1. Introduction
Lentiviral vectors (LVs) have become a prevailing gene delivery tool in cell and gene therapy in the past three decades [
1,
2,
3,
4]. LVs are primarily used for ex vivo modifications, such as transduction of T-cells for expression of chimeric antigen receptor in CAR-T cell therapies, and they are gaining popularity in in vivo applications. With 6 approved ex vivo LV products and a rapidly growing number of LV clinical trials, there is an increased urgency in addressing the persisting challenge of producing LVs at manufacturing scale to support treatments beyond early clinical trials [
5,
6,
7,
8,
9,
10,
11]. In addition to the challenge of generating a sufficient quantity of LVs, their innately labile nature poses an equally important obstacle in LV production. As LVs lose function over time and they are sensitive to environmental factors in each unit operation in the bioprocess workflow [
11,
12,
13], integrated continuous manufacturing is an attractive strategy for process intensification [
14,
15,
16].
Continuous bioprocessing has the potential to address the current challenges of biomanufacturing technology, such as reducing cost, increasing flexibility and standardization, accelerating development and scaling times, and improving product quality. There have been many discussions about the impact of continuous bioprocessing [
17,
18] and an integrated process [
19,
20] that connects the upstream and downstream, especially for unstable products. The shift from batch to continuous bioprocessing for biopharmaceuticals has been realized in antibody production, and an end-to-end integrated continuous process has been reported [
21].
In the space of viral vectors, mentions of continuous manufacturing have typically referred to the continuous nature of the perfusion cell culture for the upstream process [
15]. Usually, the perfusion harvest is pooled and frozen every 24 h, and the collected material is then processed downstream as a series of batches, where unit operations are performed and completed before the process stream moves to the next step. For the downstream process, there are two recent publications of continuous purification of adeno-associated virus [
22] and hepatitis C virus-like particles [
23] using multiple-column counter-current chromatography, utilizing upstream material produced in the batch format. Although there has been some development work in continuous manufacturing in either upstream or downstream, an integrated continuous bioprocess has yet to be reported.
Implementing the upstream process in perfusion mode increases productivity and continuously harvesting LVs from the bioreactor reduces the residence time, thus preserving their quality attributes. Executing this at manufacturing scale has been a challenge due to limitations of currently commercialized cell retention devices. To circumvent this, a scalable technology that can be used as a cell retention device that does not retain the product in perfusion mode for LV production using a stable producer cell line was demonstrated [
24], which serves as the first piece of an integrated continuous manufacturing process of LVs.
Building on that upstream work, this manuscript demonstrates (1) the implementation of a downstream process in semi-continuous mode and (2) the integration between the perfusion culture from upstream to the capture step from downstream. The Mustang Q membrane, which has been reported to work well for purifying LVs [
25,
26], is chosen for the capture step for its attributes that are advantageous at manufacturing scale: high binding capacity, ability to handle high volumetric flow rates, and scalability. The goal for the work in this manuscript is to investigate the effects of reduced processing time from a semi-continuous downstream process and reduced process hold times from the integration between the production and capture of LVs on recovery and quality. The term “semi-continuous” is used in place of “continuous” due to the fact that the Mustang Q membranes are manually moved in the downstream setup.
To characterize the LV product quality, functional vector particles and total vector particles are analyzed to convey the potency aspect, and DNA and protein contents are analyzed to convey the purity aspect. The functional vector particles are reported as transducing units, assessed by a cell-based assay that measures the GFP-transgene expression in transduced target cells. The total vector particles are reported as vector genome units, assessed by a droplet digital PCR assay. Furthermore, the product quality of LVs produced by the integrated semi-continuous process is supported by an in vitro validation using confocal microscopy.
2. Materials and Methods
2.1. LV Starting Materials
The LV harvest materials used for the downstream work in this manuscript are previously described in detail [
24]. In brief, LVs containing GFP as the transgene and VSV-G at the membrane surface were produced in batch (1 L bioreactor) and perfusion (3 L bioreactor) modes using a stable producer cell line HEK293SF-LVP-CMVGFPq-92 [
27] by inducing with doxycycline and cumate. Both inducers are required for LV production in this double switch system to ensure tighter transcription regulation, as previously described [
28]. In batch mode, the producer cells were harvested at 3 days post induction for both runs. In perfusion mode, the Tangential Flow Depth Filtration cartridge with 30 cm
2 surface area was used as the cell retention device. The producer cells were continuously harvested at around 1 VVD (vessel volume per day) over 6 days for run 1 and at 2 VVD over 4 days for run 2.
