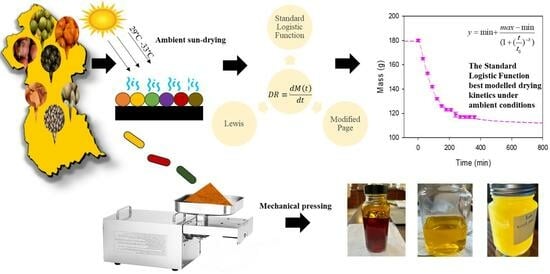
Oil Extraction and Natural Drying Kinetics of the Pulp and Seeds of Commercially Important Oleaginous Fruit from the Rainforests of Guyana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
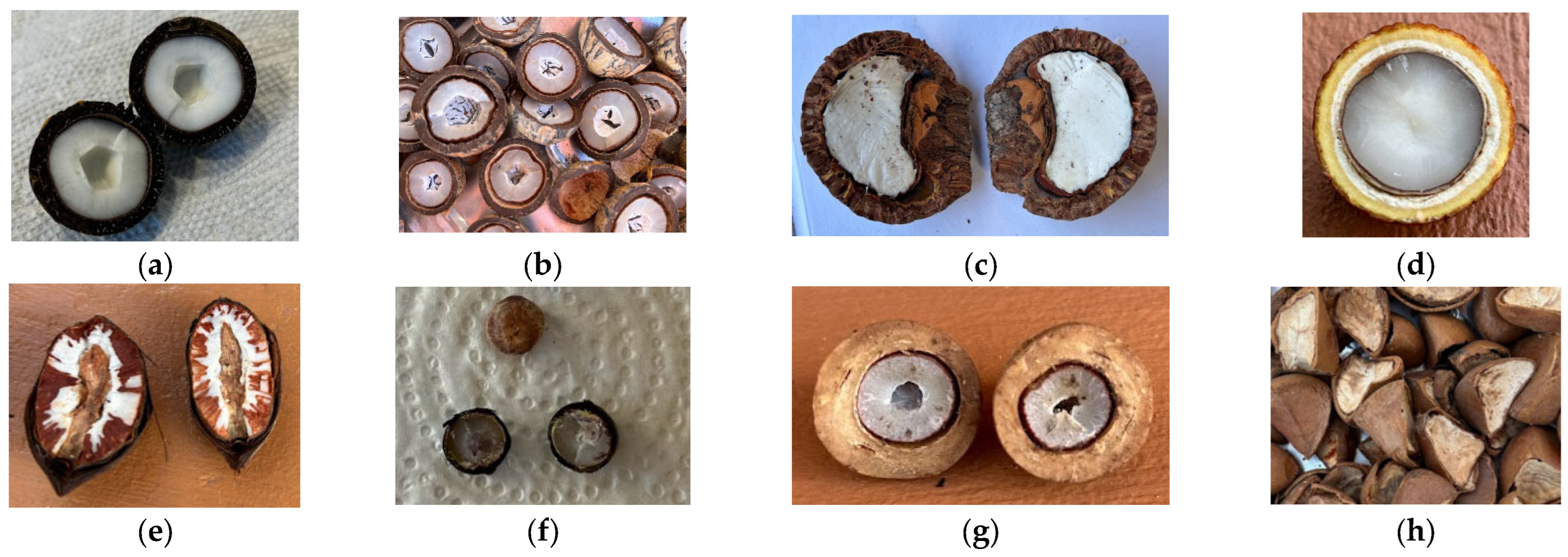
Fruit Species, Collection, and Processing
2.2. Methods
2.2.1. Drying Setup
2.2.2. Moisture Content and Pulverization
2.2.3. Oil Extraction and Mass Balance
2.2.4. Mathematical Modeling of Drying Kinetics
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Drying Kinetics and Modeling of the Experimental Data
3.1.1. Goodness of Fit
3.1.2. Drying Parameters
3.1.3. Drying Rate
3.2. Extraction of Oil from Dried Fruit
3.2.1. Fruit and Oil Description
3.2.2. Color Implication of Fresh Fruit and Oil
3.3. Mass Balance Data Table for the Eight Tropical Fruits Studied
| Fruit | Literature Oil Content (DB) w/w% | Experiment Oil Content (DB) w/w% | |||
|---|---|---|---|---|---|
| Technique | Pulp | Seed | Pulp | Seed | |
| AV | CO2 SFE | 29.8 w/w% [93] | 17–27 w/w% [94] | 31.5 w/w% | 26.8 w/w% |
| AA | CO2 SFE | 33.1 w/w% [93] | 21.9 w/w% [95] | 39.1 w/w% | 18.6 w/w% |
| OB | CO2 SFE | 45.3 w/w% [96] | -- | 12.7 w/w% | -- |
| EO | CO2 SFE | 45.4 w/w% [97] | -- | 7.8 w/w% | -- |
| MF | CO2 SFE | 41.1 w/w% [98] | 4.7 w/w% [99] | -- | -- |
| CG | PFE | -- | 17.8 w/w% [100] | -- | 43.7 w/w% |
| Soxhlet | -- | 61 w/w% [101] | -- | -- | |
| AM | Soxhlet | 8.9 w/w% [102] | 31.3 w/w% [102] | -- | 41.8 w/w% |
| CN | -- | -- | -- | -- | 26 w/w% |
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Petikirige, J.; Karim, A.; Millar, G. Effect of drying techniques on quality and sensory properties of tropical fruits. Int. J. Food Sci. Technol. 2022, 57, 6963–6979. [Google Scholar] [CrossRef]
- Filho, G.X.d.P.; Ribeiro, A.F.; Moraes, A.F.; Penha, W.F.; Borges, W.L.; Santos, R.H.S. Ethnobotanical Knowledge on Non-conventional Food and Medicinal Plants in Rio Cajari Extractivist Reserve, Amazon, Brazil. preprint 2020. [Google Scholar] [CrossRef]
- GBIF Backbone Taxonomy. Checklist Dataset. Available online: https://www.gbif.org/species/7681 (accessed on 15 May 2023).
