Novel Phospholipase C with High Catalytic Activity from a Bacillus stearothermophilus Strain: An Ideal Choice for the Oil Degumming Process
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials and Reagents
2.2. Phospholipase Production and Culture Condition Optimization
2.3. Purification of PLCBs
2.4. Protein Analysis
2.5. PLCBs Activity Measurement
2.6. Catalytic Characteristics of PLCBs
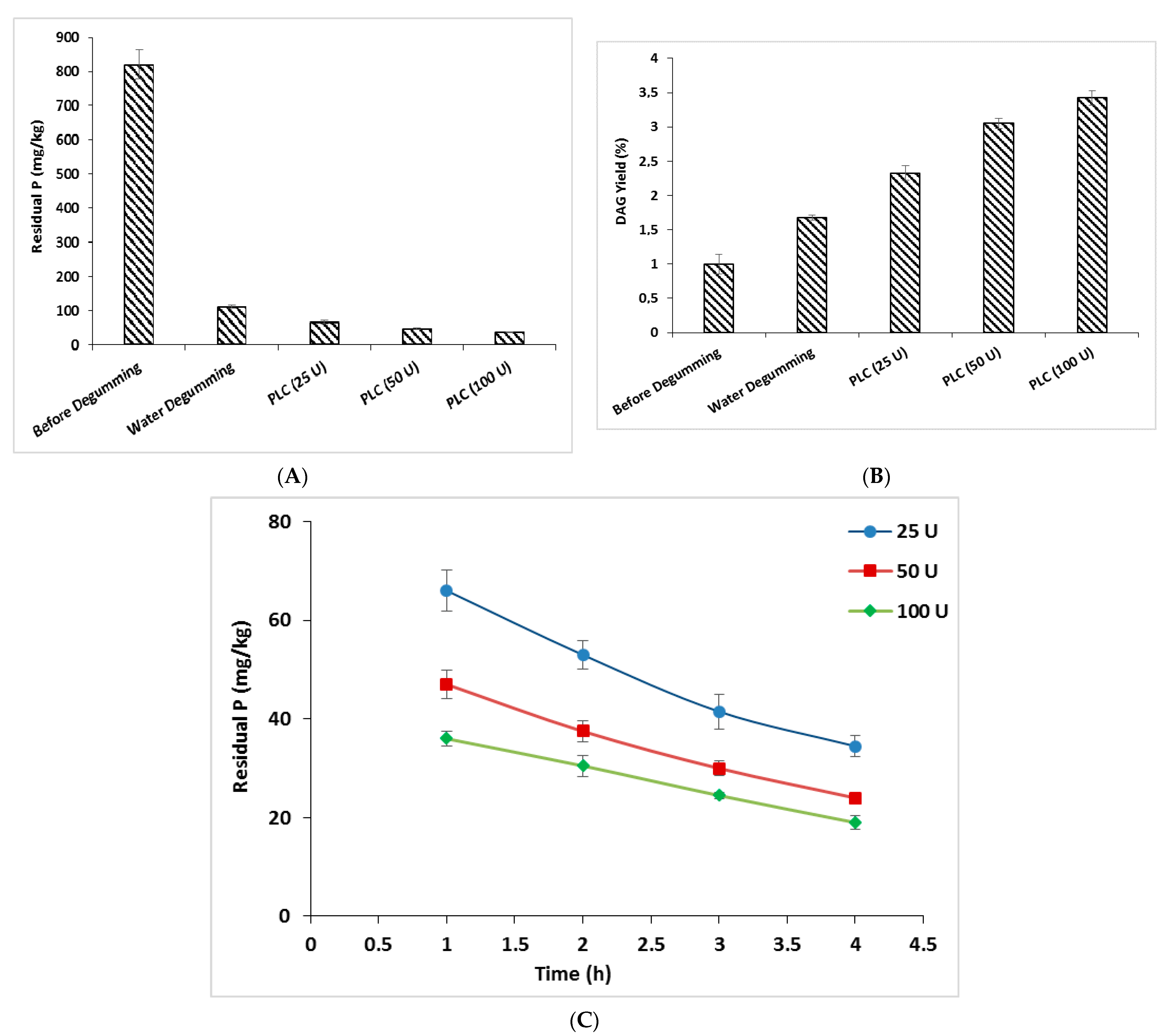
2.7. Biotechnological Potential of PLCBs Oil Degumming
2.8. Antimicrobial Activity
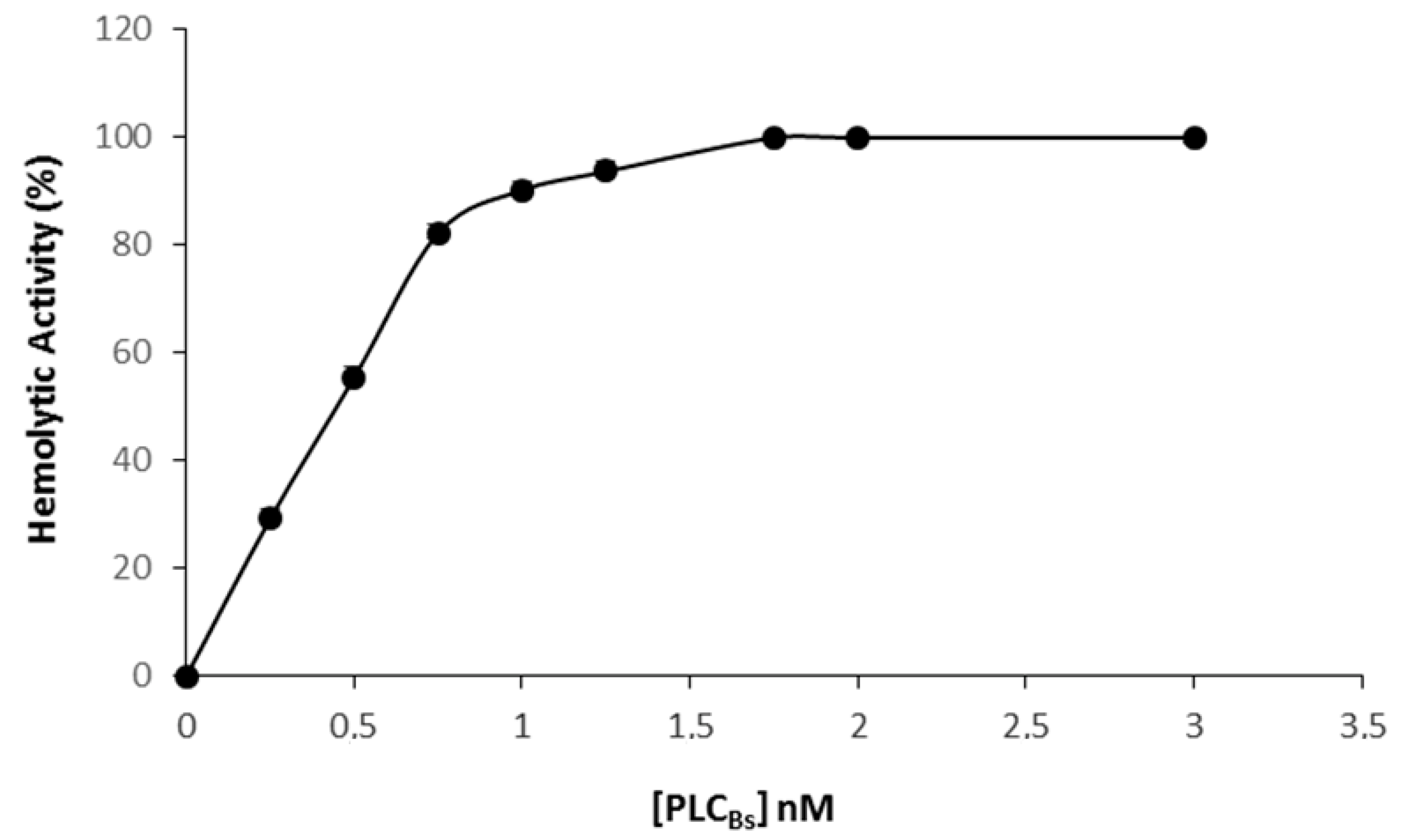
2.9. Direct Hemolytic Activity
3. Results and Discussion
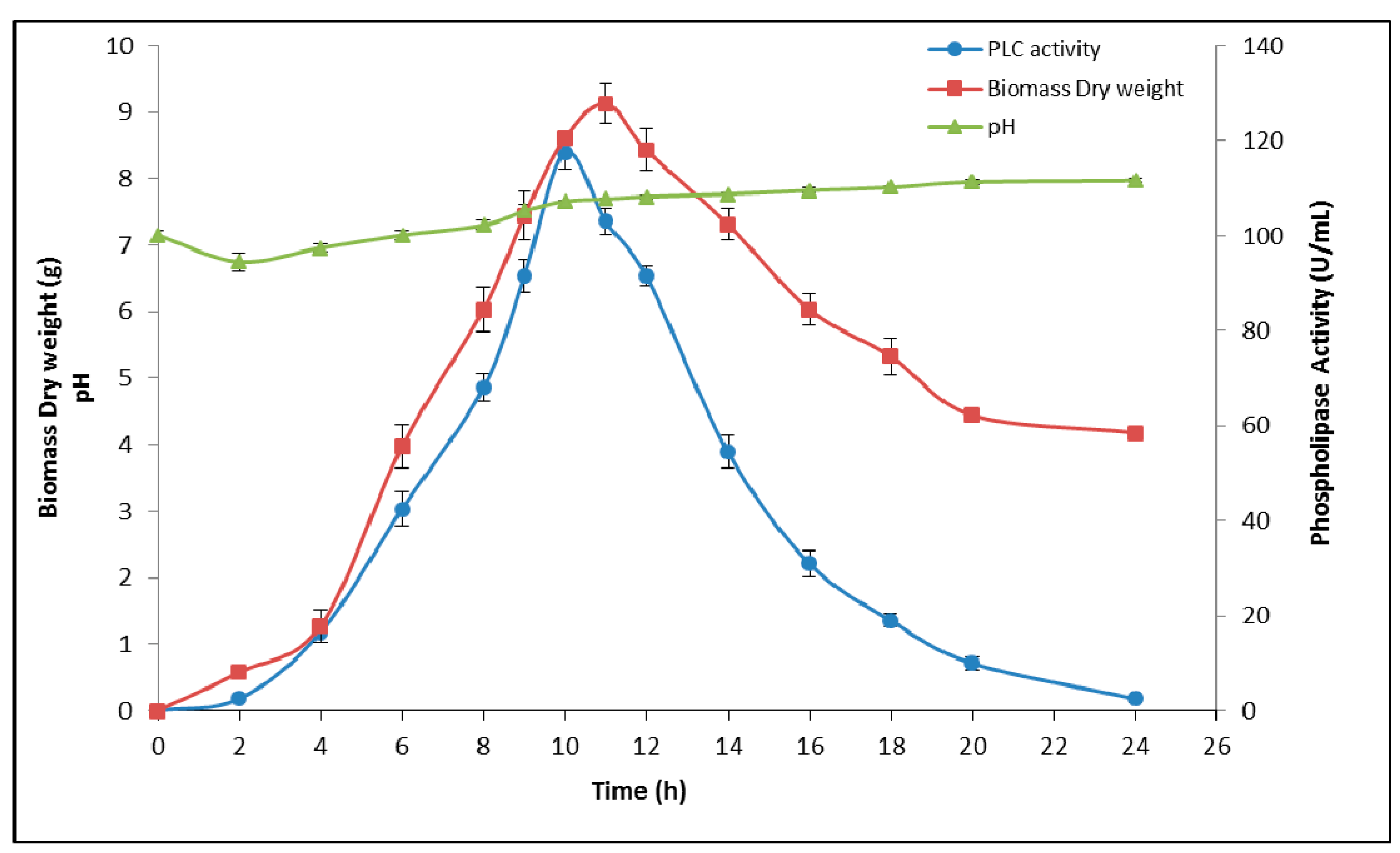
3.1. Optimization of Medium Compounds for PLCBs Production
Incubation Time
3.2. Temperature
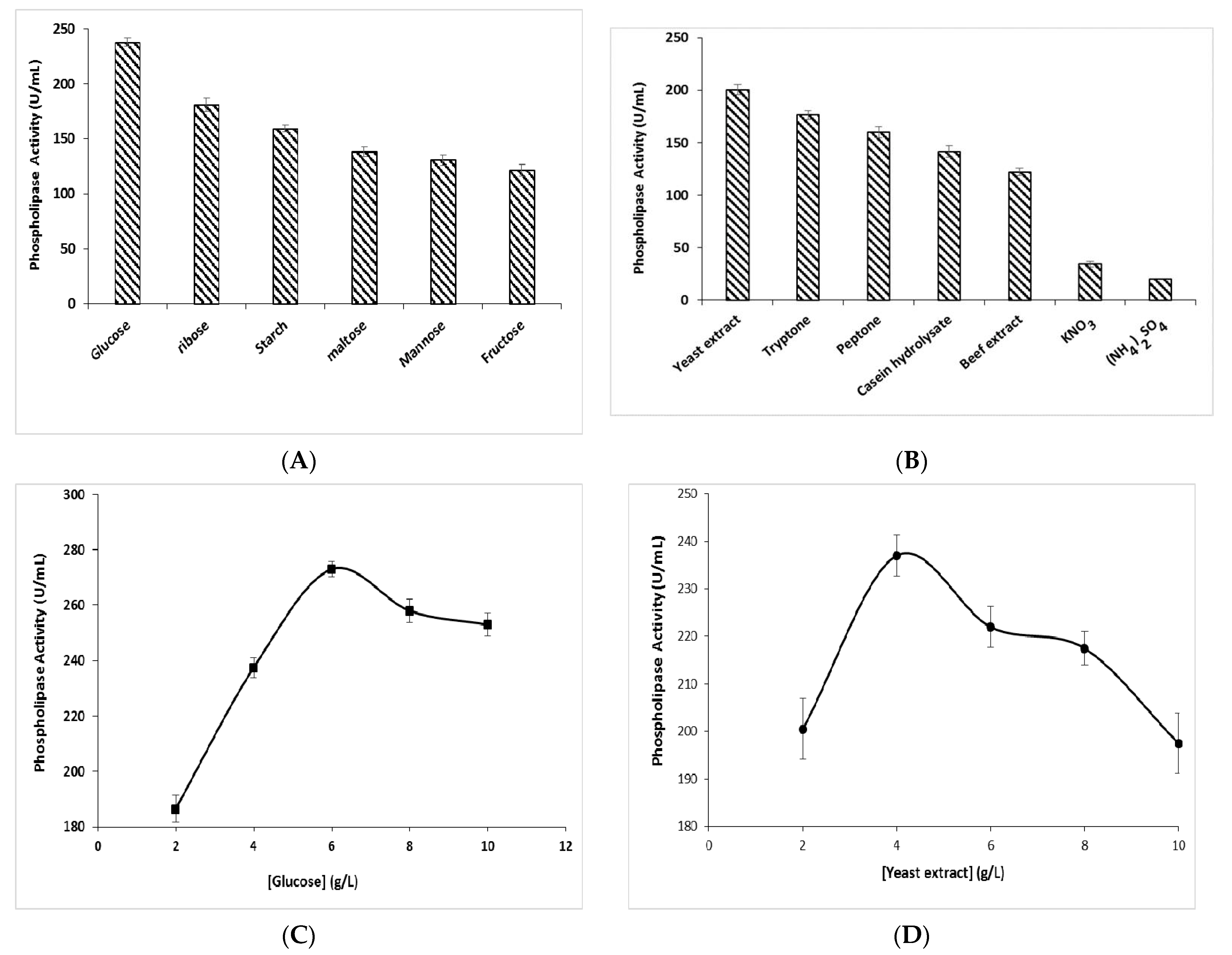
3.3. Effect of Carbon and Nitrogen Sources
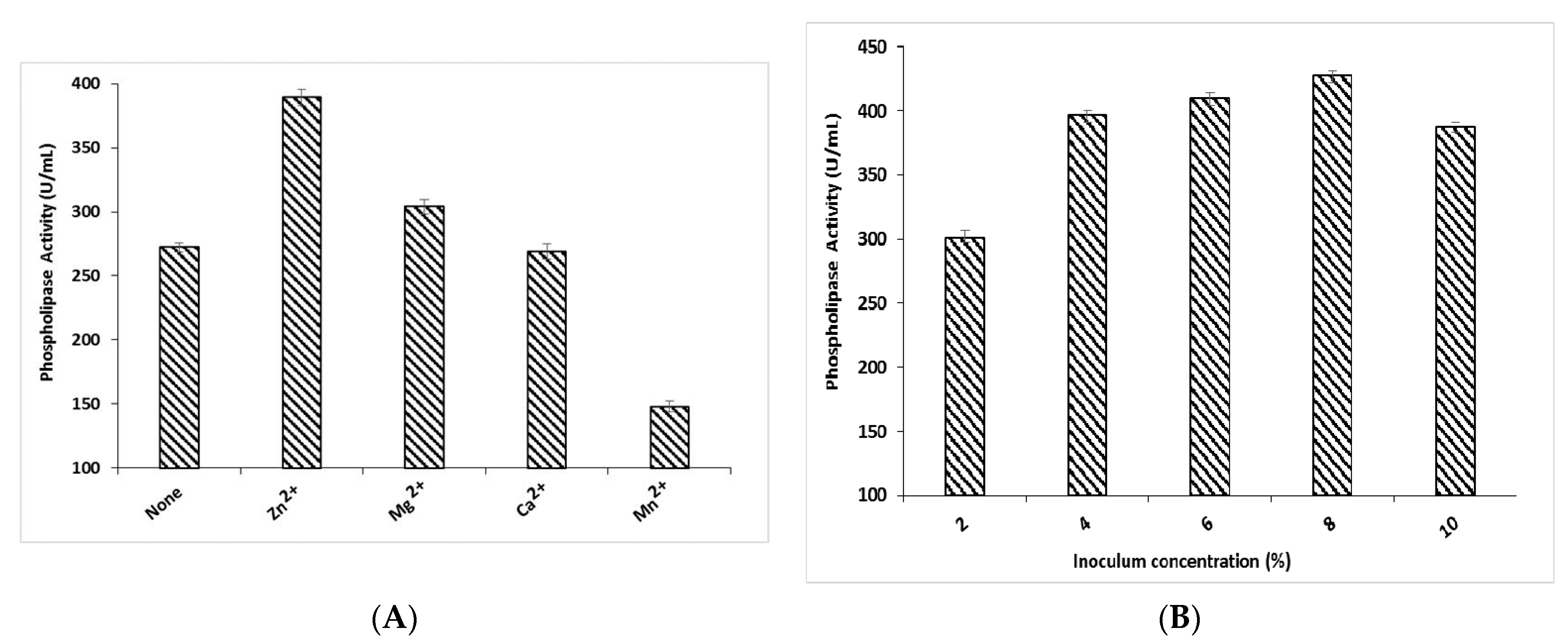
3.4. Effect of Metal Ions and Inoculum Concentration
3.5. PLCBs Purification and Biochemical Characterization
Purification of PLCBs
3.6. Biochemical Characterization of PLCBs
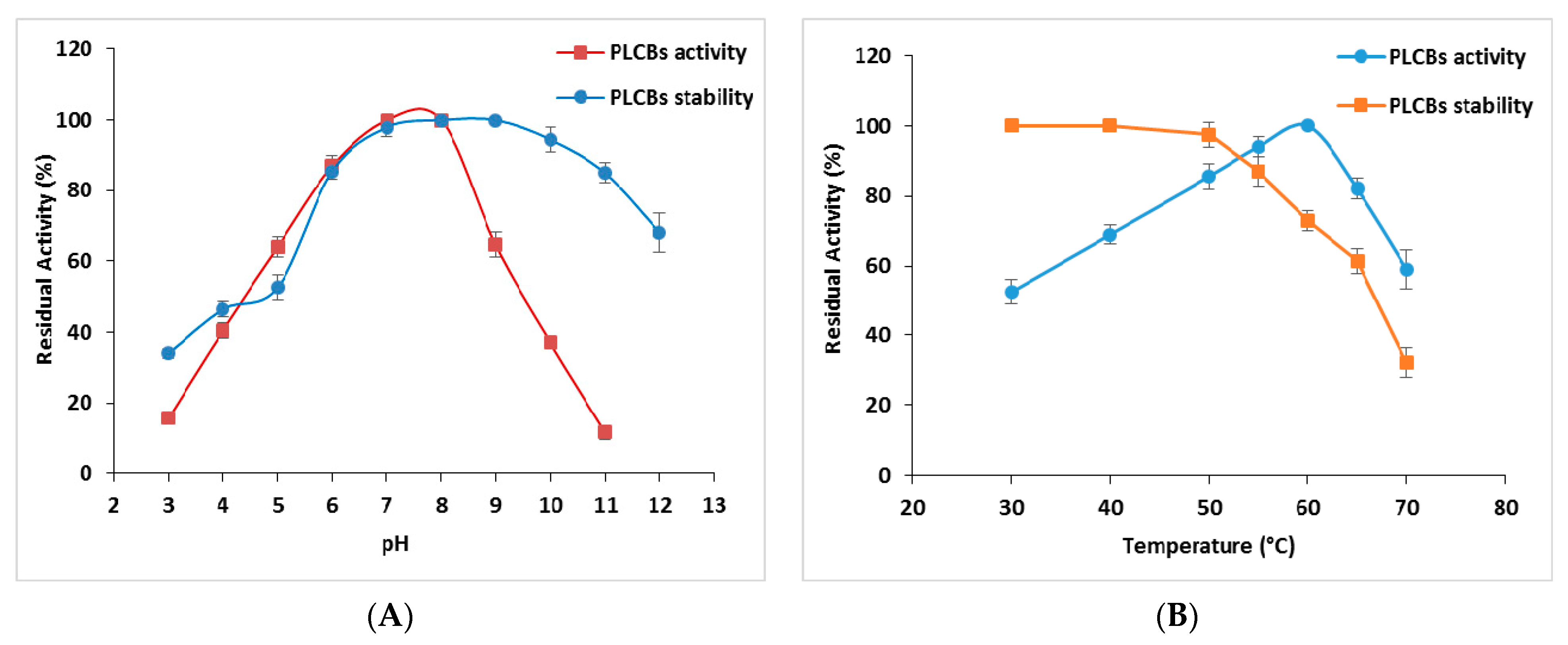
3.6.1. Effect of Temperature and pH on PLCBs Activity and Stability
3.6.2. Bile Salt Dependence and Metal Ion Requirement
3.6.3. PLCBs Apparent Kinetic Parameters
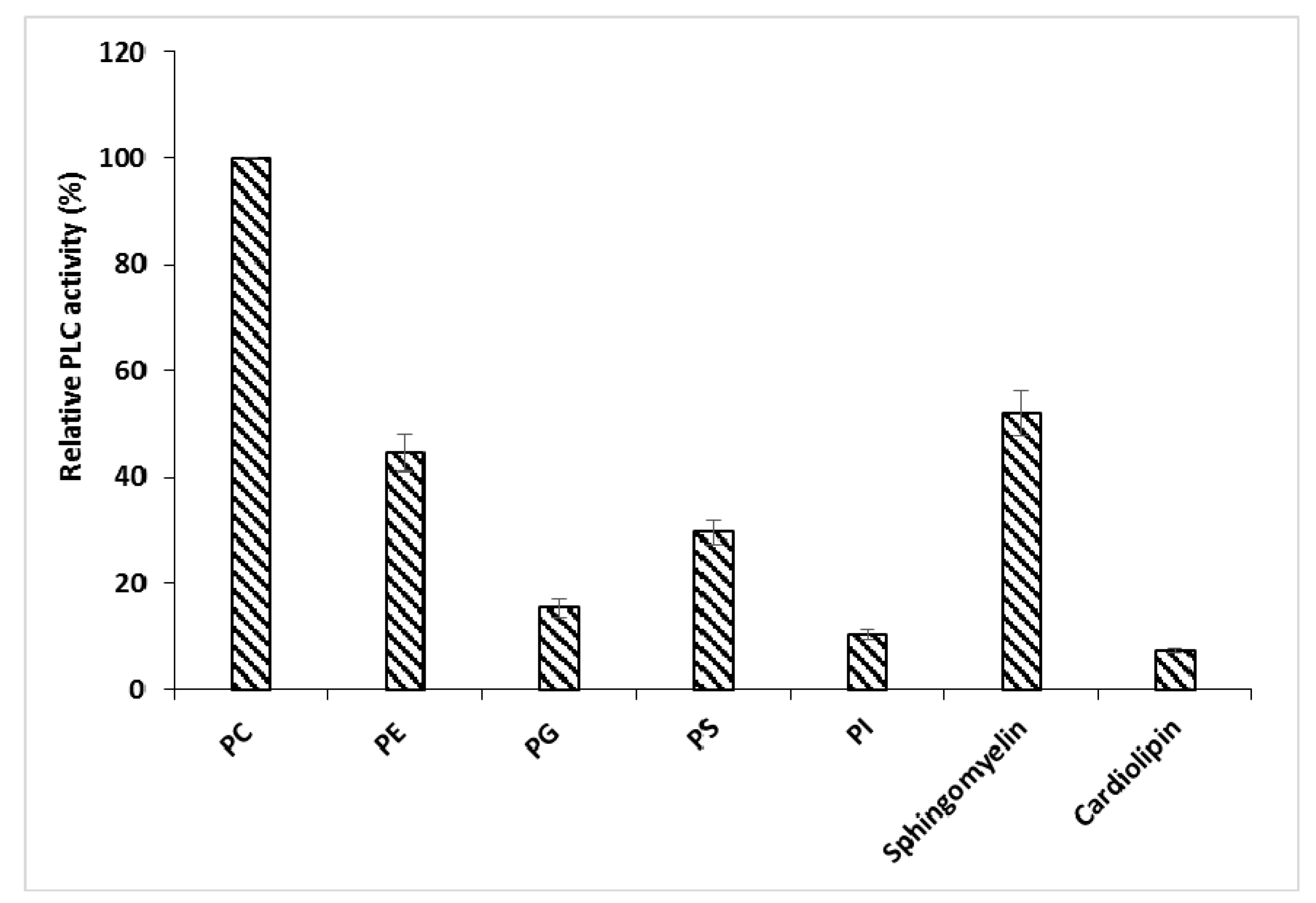
3.6.4. PLCBs Substrate Specificity
3.6.5. Correlation between Hemolytic and Antibacterial Activities and Substrate Specificity
3.6.6. Biotechnological Application of PLCBs: Oil Degumming
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aloulou, A.; Ali, Y.B.; Bezzine, S.; Gargouri, Y.; Gelb, M.H. Phospholipases: An overview. Lipases Phospholipases Methods Protoc. be 2012, 861, 63–85. [Google Scholar]