2.2. Development of the Capture Step
Mustang Q XT Acrodisc Units (Pall Life Sciences, Ann Arbor, MI, USA) with a membrane volume of 0.86 mL were used for the capture step and an AKTA Avant (GE Healthcare, Uppsala, Sweden) was used for chromatography purification. Three buffers were used: equilibration (EQ) contains 10 mM Histidine and 0.15 M Sodium Chloride (NaCl), elution A contains 20 mM Tris-HCl with 3% sucrose, and elution B contains 20 mM Tris-HCl and 1.6 M NaCl with 3% sucrose, all at pH 7.5.
The purification steps are as follows: equilibration for 25 membrane volumes (MV) with EQ buffer, LV load (variable, between 100 and 600 MV), wash for 60 MV with EQ buffer, elution 1 for 12 MV using 25% elution buffer B for 0.4 M NaCl, elution 2 for 12 MV using 75% elution buffer B for 1.2 M NaCl, and strip for 25 MV using 100% elution buffer B. Elution 2 fraction was immediately diluted with EQ buffer to achieve a lower salt concentration of 0.4 M NaCl. In this work, the combined elution fractions represent the Mustang Q elution.
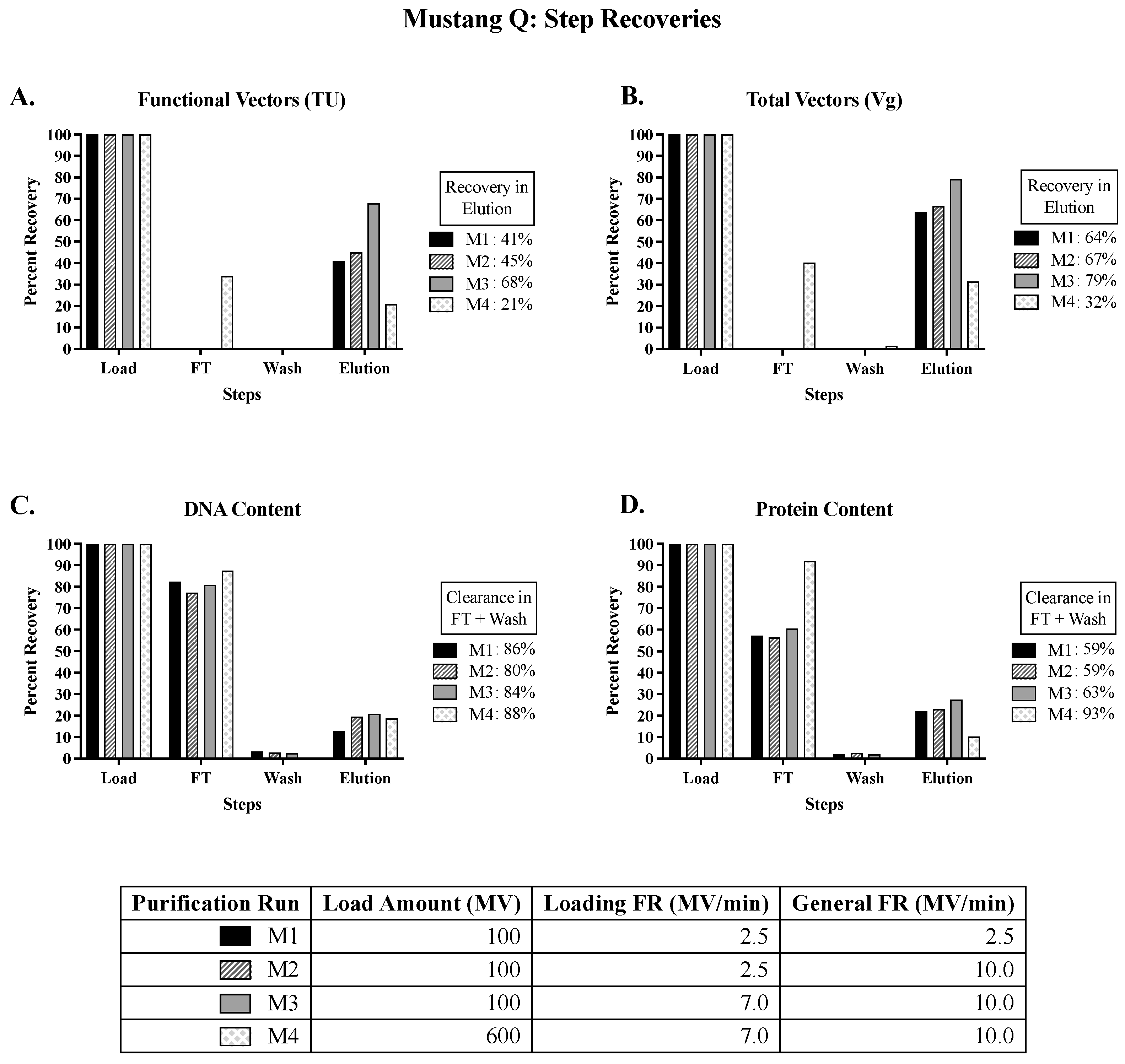
The loading flowrate (2.5 vs. 7 MV/min) and general flowrate (2.5 vs. 10 MV/min) were tested in establishing Mustang Q run conditions. For the rest of the downstream work, the flowrates used were 7 MV/min for loading and 10 MV/min for the rest of the purification steps. The conversions from MV/min to mL/min are as follows: 2.5 MV/min = 2.15 mL/min, 7 MV/min = 6.02 mL/min, and 10 MV/min = 8.60 mL/min. This work reports in the units of MV and MV/min.
