Hierarchical Porous Carbon Aerogel Derived from Sodium Alginate for High Performance Electrochemical Capacitor Electrode
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of Sodium Alginate Aerogel
2.3. Carbonization of Sodium Alginate Aerogel
2.4. Carbon Aerogel Activation
2.5. Electrochemical Measurements
2.6. Materials Characterization
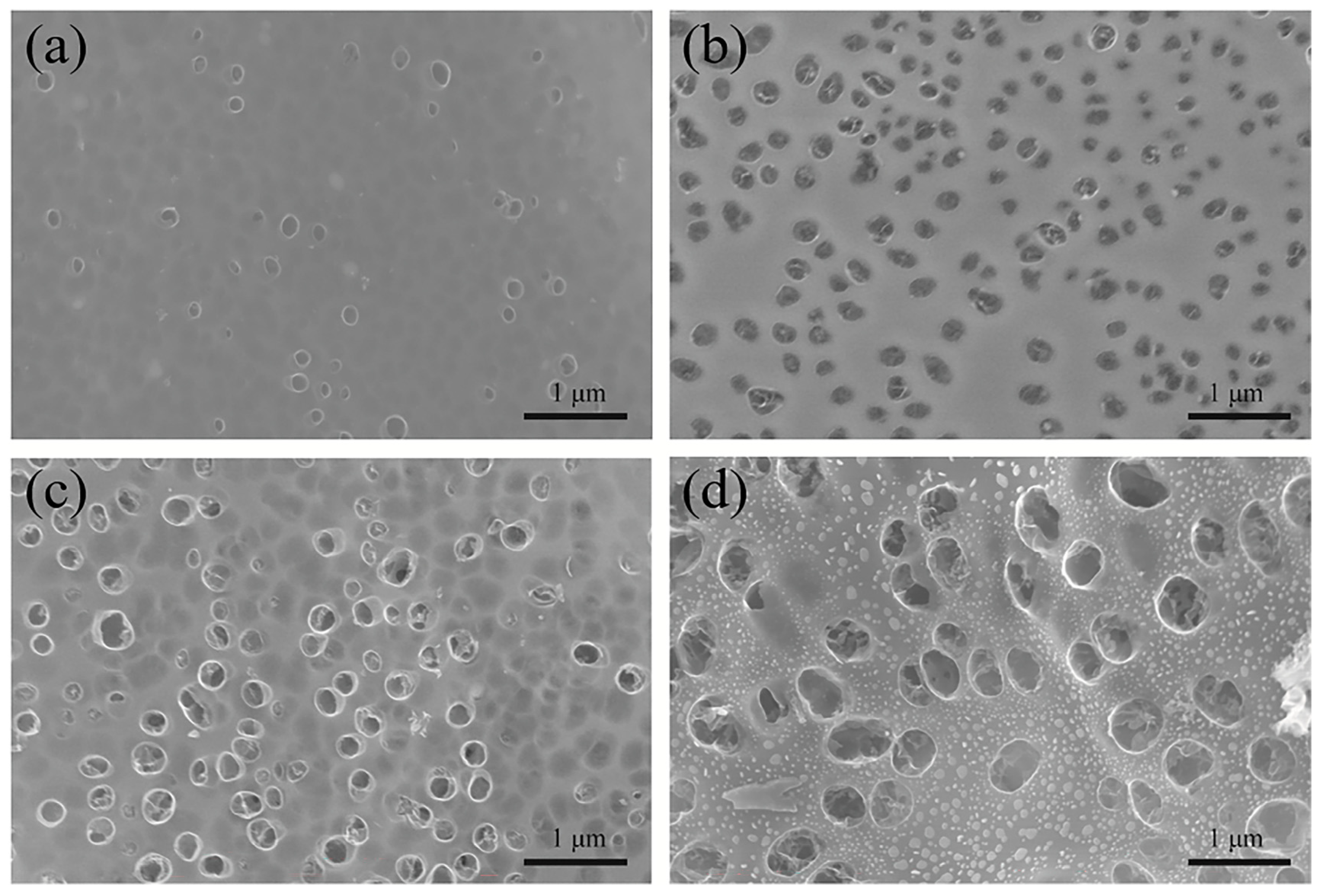
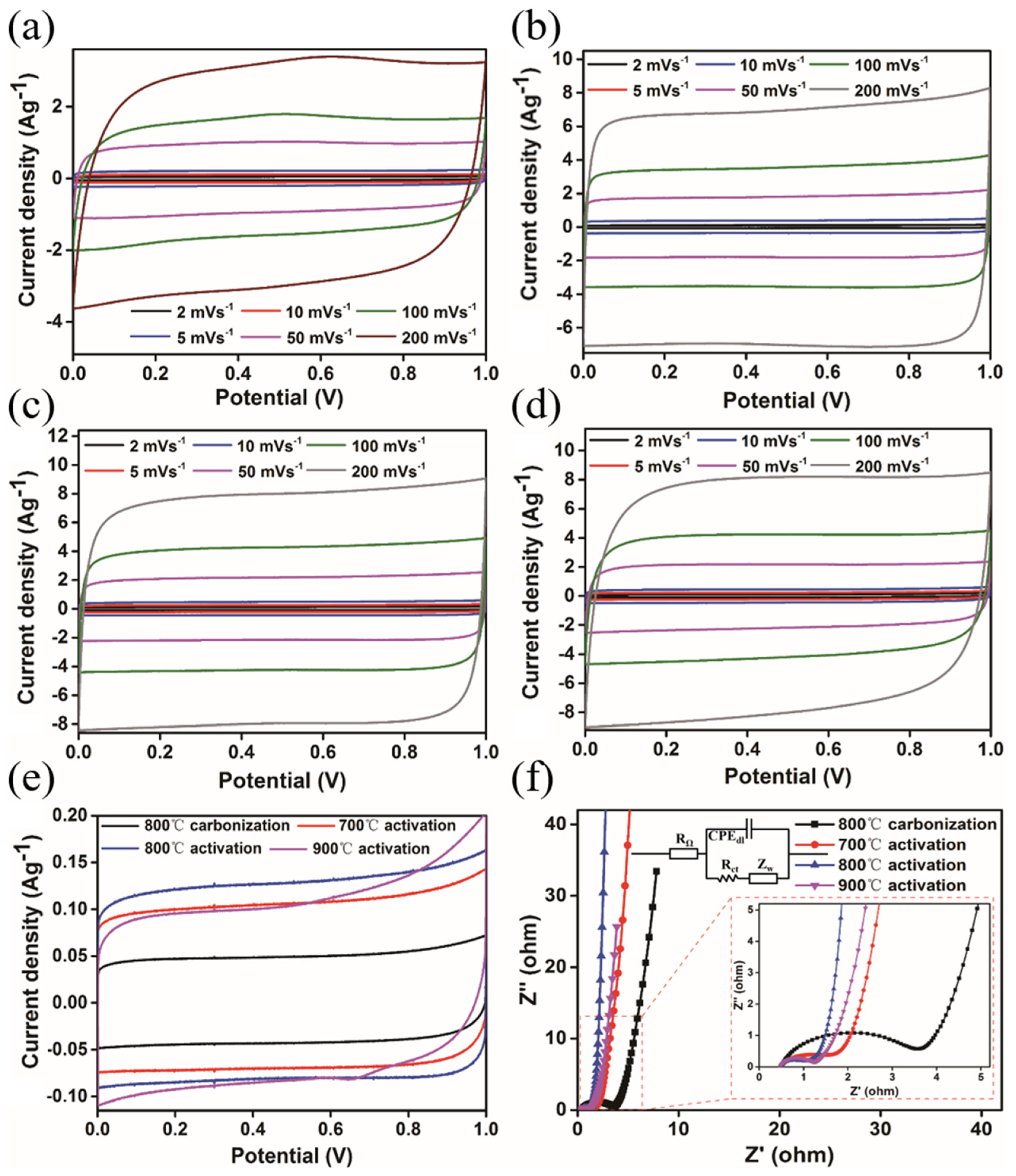
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sun, Y.; Li, S.Q.; Yang, X.R.; Zhang, Y.T.; Liao, B.Z.; Zhu, C.X.; Yang, J.J.; Xu, B.R.; Cao, F.; Guo, X.M.; et al. Bacteria-Assisted Synthesis of Fe-Co-Ni-S/Hollow Carbon Spheres as High-Performance Supercapacitor Electrode Materials. Chemistryselect 2023, 8, e202203439. [Google Scholar] [CrossRef]
- Song, Z.; Hu, H.; Shu, K.; Liu, T.; Tang, X.; Zhou, X.; Li, Y.; Zhang, Y. Novel Fe2O3 microspheres composed of triangular star-shaped nanorods as an electrode for supercapacitors. Chem. Commun. 2023, 59, 11791–11794. [Google Scholar] [CrossRef] [PubMed]
- Prasannakumar, A.T.; Mohan, R.R.V.M.; Varma, S.J. Progress in Conducting Polymer-Based Electrospun fibers for Supercapacitor Applications: A Review. ChemistrySelect 2023, 8, e202203564. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossen, M.R.; Alam, I.; Rahman, M.H.; Faruk, O.; Nurbas, M.; Rahman, M.M.; Khan, M.M.R. Synthesis of hexagonal boron nitride based PANI/h-BN and PANI-PPy/h-BN nanocomposites for efficient supercapacitors. J. Alloys Compd. 2023, 947, 169471. [Google Scholar] [CrossRef]
- Fu, X.; Li, R.; Yan, S.; Yuan, W.; Zhang, Y.; Sun, W.; Wang, X. Porous Carbons Prepared from Polyacrylonitrile Doped with Graphitic Carbon Nitride or Melamine for Supercapacitor Applications. ChemistrySelect 2023, 8, e202301801. [Google Scholar] [CrossRef]
- Benwannamas, N.; Sangtawesin, T.; Yilmaz, M.; Kanjana, K. Gamma-induced interconnected networks in microporous activated carbons from palm petiole under NaNO3 oxidizing environment towards high-performance electric double layer capacitors (EDLCs). Sci. Rep. 2023, 13, 12887. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zheng, H.; Wu, W.J.; Zhang, Y.R.; Wang, Q.; Yang, L.; Yin, H.Y.; Lu, W.; Wang, S.Y.; Yang, X. Three-Dimensional Porous Carbon Materials from Coix lacryma-jobi L. Shells for High-Performance Supercapacitor. Chemistryselect 2022, 7, e202104189. [Google Scholar] [CrossRef]