- Baker, W.; Dransfield, J. Beyond Genera Palmarum: Progress and prospects in palm systematics. Bot. J. Linn. Soc. 2016, 182, 207–213. [Google Scholar] [CrossRef]
- Yadav, R.; Pednekar, A.; Avalaskar, A.; Rathi, M.; Rewachandani, Y. A comprehensive review on Meliaceae family. World J. Pharm. Sci. 2015, 3, 1572–1577. [Google Scholar]
- Prance, G.T.; Da Silva, M.F. Caryocaraceae. Flora Neotrop. 1973, 12, 1–75. [Google Scholar]
- Xu, J.; Wang, X.; Wang, J.; Xu, L.; Zheng, X.; Zhang, Y.; Hu, C. Dominant environmental factors influencing soil metal concentrations of Poyang Lake wetland, China: Soil property, topography, plant species and wetland type. Catena 2021, 207, 105601. [Google Scholar] [CrossRef]
- Guerin, C.; Serret, J.; Montúfar, R.; Vaissayre, V.; Bastos-Siqueira, A.; Durand-Gasselin, T.; Tregear, J.; Morcillo, F.; Dussert, S. Palm seed and fruit lipid composition: Phylogenetic and ecological perspectives. Ann. Bot. 2019, 125, 157–172. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ibañez, E.; Block, J.M. Emerging Lipids from Arecaceae Palm Fruits in Brazil. Molecules 2022, 27, 4188. [Google Scholar] [CrossRef]
- Koolen, H.H.F.; da Silva, F.M.A.; Gozzo, F.C.; de Souza, A.Q.L.; de Souza, A.D.L. Antioxidant, antimicrobial activities and characterization of phenolic compounds from buriti (Mauritia flexuosa L. f.) by UPLC–ESI-MS/MS. Food Res. Int. 2013, 51, 467–473. [Google Scholar] [CrossRef]
- Lescano, C.H.; de Oliveira, I.P.; Freitas de Lima, F.; Baldivia, D.d.S.; Justi, P.N.; Cardoso, C.A.L.; Raposo Júnior, J.L.; Sanjinez-Argandoña, E.J. Nutritional and chemical characterizations of fruits obtained from Syagrus romanzoffiana, Attalea dubia, Attalea phalerata and Mauritia flexuosa. J. Food Meas. Charact. 2018, 12, 1284–1294. [Google Scholar] [CrossRef]
- Cordeiro, L.M.C.; de Almeida, C.P.; Iacomini, M. Unusual linear polysaccharides: (1→5)-α-l-Arabinan, (1→3)-(1→4)-α-d-glucan and (1→4)-β-d-xylan from pulp of buriti (Mauritia flexuosa), an edible palm fruit from the Amazon region. Food Chem. 2015, 173, 141–146. [Google Scholar] [CrossRef]
- Manhães, L.; Menezes, E.; Marques, A.; Sabaa Srur, A. Flavored Buriti Oil (Mauritia flexuosa, Mart.) for Culinary Usage: Innovation, Production and Nutrition Value. J. Culin. Sci. Technol. 2015, 13, 362–374. [Google Scholar] [CrossRef]
- Pereira-Freire, J.A.; Barros, K.B.N.T.; Lima, L.K.F.; Martins, J.M.; Araújo, Y.d.C.; da Silva Oliveira, G.L.; de Souza Aquino, J.; Ferreira, P.M.P. Phytochemistry Profile, Nutritional Properties and Pharmacological Activities of Mauritia flexuosa. J. Food Sci. 2016, 81, R2611–R2622. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.D.E.F.G.D.O.S.; Alves, R.E.; Brito, E.S.D.; Silva, S.D.M.; Silveira, M.R.S.D.A. Quality characteristics of fruits and oils of palms native to the Brazilian amazon. Rev. Bras. Frutic. 2017, 39, e-305. [Google Scholar] [CrossRef]
- Silva, A.J.B.d.; Sevalho, E.d.S.; Miranda, I.P.d.A. Potencial das palmeiras nativas da Amazônia Brasileira para a bioeconomia: Análise em rede da produção científica e tecnológica. Ciência Florest. 2021, 31, 1020–1046. [Google Scholar] [CrossRef]
- Rincón-Cervera, M.Á.; Lahlou, A.; Chileh-Chelh, T.; Lyashenko, S.; López-Ruiz, R.; Guil-Guerrero, J.L. Arecaceae Seeds Constitute a Healthy Source of Fatty Acids and Phenolic Compounds. Plants 2023, 12, 226. [Google Scholar] [CrossRef] [PubMed]
- Agostini-Costa, T.D.S. Bioactive compounds and health benefits of some palm species traditionally used in Africa and the Americas—A review. J. Ethnopharmacol. 2018, 224, 202–229. [Google Scholar] [CrossRef]