2.3. Downstream Steps in Batch Mode
After thaw, the LV materials from the perfusion bioreactor runs were processed by nuclease treatment using 50 U/mL Benzonase (MilliPore Sigma, Darmstadt, Germany) and 2 mM MgCl2 at 24 °C with 135 RPM shaking speed for 30 min, followed by clarification through a 0.45 µm syringe filter (MilliPore Sigma), and then purification on the AKTA for the Mustang Q capture step.
The LV materials from the batch bioreactor runs were previously treated with 20 U/mL Benzonase and 2 mM MgCl2 at 27 °C for 60 min with 110 RPM mixing before harvesting by centrifuging at 2000× g for 15 min. Therefore, after thaw, these materials were clarified using a 0.45 µm syringe filter and then purified on the AKTA.
2.4. Downstream Steps in Semi-Continuous Mode
Two systems were used in the semi-continuous downstream setup. In system 1, nuclease-treated material was pumped through a MD0HC23CL3 depth filter (Millipore Sigma) before being directly loaded onto two Mustang Q membranes, MA + MB, connected in series. Once MA was loaded to the targeted 86 mL, it was removed from system 1 and transferred to system 2 (the AKTA), and MC was then connected in series after MB. While the clarification and loading processes continued in system 1 for MB + MC, MA was subjected to wash, elution, and regeneration steps (i.e., strip, then EQ) in system 2. Then, MB was transferred to system 2 for purification and MA was re-transferred to system 1 behind MC. These steps were repeated until all three membranes went through two cycles each, totaling 516 mL of LV starting material.
Before commencing the semi-continuous operation, the depth filter was first flushed with milli-Q water and then with EQ buffer using the peristaltic pump in system 1, and the three Mustang Q membranes were equilibrated with EQ buffer using the AKTA in system 2.
2.5. Gene Transfer Assay for Functional LV Quantification
A flow cytometry-based gene transfer assay was used to determine functional vector titer in transducing units per milliliter (TU/mL), as previously described in detail [
24]. Downstream samples were filtered using a 0.45 µm filter and 1% Penicillin-Streptomycin was added to their respective wells to avoid contamination. Accepted GFP values range between 2 and 20% fluorescent cells to avoid signal due to super transduction.
2.6. Droplet Digital Polymerase Chain Reaction Assay for Total LV Quantification
A QX200
TM Droplet Digital PCR (ddPCR) system was used to determine total vector titer in vector genome units per milliliter (Vg/mL), as previously described in detail [
24].
2.7. Picogreen Assay for DNA Quantification
The Quant-iT PicoGreen dsDNA Assay kit (Invitrogen, Eugene, OR, USA) was used to quantify DNA in LV samples, as previously described in detail [
24].
2.8. RC DC Assay for Protein Quantification
The RC DC Protein Assay Kit (Bio-Rad, Hercules, CA, USA) was used to quantify protein content in LV samples, following the manufacturer’s protocol.
2.9. Confocal Microscopy
HEK293SF cells were plated onto 35 mm coverglass bottom dishes (MatTek, Ashland, Wilmington, DE, USA) and 24 h later were either transduced with LVs manufactured by the integrated semi-continuous process, LV supernatant produced in a shake flask, or non-induced producer cell supernatant serving as the negative control at MOI of 1. Just prior to imaging, the cells were stained with Deep Red CellMask plasma membrane stain (Invitrogen, Eugene, OR, USA). Dishes were placed onto the stage of a IX83-DSU Olympus microscope and maintained at 37 °C and 5% CO2 in a stage-top incubator. The labelling of the cell membrane was performed as per the manufacturer’s instructions. All images were acquired at 100× (oil immersion) UPLANO objectives with 1000-ms exposure uniformly for all image acquisition using the Metamorph Advanced 14 Olympus software. The acquired images were post-processed using ImageJ FIJI v1.53. The built-in fluorescence intensity per unit area plugins were used for all of the analysis.