- Eddehech, A.; Smichi, N.; Arhab, Y.; Noiriel, A.; Abousalham, A.; Gargouri, Y.; Zarai, Z. Production, purification and functional characterization of phospholipase C from Bacillus thuringiensis with high catalytic activity. Process Biochem. 2019, 83, 122–130. [Google Scholar] [CrossRef]
- Elleboudy, N.S.; ElKhatib, W.F.; Aboulwafa, M.M.; Hassouna, N.A.-H. Production and characterization of phospholipases C from some Bacillus thuringiensis isolates recovered from Egyptian soil. Int. J. Biotechnol. Wellness Ind. 2016, 5, 10. [Google Scholar]
- Martin, S.F.; Follows, B.C.; Hergenrother, P.J.; Trotter, B.K. The choline binding site of phospholipase C (Bacillus cereus): Insights into substrate specificity. Biochemistry 2000, 39, 3410–3415. [Google Scholar] [CrossRef] [PubMed]
- Hansen, S.; Hansen, L.K.; Hough, E. The Crystal Structure of Tris-inhibited Phospholipase C from Bacillus cereus at 1·9 Å Resolution: The nature of the metal ion in site 2. J. Mol. Biol. 1993, 231, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Bora, L. Characterization of novel phospholipase C from Bacillus licheniformis MTCC 7445 and its application in degumming of vegetable oils. Appl. Biochem. Microbiol. 2013, 49, 555–561. [Google Scholar] [CrossRef]
- Wang, C.G.; Chen, M.K.; Chen, T. Improved purification and some properties of a novel phospholipase C from Bacillus mycoides strain 970. Afr. J. Microbiol. Res. 2010, 4, 396–399. [Google Scholar]
- Borrelli, G.; Trono, D. Recombinant lipases and phospholipases and their use as biocatalysts for industrial applications. Int. J. Mol. Sci. 2015, 16, 20774–20840. [Google Scholar] [CrossRef]
- Elena, C.; Cerminati, S.; Ravasi, P.; Rasia, R.; Peiru, S.; Menzella, H.G.; Castelli, M.E.B. cereus phospholipase C engineering for efficient degumming of vegetable oil. Process Biochem. 2017, 54, 67–72. [Google Scholar] [CrossRef]
