of thiol–ene and thiol–acrylate photopolymerizations with real-time fourier transform infrared. J. Polym. Sci. Part. A Polym. Chem. 2001, 39, 3311–3319. [Google Scholar] [CrossRef]
- Molina-Gutiérrez, S.; Dalle Vacche, S.; Vitale, A.; Ladmiral, V.; Caillol, S.; Bongiovanni, R.; Lacroix-Desmazes, P. Photoinduced polymerization of eugenol-derived methacrylates. Molecules 2020, 25, 3444. [Google Scholar] [CrossRef] [PubMed]
- Nagelsdiek, R.; Mennicken, M.; Maier, B.; Keul, H.; Höcker, H. Synthesis of polymers containing cross-linkable groups by atom transfer radical polymerization: Poly (allyl methacrylate) and copolymers of allyl methacrylate and styrene. Macromolecules 2004, 37, 8923–8932. [Google Scholar] [CrossRef]
- Frydrych, M.; Pakuła, D.; Sztorch, B.; Brząkalski, D.; Przekop, R.E.; Marciniec, B. Novel Silsesquioxane-Derived Boronate Esters—Synthesis and Thermal Properties. Molecules 2021, 26, 4107. [Google Scholar] [CrossRef]
- Frydrych, M.; Sztorch, B.; Przekop, R.E.; Marciniec, B. New Ethynylphenylborasilsesquioxanes—Their Reactivity and Behavior during Thermal Decomposition. Int. J. Mol. Sci. 2023, 24, 13960. [Google Scholar] [CrossRef]
- Pakuła, D.; Przekop, R.E.; Sztorch, B.; Marciniec, B. Sposób Otrzymywania Modyfikowanych Żywic fotoutwardzalnych Okta(3-Tiopropylosilseskwioksanem) o Znacznie Podwyższonej Stabilności Termicznej. Poland Patent Application P.443618, 28 January 2023. [Google Scholar]
- Sun, Z.G.; Wang, S.J.; Qiao, X.J.; Li, Y.; Zheng, W.H.; Bai, P.Y. Synthesis and microwave absorbing properties of SiC nanowires. Appl. Phys. A 2018, 124, 802. [Google Scholar] [CrossRef]
- Autthawong, T.; Namsar, O.; Yu, A.; Sarakonsri, T. Cost-effective production of SiO2/C and Si/C composites derived from rice husk for advanced lithium-ion battery anodes. J. Mater. Sci. Mater. Electron. 2020, 31, 9126–9132. [Google Scholar] [CrossRef]
- Pan, J.; Ren, J.; Xie, Y.; Wei, X.; Guan, Y.; Yan, X.; Tang, H.; Cheng, X. Porous SiOC composites fabricated from preceramic polymers and wood powders for efficient dye adsorption and removal. Res. Chem. Intermed. 2017, 43, 3813–3832. [Google Scholar] [CrossRef]
- Macias-Ferrer, D.; Melo-Banda, J.A.; Silva-Rodrigo, R.; Lam-Maldonado, M.; Páramo-García, U.; Meraz-Melo, M.A. Comparative study between removers agents of silicon into the synthesis of micro/nano-structured pyrolytic carbon from refined sugar. Int. J. Sci. Res. Manag. 2018, 5, 102–111. [Google Scholar]







| Modifier Content [%] | SSQ-4SH-4R [g] | Resin [g] | Initiator DMPA [g] |
|---|---|---|---|
| Reference | 0 | 6.00 | 0.06 |
| 0.5 | 0.03 | 5.97 | 0.06 |
| 1.0 | 0.06 | 5.93 | 0.06 |
| 1.5 | 0.09 | 5.91 | 0.06 |
| 2.0 | 0.12 | 5.88 | 0.06 |
| 2.5 | 0.15 | 5.85 | 0.06 |
| 5.0 | 0.30 | 5.70 | 0.06 |
| 10 | 0.60 | 5.40 | 0.06 |
| 15 | 0.90 | 5.10 | 0.06 |
| 20 | 1.20 | 4.80 | 0.06 |
| 25 | 1.50 | 4.50 | 0.06 |
| 50 | 3.00 | 3.00 | 0.06 |
| Sample | Density [g/cm3] |
|---|---|
| SSQ-8SH oil | 1.299 |
| SSQ-4SH–4HEX | 1.198 |
| SSQ-4SH–4OCT | 1.162 |
| SSQ-4SH–4OD | 1.095 |
| SSQ-4SH–4OFP | 1.412 |
| SSQ-4SH–4STYR | 1.256 |
| Sample | Crystalline SSQ-8SH AIBN | Oil SSQ-8SH AIBN | Crystalline SSQ-8SH DMPA | Oil SSQ-8SH DMPA |
|---|---|---|---|---|
| SSQ-4SH-4HEX | oil | oil | oil | oil |
| SSQ-4SH-4OCT | oil | oil | oil | oil |
| SSQ-4SH-4OD | wax | wax | wax | wax |
| SSQ-4SH-4OFP | highly viscous oil | highly viscous oil | highly viscous oil | highly viscous oil |
| SSQ-4SH-4VTMOS | oil | resin/insoluble polymer | resin/insoluble polymer | resin/insoluble polymer |