4. Discussion
With an increasing number of successful clinical trials utilizing LVs for cell and gene therapy treatments for various conditions, an integrated manufacturing process that connects the upstream to the downstream has become more frequently discussed in the field. Integrated continuous manufacturing is a powerful strategy that has the potential to address the persisting challenges of producing a sufficient amount of LVs at manufacturing scale to support treatments for patients as well as addressing the innately labile nature of LVs. In attempting to establish an integrated continuous process for LV production that has the capability to be implemented at manufacturing scale, the work was broken into two parts. First, a scalable technology that can be used as a cell retention device that does not retain the product in perfusion mode for LV production using a stable producer cell line was demonstrated in a recently published work [
24]. Second, implementing the downstream process in a semi-continuous mode using a scalable capture step and then demonstrating the integration of the upstream and downstream, as discussed in this manuscript. The term “semi-continuous” is used in place of “continuous” due to the fact that the Mustang Q membranes are manually moved between the two systems in the downstream setup.
In the development of the Mustang Q capture step (
Figure 1), results show high step recoveries of functional vectors (represented by transducing units, TU) and total vectors (represented by vector genome units, Vg) at the higher tested loading (7 MV/min) and general (10 MV/min) flowrates. Results also show high DNA and protein clearance in the flowthrough, confirming that Mustang Q is an efficient capture step for LVs. Operating at high flowrates expedites the processing time, which poses as an asset at manufacturing scale, and doing so without compromising on product integrity is advantageous when purifying an unstable product. Further optimizing the Mustang Q capture step can potentially improve product recovery and quality.
The load amount of 100 MV was selected due to high step and cumulative recoveries of TU (
Figure 2) shown in two experiments that used different pools of harvested LVs. Fortuitously, the load time for 100 MV on the Mustang Q membrane lines up well with the time for the purification steps on the AKTA, allowing the two systems to run in parallel (
Figure 3). Combining the clarification and loading of the capture step as well as operating those steps in parallel to the purification of the capture step expedited the processing time, reducing it by 4-fold as compared to processing the same volume in batch mode using the same membrane size, where each unit operation is completed before moving onto the next step. This highlights the advantage of operating in semi-continuous mode, since it is not possible to have loading and elution occur simultaneously in batch mode. This demonstration at lab scale has potential to be applied to large scale, especially in the case where a membrane size cannot accommodate the processing volume in one run. For example, semi-continuous mode is an attractive solution for processing 500 L harvest daily from a bioreactor in perfusion mode using six 780 mL Mustang Q, whereas the next size up, a 5 L Mustang Q, is oversized.
In addition to the time saved from running the downstream process in semi-continuous mode, improvement in product recovery is observed, with a 26% increase in the recovery of functional vectors and 18% increase in the recovery of total vectors (
Figure 4A), and a lower Vg/TU ratio indicating higher potency (
Figure 4C). These results show higher LV product quality for the semi-continuous process and suggest an added benefit in loading the membranes in series. The improvements can be attributed to the efficiency of continuous flow chromatography. While the membranes were each loaded to 100 MV, given two membranes are connected in series during the loading step in the semi-continuous setup, the material that did not bind to the first membrane may have bound to the second membrane, thus pushing the capacity of the membrane a bit further.
In fact, overlaid chromatograms of the six cycles in the semi-continuous downstream run support this hypothesis, where the A280 signal for cycles 2–6 is higher than cycle 1 (
Figure S3). Given that cycle 1 occurred first, the membrane was loaded to 100 MV with no spillover, whereas cycles 2–6 had some spillover from the loading of the previous membrane. In contrast, in a different semi-continuous downstream run where the holdup volume of system 1 of the semi-continuous setup was not accounted for, thereby loading less than 100 MV in cycle 1, and the leftover LV material was loaded in cycle 7, the overlaid chromatograms show lower A280 signal for cycles 1 and 7 as compared to cycles 2–6 (
Figure S4). Given the ineffective loading of this semi-continuous downstream run, the recovered TU and Vg were lower (30% and 63%, respectively) than the correctly implemented semi-continuous downstream run (69% TU and 91% Vg recovered).
There is a possibility of further improving the product quality by optimizing the nuclease treatment and clarification steps. For example, temperature and duration can be optimized for the nuclease treatment, and the depth filtration step might be further assessed for improved performance.