- Cerminati, S.; Paoletti, L.; Aguirre, A.; Peirú, S.; Menzella, H.G.; Castelli, M.E. Industrial uses of phospholipases: Current state and future applications. Appl. Microbiol. Biotechnol. 2019, 103, 2571–2582. [Google Scholar] [CrossRef]
- Bacha, A.B.; Moubayed, N.; Abid, I. Thermostable, alkaline and detergent-tolerant lipase from a newly isolated thermophilic Bacillus stearothermophilus. Indian J. Biochem. Biophys. 2015, 52, 179–188. [Google Scholar]
- Karray, A.; Alonazi, M.; Horchani, H.; Ben Bacha, A. A novel thermostable and alkaline protease produced from Bacillus stearothermophilus isolated from olive oil mill sols suitable to industrial biotechnology. Molecules 2021, 26, 1139. [Google Scholar] [CrossRef] [PubMed]
- Price, M.F.; Wilkinson, I.D.; Gentry, L.O. Plate method for detection of phospholipase activity in Candida albicans. Sabouraudia J. Med. Vet. Mycol. 1982, 20, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Gerasimene, G.; IuP, M.; Kulene, V.; Glemzha, A. Biosynthesis of extracellular phospholipase C (lecithinase) from Bacillus cereus depending on the nutrient medium composition and pH. Prikl. Biokhimiia I Mikrobiol. 1980, 16, 523–527. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Hewick, R.M.; Hunkapiller, M.W.; Hood, L.E.; Dreyer, W.J. A gas-liquid solid phase peptide and protein sequenator. J. Biol. Chem. 1981, 256, 7990–7997. [Google Scholar] [CrossRef]
- Zwaal, R.; Roelofsen, B.; Comfurius, P.; Van Deenen, L. Complete purification and some properties of phospholipase C from Bacillus cereus. Biochim. Biophys. Acta BBA Biomembr. 1971, 233, 474–479. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, X.; Jin, Y. Non-hydrated phosphatide and its quantitative examination. Chin. J Health Lab. Technol. 2008, 18, 71–72. [Google Scholar]