- Trujillo-Gonzalez, J.M.; Torres Mora, M.A.; Santana-Castañeda, E. La palma de Moriche (Mauritia flexuosa Lf;) un ecosistema estratégico. Orinoquia 2011, 15, 62–70. [Google Scholar] [CrossRef]
- James, S.O.; Bouzidi, L.; Emery, R.J.N.; Narine, S.S. Lipid Fractionation and Physicochemical Characterization of Carapa guianensis Seed Oil from Guyana. Processes 2023, 11, 2565. [Google Scholar] [CrossRef]
- Andel, T. Non-Timber Forest Products of the North-West District of Guyana, Part II (Field Guide); Tropenbos-Guyana: Georgetown, Guayana, 2000. [Google Scholar]
- Bailão, E.F.L.C.; Devilla, I.A.; Da Conceição, E.C.; Borges, L.L. Bioactive Compounds Found in Brazilian Cerrado Fruits. Int. J. Mol. Sci. 2015, 16, 23760–23783. [Google Scholar] [CrossRef]
- Plowden, C. The ecology and harvest of andiroba seeds for oil production in the Brazilian Amazon. Conserv. Soc. 2004, 2, 251–272. [Google Scholar]
- Dantas, A.R.; Lira-Guedes, A.C.; Mustin, K.; Aparício, W.; Guedes, M.C. Phenology of the multi-use tree species Carapa guianensis in a floodplain forest of the Amazon Estuary. Acta Bot. Bras. 2016, 30, 618–627. [Google Scholar] [CrossRef]
- Almeida-Bezerra, J.W.; Bezerra, J.J.L.; Silva, V.B.d.; Coutinho, H.D.M.; Costa, J.G.M.d.; Cruz-Martins, N.; Hano, C.; Menezes, S.A.d.; Morais-Braga, M.F.B.; Oliveira, A.F.M.d. Caryocar coriaceum Wittm.(Caryocaraceae): Botany, Ethnomedicinal Uses, Biological Activities, Phytochemistry, Extractivism and Conservation Needs. Plants 2022, 11, 1685. [Google Scholar] [CrossRef] [PubMed]
- Wickens, G.E.; Haq, N.; Day, P.R. New Crops for Food and Industry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 1989. [Google Scholar]
- Sidhu, J.S.; Zafar, T.A. Super Fruits: Pomegranate, Wolfberry, Aronia (Chokeberry), Acai, Noni, and Amla. In Handbook of Fruits and Fruit Processing; Wiley: Hoboken, NJ, USA, 2012; pp. 653–679. [Google Scholar] [CrossRef]
- Prance, G.T. The Genus Caryocar L. (Caryocaraceae): An Underexploited Tropical Resource. Adv. Econ. Bot. 1990, 8, 177–188. [Google Scholar]
- Ascari, J.; Takahashi, J.; Boaventura, M. The phytochemistry and biological aspects of Caryocaraceae family. Rev. Bras. Plantas Med. 2013, 15, 293–308. [Google Scholar] [CrossRef]
- Lima, A.d.; Silva, A.M.d.O.; Trindade, R.A.; Torres, R.P.; Mancini-Filho, J. Composição química e compostos bioativos presentes na polpa e na amêndoa do pequi (Caryocar brasiliense, Camb.). Rev. Bras. Frutic. 2007, 29, 695–698. [Google Scholar] [CrossRef]
- De Araujo, F.D. A Review of Caryocar brasiliense (Caryocaraceae): An Economically Valuable Species of the Central Brazilian Cerrados. Econ. Bot. 1995, 49, 40–48. [Google Scholar] [CrossRef]
- Raj, S.P.; Solomon, P.R.; Thangaraj, B.; Raj, S.P.; Solomon, P.R.; Thangaraj, B. Caryocaraceae. In Biodiesel from Flowering Plants; Springer: Berlin/Heidelberg, Germany, 2022; pp. 131–134. [Google Scholar]
- Sablani, S.S. Drying of Fruits and Vegetables: Retention of Nutritional/Functional Quality. Dry. Technol. 2006, 24, 123–135. [Google Scholar] [CrossRef]
- Roberts, J.S.; Kidd, D.R.; Padilla-Zakour, O. Drying kinetics of grape seeds. J. Food Eng. 2008, 89, 460–465. [Google Scholar] [CrossRef]
- Gokhale, S.B. Pharmacognosy; Nirali Prakashan: Pune, India, 2008. [Google Scholar]
- Maisnam, D.; Rasane, P.; Dey, A.; Kaur, S.; Sarma, C. Recent advances in conventional drying of foods. J. Food Technol. Preserv. 2017, 1, 25–34. Available online: http://www.alliedacademies.org/food-technology-and-preservation/ (accessed on 15 May 2023).