| SSQ-4SH-4AGE | oil | resin/insoluble polymer | resin/insoluble polymer | resin/insoluble polymer |
| SSQ-4SH-4STYR | oil | oil | oil | oil |
| N2 | 1% Mass Loss [°C] | 5% Mass Loss [°C] | Onset Temperature [°C] | Temperature at the Maximum Rate of Mass Loss [°C] | Onset Temperature [°C] | Temperature at the Maximum Rate of Mass Loss [°C] |
|---|---|---|---|---|---|---|
| SSQ-8SH oil | 243.0 | 337.5 | 343.6 | 372.8 | 465.9 | 506.5 |
| SSQ-4SH-4HEX | 275.3 | 341.9 | 356.2 | 377.2 | 470.6 | 507.6 |
| SSQ-4SH-4OCT | 295.1 | 340.5 | 355.1 | 347.4 | 471.4 | 512 |
| SSQ-4SH-4OD | 137.6 | 281.6 | 357.4 | 377.3 | 480.3 | 502.2 |
| SSQ-4SH-4OFP | 288.5 | 336.7 | 355.8 | 375.1 | 483.7 | 502.0 |
| SSQ-4SH-4STYR | 174.8 | 290.7 | 299.7 | 328.4 | 476.8 | 511.6 |
| SSQ-4SH-4MA | 123.9 | 251.5 | 352.6 | 372.1 | 480.0 | 487.1 |
| SSQ-4SH-4AGE | 126.1 | 291.9 | 336.5 | 363.5 | 492.5 | 503.5 |
| SSQ-4SH-4TMOS | 88.9 | 284.2 | 352.1 | 378.5 | 497.1 | 521.4 |
| Sample | Contact Angle [°] |
|---|---|
| Reference | 58.1 ± 0.5 |
| SSQ-8SH oil | 85.7 ± 1.5 |
| SSQ-4SH-4HEX | 63.7 ± 1.0 |
| SSQ-4SH-4OCT | 69.2 ± 1.2 |
| SSQ-4SH-4OD | 96.9 ± 2.1 |
| SSQ-4SH-4OFP | 79.1 ± 1.0 |
| SSQ-4SH-4TMOS | 60.5 ± 2.4 |
| SSQ-4SH-4AGE | 98.4 ± 2.6 |
| SSQ-4SH-4STYR | 74.2 ± 1.3 |
| The Temperature at 5% Mass Change [°C] | Onset Temperature [°C] | Temperature at the Maximum Rate of Mass Loss [°C] | ||||
|---|---|---|---|---|---|---|
| N2 | ΔT | N2 | ΔT | N2 | ΔT | |
| Ebecryl | 290.4 | - | 306.0 | - | 345.3 | - |
| 0.5% SSQ-4SH-4OFP | 297.5 | 7.1 | 321.1 | 15.1 | 358.8 | 13.5 |
| 1.0% SSQ-4SH-4OFP | 288.0 | - | 318.8 | 12.8 | 351.2 | 5.9 |
| 1.5% SSQ-4SH-4OFP | 292.1 | 1.7 | 319.2 | 13.2 | 351.8 | 6.5 |
| 2.5% SSQ-4SH-4OFP | 283.7 | - | 314.7 | 8.7 | 349.1 | 3.8 |
| 5% SSQ-4SH-4OFP | 284.0 | - | 313.9 | 7.9 | 350.1 | 4.8 |
| 10% SSQ-4SH-4OFP | 285.0 | - | 312.8 | 6.8 | 345.6 | 0.3 |
| 15% SSQ-4SH-4OFP | 301.7 | 11.3 | 322.7 | 16.7 | 354.4 | 9.1 |
| 20% SSQ-4SH-4OFP | 304.2 | 13.8 | 323.9 | 17.9 | 361.7 | 16.4 |
| 50% SSQ-4SH-4OFP | 304.4 | 14.0 | 325.2 | 19.2 | 360.2 | 14.9 |
| 0.5% SSQ-4SH-4OCT | 273.5 | - | 307.0 | 1.0 | 341.1 | - |
| 1.0% SSQ-4SH-4OCT | 277.4 | - | 310.9 | 4.9 | 341.8 | - |
| 1.5% SSQ-4SH-4OCT | 278.8 | - | 312.9 | 6.9 | 341.3 | - |
| 2.5% SSQ-4SH-4OCT | 280.7 | - | 312.5 | 6.5 | 341.0 | - |
| 5% SSQ-4SH-4OCT | 285.1 | - | 316.1 | 10.1 | 348.2 | 2.9 |
| 10% SSQ-4SH-4OCT | 291.1 | 0.7 | 318.0 | 12.0 | 348.8 | 3.5 |
| 15% SSQ-4SH-4OCT | 285.8 | - | 314.7 | 8.7 | 348.1 | 2.8 |
| 20% SSQ-4SH-4OCT | 285.7 | - | 314.6 | 8.6 | 348.3 | 3.0 |
| 50% SSQ-4SH-4OCT | 306.0 | 15.6 | 317.9 | 11.9 | 353.6 | 8.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pakuła, D.; Sztorch, B.; Przekop, R.E.; Marciniec, B. Click Addition Reaction of Urethane–Acrylate Resin Using Octa(3-thiopropyl)silsesquioxane Derivatives as Cross-Linking Agents. Processes 2023, 11, 3285. https://doi.org/10.3390/pr11123285
Pakuła D, Sztorch B, Przekop RE, Marciniec B. Click Addition Reaction of Urethane–Acrylate Resin Using Octa(3-thiopropyl)silsesquioxane Derivatives as Cross-Linking Agents. Processes. 2023; 11(12):3285. https://doi.org/10.3390/pr11123285
Chicago/Turabian StylePakuła, Daria, Bogna Sztorch, Robert E. Przekop, and Bogdan Marciniec. 2023. "Click Addition Reaction of Urethane–Acrylate Resin Using Octa(3-thiopropyl)silsesquioxane Derivatives as Cross-Linking Agents" Processes 11, no. 12: 3285. https://doi.org/10.3390/pr11123285