- Jiang, X.; Chang, M.; Jin, Q.; Wang, X. Application of phospholipase A1 and phospholipase C in the degumming process of different kinds of crude oils. Process Biochem. 2015, 50, 432–437. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G.; Kaletta, U. Chromatography of rattlesnake venom A separation of three phosphodiesterases. Biochim. Biophys. Acta 1957, 24, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Contesini, F.J.; Melo, R.R.d.; Sato, H.H. An overview of Bacillus proteases: From production to application. Crit. Rev. Biotechnol. 2018, 38, 321–334. [Google Scholar] [CrossRef]
- Sharma, K.M.; Kumar, R.; Panwar, S.; Kumar, A. Microbial alkaline proteases: Optimization of production parameters and their properties. J. Genet. Eng. Biotechnol. 2017, 15, 115–126. [Google Scholar] [CrossRef]
- Palvannan, T.; Boopathy, R. Phosphatidylinositol-specific phospholipase C production from Bacillus thuringiensis serovar. kurstaki using potato-based media. World J. Microbiol. Biotechnol. 2005, 21, 1153–1155. [Google Scholar] [CrossRef]
- Ryo, T.; Yoshinari, A.; Hiroh, I. Purification and properties of phosphatidylinositol-specific phospholipase C of Bacillus thuringiensis. Biochim. Biophys. Acta BBA Lipids Lipid Metab. 1980, 619, 48–57. [Google Scholar] [CrossRef]
- Fisher, S.H.; Sonenshein, A.L. Control of carbon and nitrogen metabolism in Bacillus subtilis. Annu. Rev. Microbiol. 1991, 45, 107–135. [Google Scholar] [CrossRef]
- Stülke, J.; Hillen, W. Regulation of carbon catabolism in Bacillus species. Annu. Rev. Microbiol. 2000, 54, 849–880. [Google Scholar] [CrossRef]