- Mühlbauer, W.; Müller, J. Chapter 2.1-Drying kinetics. In Drying Atlas; Mühlbauer, W., Müller, J., Eds.; Woodhead Publishing: Sawstone, UK, 2020; pp. 53–61. [Google Scholar] [CrossRef]
- Brennan, J.G. Evaporation and dehydration. In Food Processing Handbook; Wiley: Hoboken, NJ, USA, 2006; pp. 71–124. [Google Scholar]
- Kahyaoglu, L.N.; Sahin, S.; Sumnu, G. Spouted bed and microwave-assisted spouted bed drying of parboiled wheat. Food Bioprod. Process. 2012, 90, 301–308. [Google Scholar] [CrossRef]
- Falade, K.; Igbeka, J. Osmotic dehydration of tropical fruits and vegetables. Food Rev. Int. 2007, 23, 373–405. [Google Scholar] [CrossRef]
- Silva, M.B.; Perez, V.H.; Pereira, N.R.; Silveira, T.d.C.; da Silva, N.R.F.; de Andrade, C.M.; Sampaio, R.M. Drying kinetic of tucum fruits (Astrocaryum aculeatum Meyer): Physicochemical and functional properties characterization. J. Food Sci. Technol. 2018, 55, 1656–1666. [Google Scholar] [CrossRef] [PubMed]
- da Silva, R.S.; Aguiar, N.V.; Durigon, A.; de Oliveira, A.M., Jr. Cast-tape drying and freeze drying of tucumã pulp into powder. J. Food Process Eng. 2022, 45, e13956. [Google Scholar] [CrossRef]
- Pereira, C.B.; de Souza, J.B.; da Costa Santos, D.; de Farias Leite, D.D.; de Lima Ferreira, J.P.; de Oliveira, E.N.A. Modelagem e propriedades termodinâmicas da secagem do epicarpo, mesocarpo e endocarpo do tucumã (Astrocaryum aculeatum). Braz. J. Food Technol. 2022, 1, 1–18. [Google Scholar] [CrossRef]
- Oliveira, N.L.; Silva, S.H.; Figueiredo, J.d.A.; Norcino, L.B.; Resende, J.V.d. Infrared-assisted freeze-drying (IRFD) of açai puree: Effects on the drying kinetics, microstructure and bioactive compounds. Innov. Food Sci. Emerg. Technol. 2021, 74, 102843. [Google Scholar] [CrossRef]
- Simão, R.d.S.; Zhang, L.; Moraes, J.O.d.; Schröder, A.; Laurindo, J.B.; Schutyser, M.A.I. Low-pressure conductive thin film drying of açaí pulp. LWT 2022, 164, 113695. [Google Scholar] [CrossRef]
- Nagata, G.A.; Costa, T.V.; Perazzini, M.T.B.; Perazzini, H. Coupled heat and mass transfer modelling in convective drying of biomass at particle-level: Model validation with experimental data. Renew. Energy 2020, 149, 1290–1299. [Google Scholar] [CrossRef]
- Morais, M.F.d.; dos Santos, J.R.; Santos, M.P.d.; Santos, D.d.C.; Costa, T.N.d.; Lima, J.B. Modeling and thermodynamic properties of ‘bacaba’ pulp drying. Rev. Bras. Eng. Agrícola Ambient. 2019, 23, 702–708. [Google Scholar] [CrossRef]
- Resende, L.M.; Franca, A.S.; Oliveira, L.S. Buriti (Mauritia flexuosa L. f.) fruit by-products flours: Evaluation as source of dietary fibers and natural antioxidants. Food Chem. 2019, 270, 53–60. [Google Scholar] [CrossRef]
- Chen, C.; Pan, Z. An overview of progress, challenges, needs and trends in mathematical modeling approaches in food drying. Dry. Technol. 2023, 1–20. [Google Scholar] [CrossRef]
- Akter, F.; Muhury, R.; Sultana, A.; Deb, U.K. A Comprehensive Review of Mathematical Modeling for Drying Processes of Fruits and Vegetables. Int. J. Food Sci. 2022, 2022, 6195257. [Google Scholar] [CrossRef] [PubMed]
- Buzrul, S. Reassessment of Thin-Layer Drying Models for Foods: A Critical Short Communication. Processes 2022, 10, 118. [Google Scholar] [CrossRef]
- Lewis, W.K. The Rate of Drying of Solid Materials. J. Ind. Eng. Chem. 1921, 13, 427–432. [Google Scholar] [CrossRef]
- Hutchinson, D.; Otten, L. Thin-layer air drying of soybeans and white beans. Int. J. Food Sci. Technol. 1983, 18, 507–522. [Google Scholar] [CrossRef]
- Alibas, I. Microwave, air and combined microwave–air-drying parameters of pumpkin slices. LWT-Food Sci. Technol. 2007, 40, 1445–1451. [Google Scholar] [CrossRef]
- Page, G.E. Factors Influencing the Maximum Rates of Air Drying Shelled Corn in Thin Layers; Purdue University: West Lafayette, IN, USA, 1949. [Google Scholar]
- Overhults, D.G.; White, G.; Hamilton, H.; Ross, I. Drying soybeans with heated air. Trans. ASAE 1973, 16, 112–113. [Google Scholar] [CrossRef]
- Corzo, O.; Bracho, N.; Pereira, A.; Vásquez, A. Weibull distribution for modeling air drying of coroba slices. LWT-Food Sci. Technol. 2008, 41, 2023–2028. [Google Scholar] [CrossRef]
- Karacabey, E.; Buzrul, S. Modeling and predicting the drying kinetics of apple and pear: Application of the Weibull model. Chem. Eng. Commun. 2017, 204, 573–579. [Google Scholar] [CrossRef]
- Johnson, M. Parameter correlations while curve fitting. Methods Enzymol. 2000, 321, 424–446. [Google Scholar] [CrossRef]
- Whitaker, S. Simultaneous Heat, Mass, and Momentum Transfer in Porous Media: A Theory of Drying. In Advances in Heat Transfer; Hartnett, J.P., Irvine, T.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1977; Volume 13, pp. 119–203. [Google Scholar]
- Erbay, Z.; Icier, F. A Review of Thin Layer Drying of Foods: Theory, Modeling, and Experimental Results. Crit. Rev. Food Sci. Nutr. 2010, 50, 441–464. [Google Scholar] [CrossRef] [PubMed]
- The-Weather-Channel. Weather in Georgetown, Demerara-Mahaica, Guyana. USA. 2023. Available online: https://weather.com/weather/today/l/6.80,-58.15?par=google (accessed on 17 September 2023).