- Demir, M.; Doguscu, M. Preparation of Porous Carbons Using NaOH, K2CO3, Na2CO3 and Na2S2O3 Activating Agents and Their Supercapacitor Application: A Comparative Study. Chemistryselect 2022, 7, e202104295. [Google Scholar] [CrossRef]
- Paalo, M.; Härmas, M.; Romann, T.; Jänes, A.; Lust, E. Modification of micro/mesoporous carbon synthesis method from well decomposed peat using ZnCl2 additional activation step. Electrochem. Commun. 2023, 153, 107543. [Google Scholar] [CrossRef]
- Jha, M.K.; Shah, D.; Mulmi, P.; Joshi, S.; Sharma, R.K.; Pant, B.; Park, M.; Pant, H.R. Development of activated carbon from bhang (Cannabis) stems for supercapacitor electrodes. Mater. Lett. 2023, 344, 134436. [Google Scholar] [CrossRef]
- Pourhosseini, S.E.M.; Norouzi, O.; Salimi, P.; Naderi, H.R. Synthesis of a Novel Interconnected 3D Pore Network Algal Biochar Constituting Iron Nanoparticles Derived from a Harmful Marine Biomass as High-Performance Asymmetric Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2018, 6, 4746–4758. [Google Scholar] [CrossRef]
- Wu, J.; Ma, Z.; Wang, G.; Chen, T. Biomass Porous Carbon Derived from Celery Leaves with High Capacitance for Supercapacitor. ChemistrySelect 2023, 8, e202204616. [Google Scholar] [CrossRef]
- Huang, J.; Wu, J.G.; Dai, F.Y.; Li, C.M. 3D honeycomb-like carbon foam synthesized with biomass buckwheat flour for high-performance supercapacitor electrodes. Chem. Commun. 2019, 55, 9168–9171. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, Z.; Ma, X.; Feng, Q.; Wang, D.; Ma, D. A combination of heteroatom doping engineering assisted by molten salt and KOH activation to obtain N and O co-doped biomass porous carbon for high performance supercapacitors. J. Alloys Compd. 2023, 960, 170785. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Cai, M.; Zhou, Y.; Guo, C.; Han, Y.; Zhang, L. Valorization of Biomass-Derived Polymers to Functional Biochar Materials for Supercapacitor Applications via Pyrolysis: Advances and Perspectives. Polymers 2023, 15, 2741. [Google Scholar] [CrossRef] [PubMed]