- He, H.; Li, Y.; Zhang, L.; Ding, Z.; Shi, G. Understanding and application of Bacillus nitrogen regulation: A synthetic biology perspective. J. Adv. Res. 2022, 49, 1–14. [Google Scholar] [CrossRef]
- Jacob, F.; Michel, M.; Zarnkow, M.; Hutzler, M.; Methner, F. The complexity of yeast extracts and its consequences on the utility in brewing: A review. BrewingScience 2019, 72, 50–62. [Google Scholar]
- Im, H.; An, T.; Kwon, R.; Park, S.; Kim, Y.-K. Effect of Organic Nitrogen Supplements on Syngas Fermentation Using Clostridium Autoethanogenum. Biotechnol. Bioprocess Eng. 2021, 26, 476–482. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast Extract: Characteristics, Production, Applications and Future Perspectives. J. Microbiol. Biotechnol. 2023, 33, 151. [Google Scholar] [CrossRef]
- Wu, C.-Y.; Liang, Z.-C.; Lu, C.-P.; Wu, S.-H. Effect of carbon and nitrogen sources on the production and carbohydrate composition of exopolysaccharide by submerged culture of Pleurotus citrinopileatus. J. Food Drug Anal. 2008, 16, 6. [Google Scholar] [CrossRef]
- Hughes, M.N.; Poole, R.K. Metal speciation and microbial growth—The hard (and soft) facts. Microbiology 1991, 137, 725–734. [Google Scholar] [CrossRef]
- Dedyukhina, E.G.; Eroshin, V.K. Essential metal ions in the control of microbial metabolism. Process Biochem. 1991, 26, 31–37. [Google Scholar] [CrossRef]
- Khusro, A. One Factor at A Time based optimization of protease from poultry associated Bacillus licheniformis. J. Appl. Pharm. Sci. 2016, 6, 088–095. [Google Scholar] [CrossRef]
- Epperson, J.D.; Ming, L.-J. Cobalt (II) and copper (II) binding of Bacillus cereus trinuclear phospholipase C: A novel 1H NMR spectrum of a ‘Tri-Cu (II)’center in protein. J. Inorg. Biochem. 2001, 87, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Guan, P.; Ai, P.; Dai, X.; Zhang, J.; Xu, L.; Zhu, J.; Li, Q.; Deng, Q.; Li, S.; Wang, S. Complete genome sequence of Bacillus thuringiensis serovar Sichuansis strain MC28. J. Bacteriol. 2012, 194, 6975. [Google Scholar] [CrossRef]