- Mujumdar, A.S. Handbook of Industrial Drying; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Henderson, S. Grain drying theory, I. Temperature effect on drying coefficient-No Paper Link Available. J. Agr. Eng. Res. 1961, 6, 169–173. [Google Scholar]
- Tsoularis, A.; Wallace, J. Analysis of logistic growth models. Math. Biosci. 2002, 179, 21–55. [Google Scholar] [CrossRef]
- Burgos-Simón, C.; Cortés, J.-C.; Martínez-Rodríguez, D.; Villanueva, R.J. Modeling breast tumor growth by a randomized logistic model: A computational approach to treat uncertainties via probability densities. Eur. Phys. J. Plus 2020, 135, 826. [Google Scholar] [CrossRef]
- Yin, X.; Zelenay, P. (Invited) Kinetic Models for the Degradation Mechanisms of PGM-Free ORR Catalysts. ECS Trans. 2018, 85, 1239. [Google Scholar] [CrossRef]
- Pelinovsky, E.; Kurkin, A.; Kurkina, O.; Kokoulina, M.; Epifanova, A. Logistic equation and COVID-19. Chaos Solitons Fractals 2020, 140, 110241. [Google Scholar] [CrossRef]
- Reed, L.J.; Berkson, J. The Application of the Logistic Function to Experimental Data. J. Phys. Chem. 1929, 33, 760–779. [Google Scholar] [CrossRef]
- Cunha, L.M.; Oliveira, F.A.R.; Oliveira, J.C. Optimal experimental design for estimating the kinetic parameters of processes described by the Weibull probability distribution function. J. Food Eng. 1998, 37, 175–191. [Google Scholar] [CrossRef]
- Motulsky, H.; Christopoulos, A. Fitting Models to Biological Data Using Linear and Nonlinear Regression: A Practical Guide to Curve Fitting; Oxford University Press: Oxford, UK, 2004. [Google Scholar]
- Van Boekel, M. To pool or not to pool: That is the question in microbial kinetics. Int. J. Food Microbiol. 2021, 354, 109283. [Google Scholar] [CrossRef]
- Zelinka, S.L.; Kirker, G.T.; Bishell, A.B.; Glass, S.V. Effects of Wood Moisture Content and the Level of Acetylation on Brown Rot Decay. Forests 2020, 11, 299. [Google Scholar] [CrossRef]
- Barrett, J.P. The Coefficient of Determination—Some Limitations. Am. Stat. 1974, 28, 19–20. [Google Scholar] [CrossRef]
- Cunha, L.M.; Oliveira, F.A.; Aboim, A.P.; Frías, J.M.; Pinheiro-Torres, A. Stochastic approach to the modelling of water losses during osmotic dehydration and improved parameter estimation. Int. J. Food Sci. Technol. 2001, 36, 253–262. [Google Scholar] [CrossRef]
- Surendhar, A.; Sivasubramanian, V.; Vidhyeswari, D.; Deepanraj, B. Energy and exergy analysis, drying kinetics, modeling and quality parameters of microwave-dried turmeric slices. J. Therm. Anal. Calorim. 2019, 136, 185–197. [Google Scholar] [CrossRef]
- Motevali, A.; Minaei, S.; Banakar, A.; Ghobadian, B.; Darvishi, H. Energy analyses and drying kinetics of chamomile leaves in microwave-convective dryer. J. Saudi Soc. Agric. Sci. 2016, 15, 179–187. [Google Scholar] [CrossRef]
- Soysal, Y.; Öztekin, S.; Eren, Ö. Microwave drying of parsley: Modelling, kinetics, and energy aspects. Biosyst. Eng. 2006, 93, 403–413. [Google Scholar] [CrossRef]
- Darvishi, H.; Asl, A.R.; Asghari, A.; Azadbakht, M.; Najafi, G.; Khodaei, J. Study of the drying kinetics of pepper. J. Saudi Soc. Agric. Sci. 2014, 13, 130–138. [Google Scholar] [CrossRef]
- Zarein, M.; Samadi, S.H.; Ghobadian, B. Investigation of microwave dryer effect on energy efficiency during drying of apple slices. J. Saudi Soc. Agric. Sci. 2015, 14, 41–47. [Google Scholar] [CrossRef]
- Noronha Matos, K.A.; Praia Lima, D.; Pereira Barbosa, A.P.; Zerlotti Mercadante, A.; Campos Chisté, R. Peels of tucumã (Astrocaryum vulgare) and peach palm (Bactris gasipaes) are by-products classified as very high carotenoid sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- do Nascimento Silva, N.R.R.; Cavalcante, R.B.M.; da Silva, F.A. Nutritional properties of Buriti (Mauritia flexuosa) and health benefits. J. Food Compos. Anal. 2023, 117, 105092. [Google Scholar] [CrossRef]