An additional characterization of the ISC LV (i.e., LVs manufactured by the integrated semi-continuous process) was implemented to visually assess product quality. The functionality of LVs is defined as their ability to transduce host cells to effectively deliver genetic material to be integrated in the host cell genome. Given that the transgene of the LVs in this work is GFP, it is possible to qualitatively assess the functionality in terms of GFP expression using confocal microscopy (
Figure 5). These results allow for a visual assessment of the product quality in this experiment, and they indicate the potential of leveraging imaging techniques as additional analytical tools for assessing the quality of LVs, such as a validation assay to assess transduction effectiveness for LVs used in ex vivo applications.
In investigating the effect of process hold times, results show that perfusion LV harvest held at 4 °C for 6 days versus 1 day post thaw decreases the TU recovery by 9% and the Vg recovery by 10% (
Figure 4B) and increases the Vg/TU ratio indicating lower potency (
Figure 4C). These results are in line with what has been shown in the literature, where LVs lose function over time, and they support the implication that reducing process hold times can help in maintaining a higher number of the produced functional vectors. These results highlight the relevance of integrating a continuous upstream process (i.e., perfusion) that increases LV productivity and a semi-continuous downstream process that preserves LV quality attributes by reducing both the processing time and processing hold times.
The presented case study demonstrates the feasibility and practicality of an integrated semi-continuous process for LV production and capture using upstream data from a previous publication [
24] and downstream data from this manuscript to generate projected processing times (
Figure 6) and recovered functional yields (
Figure 7). The assumption of repeating the batch bioreactor to produce the same amount of functional vectors as in one perfusion bioreactor is used for comparison with precaution. The projected processing time for the batch upstream and batch downstream process (Case I) is an underestimation, since it does not take into account the necessary time for cleaning and set up between batch operations. In addition, the need to operate multiple batch bioreactors increases the risk of contamination.
The case study shows reduced processing time and improved product recovery for both the semi-continuous downstream process (Case II) and the integrated semi-continuous manufacturing (Case III). The integrated semi-continuous process results in the shortest processing time for the highest amount of recovered functional vectors, which has great implications on reducing cost and increasing productivity of the manufacturing plant. Depending on the layout of the manufacturing plant, LVs harvested from the perfusion bioreactor can be processed downstream every 12 h or 24 h, which gives flexibility in operation.
The downstream processing times in batch mode and semi-continuous mode from the lab scale experiment with 0.86 mL Mustang Q membrane was applied to the calculations in the case study. The caveat in this simulation is that a larger membrane would be more appropriately sized to process larger harvest volumes. Given that the exact time calculations for a larger membrane cannot be simulated, the case study used the processing times from the lab scale demonstration. Future experiments using larger membranes in semi-continuous mode would be helpful in validating the effect of time savings and product quality at large scale. However, employing a larger membrane for both batch and semi-continuous mode would still result in the same time savings advantage by combining the clarification and loading of the capture step as well as operating those steps in parallel to the purification of the capture step.
The two highly discussed drivers behind the current interest in continuous bioprocessing are cost and improvements in product quality. In terms of cost, an article discussing the LV bioprocess economics for cell and gene therapy reported high treatment costs for gene-modifying cell therapy products that use LVs as the gene delivery method: USD 473k for Kymriah approved in 2017 to treat acute lymphoblastic leukemia and USD 1.8M projected for Zynteglo approved in 2022 to treat beta-thalassemia [
9]. There is an increased interest in reducing the price, especially in the effort to make life-saving therapies available to patients in developing countries. In terms of product quality, continuous processing has the potential to provide improvements through enhanced control of the manufacturing process.
In the context of LVs, the main motivation for an integrated continuous process is the instability of LVs. While increasing the total vector yield through process intensification in the upstream process contributes to the scalability and mass production of LVs, improving the functional yield in the downstream process will improve the transducibility ratio, which means less LVs are needed for an effective treatment. Together, increasing vector yield and improving functional yield will lead to higher quantity and quality of viral vectors, contributing to improved process performance and robustness. Overall, these improvements will lead to lower drug prices for consumers and allow manufacturers to respond much quicker to changes in demand, contributing to the prevention of drug shortages.
Until limitations such as hardware and software integration are addressed, the direct link between the bioreactor and the capture step cannot be made. However, this investigation of a proof-of-concept for the workflow in a semi-continuous manner shows that implementing such strategy would be successful in maintaining the functionality of LVs produced. Ultimately, the work shows that there is an advantage to implementing an integrated semi-continuous manufacturing process for LV production to support late-stage clinical trials and treatments.