- Gilmore, M.S.; Cruz-Rodz, A.L.; Leimeister-Wächter, M.; Kreft, J.; Goebel, W. A Bacillus cereus cytolytic determinant, cereolysin AB, which comprises the phospholipase C and sphingomyelinase genes: Nucleotide sequence and genetic linkage. J. Bacteriol. 1989, 171, 744–753. [Google Scholar] [CrossRef]
- Yuan, C.; Byeon, I.-J.L.; Poi, M.-J.; Tsai, M.-D. Structural analysis of phospholipase A2 from functional perspective. 2. Characterization of a molten globule-like state induced by site-specific mutagenesis. Biochemistry 1999, 38, 2919–2929. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- El-Sayed, M.; Roberts, M. Charged detergents enhance the activity of phospholipase C (Bacillus cereus) towards micellar short-chain phosphatidylcholine. Biochim. Biophys. Acta BBA Protein Struct. Mol. Enzymol. 1985, 831, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hough, E.; Hansen, L.K.; Birknes, B.; Jynge, K.; Hansen, S.; Hordvik, A.; Little, C.; Dodson, E.; Derewenda, Z. High-resolution (1.5 Å) crystal structure of phospholipase C from Bacillus cereus. Nature 1989, 338, 357–360. [Google Scholar] [CrossRef]
- Lyu, Y.; Ye, L.; Xu, J.; Yang, X.; Chen, W.; Yu, H. Recent research progress with phospholipase C from Bacillus cereus. Biotechnol. Lett. 2016, 38, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Hergenrother, P.J.; Martin, S.F. Determination of the kinetic parameters for phospholipase C (Bacillus cereus) on different phospholipid substrates using a chromogenic assay based on the quantitation of inorganic phosphate. Anal. Biochem. 