- Finco, F.; Kammerer, D.R.; Carle, R.; Tseng, W.-H.; Böser, S.; Graeve, L. Antioxidant activity and characterization of phenolic compounds from bacaba (Oenocarpus bacaba Mart.) fruit by HPLC-DAD-MS. J. Agric. Food Chem. 2012, 60, 7665–7673. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Matta, F.V.; Grace, M.; Lila, M.A.; Ward, N.I.; Felipe-Sotelo, M.; Esposito, D. Phenolic content, anti-inflammatory properties, and dermal wound repair properties of industrially processed and non-processed acai from the Brazilian Amazon. Food Funct. 2020, 11, 4903–4914. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food.Chem 2011, 128, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sun, S. Color Reversion of Refined Vegetable Oils: A Review. Molecules 2023, 28, 5177. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.T.A.; Pereira, R.R.; Duarte Junior, A.P.; da Cruz Rodrigues, A.M.; Remédios, C.M.R.; Brasil, D.d.S.B.; Morais, L.R.B.; Silva-Júnior, J.O.C.; Ribeiro-Costa, R.M. Tucumã (Astrocaryum vulgare) fat: An Amazonian material as a pharmaceutical input for lipid nanoparticle production. J. Therm. Anal. Calorim. 2020, 147, 355–365. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, X.; Liu, S.Q. Aroma modulation of vegetable oils—A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F. (Ed.) Chapter 1-Introduction to cold pressed oils: Green technology, bioactive compounds, functionality, and applications. In Cold Pressed Oils; Academic Press: Cambridge, MA, USA, 2020; pp. 1–5. [Google Scholar] [CrossRef]
- Danlami, J.M.; Arsad, A.; Ahmad Zaini, M.A.; Sulaiman, H. A comparative study of various oil extraction techniques from plants. Rev. Chem. Eng. 2014, 30, 605–626. [Google Scholar] [CrossRef]
- Mehalaine, S.; Chenchouni, H. Quantifying how climatic factors influence essential oil yield in wild-growing plants. Arab. J. Geosci. 2021, 14, 1257. [Google Scholar] [CrossRef]
- Fornasari, C.H.; Secco, D.; Santos, R.F.; da Silva, T.R.B.; Lenz, N.B.G.; Tokura, L.K.; Lenz, M.L.; Souza, S.N.M.d.; Junior, L.A.Z.; Gurgacz, F. Efficiency of the use of solvents in vegetable oil extraction at oleaginous crops. Renew. Sustain. Energy Rev. 2017, 80, 121–124. [Google Scholar] [CrossRef]
- Costa, B.E.T.; Santos, O.V.d.; CorrÊA, N.C.F.; FranÇA, L.F.d. Comparative study on the quality of oil extracted from two tucumã varieties using supercritical carbon dioxide. Food Sci. Technol. 2016, 36, 322–328. [Google Scholar] [CrossRef]
- Didonet, A.A.; Antoniassi, R.; Back, G.R.; de Faria-Machado, A.F.; Wilhelm, A.E.; Ferraz, I.D.K. Characterization of Amount and Quality of Tucuman Kernel Oil as a Potential Biomass. J. Am. Oil Chem. Soc. 2020, 97, 955–962. [Google Scholar] [CrossRef]
- Zaninetti, R.; Moreira, A.; Ferraudo, A.; Teixeira, S. Population variability in production of oil, total lipids in kernel and pulp of tucumã (Astrocaryum aculeatum) collected in the Acre State, Brazil. Agrotrópica 2016, 28, 179–184. [Google Scholar] [CrossRef]
- Cunha, V.M.B.; Silva, M.P.d.; Sousa, S.H.B.d.; Bezerra, P.d.N.; Menezes, E.G.O.; Silva, N.J.N.d.; Banna, D.A.D.d.S.; Araújo, M.E.; Carvalho Junior, R.N.d. Bacaba-de-leque (Oenocarpus distichus Mart.) oil extraction using supercritical CO2 and bioactive compounds determination in the residual pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- de Cássia Rodrigues Batista, C.; de Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2016, 107, 364–369. [Google Scholar] [CrossRef]
- Best, I.; Cartagena-Gonzales, Z.; Arana-Copa, O.; Olivera-Montenegro, L.; Zabot, G. Production of Oil and Phenolic-Rich Extracts from Mauritia flexuosa L.f. Using Sequential Supercritical and Conventional Solvent Extraction: Experimental and Economic Evaluation. Processes 2022, 10, 459. [Google Scholar] [CrossRef]
- Balick, M.J. Amazonian oil palms of promise: A survey. Econ. Bot. 1979, 33, 11–28. [Google Scholar] [CrossRef]
- Novello, Z.; Scapinello, J.; Magro, J.D.; Zin, G.; Luccio, M.D.; Tres, M.V.; Oliveira, J.V. Extraction, chemical characterization and antioxidant activity of andiroba seeds oil obtained from pressurized n-butane. Ind. Crops Prod. 2015, 76, 697–701. [Google Scholar] [CrossRef]
- Araujo-Lima, C.F.; Fernandes, A.S.; Gomes, E.M.; Oliveira, L.L.; Macedo, A.F.; Antoniassi, R.; Wilhelm, A.E.; Aiub, C.A.F.; Felzenszwalb, I. Antioxidant Activity and Genotoxic Assessment of Crabwood (Andiroba, Carapa guianensis Aublet) Seed Oils. Oxidative Med. Cell. Longev. 2018, 2018, 3246719. [Google Scholar] [CrossRef] [PubMed]
- Bereau, D.; Benjelloun-Mlayah, B.; Delmas, M. Maximiliana maripa drude mesocarp and kernel oils: Fatty acid and total tocopherol compositions. J. Am. Oil Chem. Soc. 2001, 78, 213–214. [Google Scholar] [CrossRef]









| Scientific Name | Fruit Acronym | Fruit Material | Material Acronym |
|---|---|---|---|
| Astrocaryum vulgare | AV | Pulp | AVP |
| Seed | AVS | ||
| Kernel | AVK | ||
| Astrocaryum aculeatum | AA | Pulp | AAP |
| Seed | AAS | ||
| Kernel | AAK | ||
| Oenocarpus bacaba | OB | Pulp | OBP |
| Seed | OBS | ||
| Euterpe oleracea | EO | Pulp | EOP |
| Seed | EOS | ||
| Mauritia flexuosa | MF | Pulp | MFP |
| Seed | MFS | ||
| Attalea maripa | AM | Seed | AMS |
| Kernel | AMK | ||
| Carapa guianensis | CG | Seed | CGS |
| Kernel | CGK | ||
| Caryocar nuciferum | CN | Seed | CNS |
| Kernel | CNK |
| Class | Model Name | Parameter Correlation | Comment | Ref | |
|---|---|---|---|---|---|
| Logarithmic |
| Simplest but fails to model many drying data | [52] | ||
| Mild to strong | [53,54] | |||
| Sigmoidal |
| Strong | Simple, describes many drying data | [55] | |
| Low | Same as Page model with fewer errors on the rate parameter | [56] | ||
| Low | Simple, describes many drying data | [57] | ||
| Mild | Simple, describes many drying data. | [58] | |||
| Mild | Can describe many experimental data | This work |
| (a) Sun-Dried Pulp | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| AAP | AVP | EOP | |||||||
| SLF | M. Page | Lewis | SLF | M. Page | Lewis | SLF | M. Page | Lewis | |
| 2 | 0.373 | 0.445 | 0.385 | 0.211 | 1.3851 | 1.56 | 0.150 | 0.089 | 0.215 |
| 0.996 | 0.991 | 0.987 | 0.988 | 0.994 | 0.986 | 0.996 | 0.998 | 0.994 | |
| RSS | 56.7 ± 12.6 | 49.2 ± 12.0 | 88.2 ± 36.8 | 335.4 ± 320.3 | 60.6 ± 12.2 | 196.5 ± 47.6 | 21.4 ± 4.9 | 11.0 ± 0.6 | 33.1 ± 8.7 |
| (min) | 283.3 ± 59.9 | 260.7 ± 30.8 | 347.1 ± 22.1 | 201.6 ± 15.1 | 222.5 ± 7.9 | 288.6 ± 26.6 | 76.5 ± 1.2 | 95.1 ± 1.2 | 98.5 ± 2.4 |
| 1.3 ± 0.2 | 1.2 ± 0.1 | 1.0 | 0.9 ± 0.1 | 1.2 ± 0.2 | 1.0 | 1.7 ± 0.1 | 0.95 ± 0.1 | 1.0 | |
| (%) | 63.1± 9.7 | 47.8 ± 4.7 | 39.3 ± 1.4 | 56.5 ± 0.2 | 35.2 ± 9.4 | 45.7 ± 1.4 | 61.7 ± 0.3 | 61.8 ± 0.3 | 63.3 ± 0.6 |
| OBP | MFP | ||||||||
| SLF | M. Page | Lewis | SLF | M. Page | Lewis | ||||
| 2 | 0.362 | 0.514 | 0.5888 | 2.23 | 1.277 | 2.587 | |||
| 0.992 | 0.9916 | 0.989 | 0.991 | 0.993 | 0.99 | ||||
| RSS | 41.8 ± 19.7 | 65.1 ± 33.1 | 58.6 ± 34.2 | 513.3 ± 106.5 | 408.4 ± 100.5 | 573.4 ± 103.9 | |||
| (min) | 66.1 ± 2.1 | 91.4 ± 3.2 | 91.4 ± 2.3 | 454.4 ± 25.1 | 470.5 ± 22.5 | 586.5 ± 74.7 | |||
| 1.3 ± 0.1 | 0.9 ± 0 | 1 | 1.5 ± 0.1 | 1.4 ± 0.1 | 1 | ||||
| (%) | 44.6 ± 30.7 | 64.8 ± 0.5 | 65.9 ± 1.3 | 61.7 ± 0.3 | 25.7 ± 0.4 | 13.1 ± 2.41 | |||
| (b) Sun-dried seeds | |||||||||
| AAS | CGS | ||||||||
| SLF | M. Page | Lewis | SLF | M. Page | Lewis | ||||
| 2 | 0.659 | 0.793 | 0.859 | 0.151 | 0.151 | 0.211 | |||
| 0.995 | 0.994 | 0.99 | 0.991 | 0.991 | 0.989 | ||||
| RSS | 93 ± 47.8 | 24,968.6 ± 42,209.4 | 672.5 ± 977.5 | 94.7 ± 27.4 | 94.7 ± 27.9 | 119.0 ± 43.9 | |||
| (min) | 475.5 ± 191.1 | 359.3 ± 56.13 | 395.3 ± 56.6 | 2315.5 ± 1520.5 | 1361.6 ± 755.7 | 535.6 ± 20.9 | |||
| 1.3 ± 0 | 1.242 ± 0.249 | 1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 1 | ||||
| (%) | 67.3 ± 5.3 | 70.7 ± 3.7 | 69.1 ± 2.7 | 50.6 ± 15.3 | 66.1 ± 8.8 | 78.4 ± 0.7 | |||
| Materials | Mass Loss (%) | |||
|---|---|---|---|---|
| Experimental | SLF | M. Page | Lewis | |
| Sun dried pulps | ||||