1997, 251, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Elleboudy, N.S.; Aboulwafa, M.; Hassouna, N. Characterization of Phospholipase C Productivity by Pseudomonas aeruginosa, Bacillus cereus and Staphylococcus aureus isolates. J. Am. Sci. 2011, 7, 545–566. [Google Scholar]
- Devaux, P.F. Static and dynamic lipid asymmetry in cell membranes. Biochemistry 1991, 30, 1163–1173. [Google Scholar] [CrossRef]
- Geiger, O.; Gonzalez-Silva, N.; Lopez-Lara, I.M.; Sohlenkamp, C. Amino acid-containing membrane lipids in bacteria. Prog. Lipid Res. 2010, 49, 46–60. [Google Scholar] [CrossRef]
- Titball, R.W. Bacterial phospholipases C. Microbiol. Rev. 1993, 57, 347–366. [Google Scholar] [CrossRef]
- López-Lara, I.M.; Geiger, O. Bacterial lipid diversity. BBA Mol. Cell Biol. Lipids 2017, 1862, 1287–1299. [Google Scholar] [CrossRef]
- Epand, R.M.; Epand, R.F. Bacterial membrane lipids in the action of antimicrobial agents. J. Pept. Sci. 2011, 17, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Eddehech, A.; Rahier, R.; Donnarumma, D.; Rigano, F.; Noiriel, A.; Abousalham, A.; Cacciola, F.; Mondello, L.; Zarai, Z. Development of a highly efficient oil degumming process using a novel phosphatidylinositol-specific phospholipase C enzyme from Bacillus thuringiensis PL14. Food Biosci. 2023, 53, 102579. [Google Scholar] [CrossRef]




| Purification Step | Total Activity (U) a | Protein (mg) b | Specific Activity (U/mg) | Activity Recovery (%) | Purification Factor |
|---|---|---|---|---|---|
| Culture supernantant | 204,000 | 425 | 480 | 100 | 1 |
| Heat treatment (70 °C, 10 min) | 163,200 | 115 | 1419 | 80 | 2.95 |
| (NH4)2 SO4 fractionation (40–70%) | 122,400 | 41.5 | 2950 | 60 | 6.14 |
| Mono Q-Sepharose | 79,560 | 9.41 | 8450 | 39 | 17.6 |
| Strain | Gram | Inhibition Zone (mm) | IC50 (µg/mL) | |
|---|---|---|---|---|
| PLCBs | Ampicillin | |||
| Escherichia coli (ATCC 25922) | − | 10.6 ± 0.57 | 22.6 ± 1.5 | − |
| Pseudomonas aeruginosa (ATCC 27853) | − | 5.6 ± 0.58 | 20 ± 0.7 | − |
| Enterococcus faecalis (ATCC 29122) | + | 21.6 ± 0.57 | 24.2 ± 0.7 | 15 ± 1.51 |
| Staphylococcus epidermidis (ATCC 14990) | + | 19.3 ± 1.52 | 26 ± 0.5 | 17.5 ± 1.61 |
| Staphylococcus aureus (ATCC 25923) | + | 20 ± 1 | 21.5 ± 1.4 | 11 ± 1.65 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonazi, M.; Krayem, N.; Alzahrani, A.A.; Horchani, H.; Ben Bacha, A. Novel Phospholipase C with High Catalytic Activity from a Bacillus stearothermophilus Strain: An Ideal Choice for the Oil Degumming Process. Processes 2023, 11, 3310. https://doi.org/10.3390/pr11123310
Alonazi M, Krayem N, Alzahrani AA, Horchani H, Ben Bacha A. Novel Phospholipase C with High Catalytic Activity from a Bacillus stearothermophilus Strain: An Ideal Choice for the Oil Degumming Process. Processes. 2023; 11(12):3310. https://doi.org/10.3390/pr11123310
Chicago/Turabian StyleAlonazi, Mona, Najeh Krayem, Areej A. Alzahrani, Habib Horchani, and Abir Ben Bacha. 2023. "Novel Phospholipase C with High Catalytic Activity from a Bacillus stearothermophilus Strain: An Ideal Choice for the Oil Degumming Process" Processes 11, no. 12: 3310. https://doi.org/10.3390/pr11123310
APA StyleAlonazi, M., Krayem, N., Alzahrani, A. A., Horchani, H., & Ben Bacha, A. (2023). Novel Phospholipase C with High Catalytic Activity from a Bacillus stearothermophilus Strain: An Ideal Choice for the Oil Degumming Process. Processes, 11(12), 3310. https://doi.org/10.3390/pr11123310