| AAP | 46.5 ± 0.8 | 63.1 ± 9.7 | 49.6 ± 9.4 | 60.7 ± 1.4 |
| AVP | 47.0 ± 0.6 | 43.5 ± 0.2 | 68.0 ± 9.4 | 54.3 ± 1.4 |
| EOP | 35.0 ± 0.6 | 38.0 ± 0.3 | 35.1 ± 0.3 | 36.7 ± 0.6 |
| MFP | 69.6 ± 0.6 | 90.6 ± 0.7 | 74.5 ± 0.4 | 86.9 ± 4.9 |
| OBP | 34 ± 1.0 | 36.6 ± 1.7 | 34.4 ± 1.5 | 34.1 ± 1.3 |
| Sun-dried seeds | ||||
| AAS | 24.1 ± 3.1 | 29.4 ± 2.3 | 26.2 ± 1.9 | 26.6 ± 2.7 |
| CGS | 17 ± 0.1 | 61.5 ± 6.2 | 38.1 ± 8.7 | 38.1 ± 8.1 |
| Fruit | Fruit Colour | Shape | Length (cm) | Width (cm) | Fruit Mass (g) | Pulp Mass (g) | Kernel Mass (g) | |
|---|---|---|---|---|---|---|---|---|
| (i) | AV | Orange | Oval-spherical | 4.4 ± 0.8 | 3.2 ± 0.6 | 31.8 ± 11.3 | 18.0 ± 7.6 | 17 ± 3.2 |
| (ii) | AA | Yellow-green | Oval-spherical | 5.6 ± 2.3 | 5.2 ± 1.2 | 41.1 ± 13 | 25.9 ± 1.0 | 21.3 ± 4.6 |
| (iii) | OB | Purple | Elliptical-globular | 3.54 ± 0.1 | 3.04 ± 0.5 | 11.2 ± 2.4 | 4.2 ± 0.3 | 6.66 ± 1.7 |
| (iv) | EO | Purple | Globular | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.32 ± 0.1 | 0.42 ± 0.1 | 0.9 ± 0.1 |
| (v) | MF | Chestnut | Globular-elongated | 5.75 ± 0.4 | 4.70 ± 0.5 | 55.1 ± 9.8 | 18.7 ± 3.3 | 20.83 ± 7.4 |
| (vi) | CG | Yellow-brown | Irregular quadrilateral | 7.1 ± 0.4 | 5.8 ± 0.1 | 19.6 ± 4.4 | - | 14.8 ± 1.5 |
| (vii) | CN | Yellow-brown | Kidney-shaped | 7.46 ± 0.2 | 6.56 ± 0.2 | 123.6 ± 10.5 | - | 23 ± 3.8 |
| (viii) | AM | Cream-yellow | Oblong ovoid | 4.9 ± 0.2 | 2.3 ± 0.1 | 20.3 ± 1.5 | 4.7 ± 1.4 | 9.8 ± 0.6 |
| Parameters | AV | AA | OB | EO | MF | CG | CN | AM |
|---|---|---|---|---|---|---|---|---|
| Fresh Fruit Mass (FFM) (g) | 29,800 | 12,112 | 24,000 | 9500 | 38,000 | 14,165 | 1485 | 22,700 |
| Fresh Pulp Mass (g) | 16,344 | 5975 | 6510 | 2542 | 20,519 | -- | -- | -- |
| Dried Pulp Mass (g) | 11,510 | 3337 | 4770 | 1757 | 6675 | -- | -- | -- |
| Oil Pulp Mass (g) | 2533 | 1471 | 682 | 137 | -- | -- | -- | -- |
| Fresh Kernel Mass (g) | 12,678 | 6137 | 16,843 | 5848 | 9130 | -- | 313 | 22,400 |
| Dried Kernel Mass (g) | 6238 | 4481 | -- | -- | -- | 8989 | 173 | 1632 |
| Deshelled Kernel Mass (g) | 5178 | 1695 | -- | -- | -- | 5894 | -- | 3904 |
| Kernel Oil Mass (g) | 1526 | 239 | -- | -- | -- | 2576 | 45 | 1632 |
| Pulp Flour Mass (g) | 6900 | 900 | 3336 | 1417 | -- | -- | -- | -- |
| Kernel Flour Mass (g) | 2764 | 487 | -- | -- | 1885 | 56 | 1318 | |
| Filter Paper Residue Mass (g) | 290 | - | 435 | 89 | -- | 624 | -- | 747 |
| Fresh pulp moisture (w/w%) | 53.5 ± 2.0 | 29.8 ± 1.1 | 35.4 ± 1.5 | 41.2 ± 1.0 | 71.0 ± 1.1 | -- | -- | -- |
| Dried pulp moisture (w/w%) | 5.5 ± 1.0 | 5.0 ± 1.0 | 5.0 ± 1.0 | 5.0 ± 1.1.0 | 5.9 ± 1.0 | -- | -- | -- |
| Fresh kernel moisture (w/w%) | 24.4 ± 2.0 | 37.2 ± 2.5 | -- | -- | -- | 50.8 ± 5.2 | 35.5 ± 1.0 | -- |
| Dried kernel moisture (w/w%) | -- | -- | -- | -- | -- | 5.2 ± 1.0 | 5.0 ± 1.0 | 4.7 ± 1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deonarine, S.; Soodoo, N.; Bouzidi, L.; Narine, S.S. Oil Extraction and Natural Drying Kinetics of the Pulp and Seeds of Commercially Important Oleaginous Fruit from the Rainforests of Guyana. Processes 2023, 11, 3292. https://doi.org/10.3390/pr11123292
Deonarine S, Soodoo N, Bouzidi L, Narine SS. Oil Extraction and Natural Drying Kinetics of the Pulp and Seeds of Commercially Important Oleaginous Fruit from the Rainforests of Guyana. Processes. 2023; 11(12):3292. https://doi.org/10.3390/pr11123292
Chicago/Turabian StyleDeonarine, Shaveshwar, Navindra Soodoo, Laziz Bouzidi, and Suresh S. Narine. 2023. "Oil Extraction and Natural Drying Kinetics of the Pulp and Seeds of Commercially Important Oleaginous Fruit from the Rainforests of Guyana" Processes 11, no. 12: 3292. https://doi.org/10.3390/pr11123292
APA StyleDeonarine, S., Soodoo, N., Bouzidi, L., & Narine, S. S. (2023). Oil Extraction and Natural Drying Kinetics of the Pulp and Seeds of Commercially Important Oleaginous Fruit from the Rainforests of Guyana. Processes, 11(12), 3292. https://doi.org/10.3390/pr11123292