- Thi, V.M.; Chao, H.-P.; Van, T.T.; Thi, L.T.; Lin, C.-C.; Nguyen, T.H. Removal of ammonium from groundwater using NaOH-treated activated carbon derived from corncob wastes: Batch and column experiments. J. Clean. Prod. 2018, 180, 560–570. [Google Scholar]
- Qiu, Z.; Wang, Y.; Bi, X.; Zhou, T.; Zhou, J.; Zhao, J.; Miao, Z.; Yi, W.; Fu, P.; Zhuo, S. Biochar-based carbons with hierarchical micro-meso-macro porosity for high rate and long cycle life supercapacitors. J. Power Sources 2018, 376, 82–90. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Chen, X.; Hou, X.; Lin, H.; Jia, L. One step synthesis of N, P co-doped hierarchical porous carbon nanosheets derived from pomelo peel for high performance supercapacitors. J. Colloid Interface Sci. 2021, 605, 71–81. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Sun, X.; Zhan, J.; Zhang, H.; Zhao, X. Nitrogen-doped hierarchical porous carbon using biomass-derived activated carbon/carbonized polyaniline composites for supercapacitor electrodes. J. Electroanal. Chem. 2018, 827, 213–220. [Google Scholar] [CrossRef]
- Charoensook, K.; Huang, C.-L.; Tai, H.-C.; Lanjapalli, V.K.; Chiang, L.-M.; Hosseini, S.; Lin, Y.-T.; Li, Y.-Y. Preparation of porous nitrogen-doped activated carbon derived from rice straw for high-performance supercapacitor application. J. Taiwan Inst. Chem. Eng. 2021, 120, 246–256. [Google Scholar] [CrossRef]
- Quan, L.H.; Thuy, U.T.D.; Nam, P.V.; Van Chi, N.; Duong, T.X.; Van Hoa, N. Chitosan-derived carbon aerogel nanocomposite as an active electrode material for high-performance supercapacitors. J. Sci. Adv. Mater. Devices 2023, 8, 100586. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Duong, T.T.T.; Phan, T.V.T.; Truong, C.C.; Nguyen, V.V.; Tran, T.N.; Nguyen, T.T.; Anh, T.H.; Hoang, S.M.T.; Ngo, H.L.; et al. Water hyacinth as a green and sustainable material for carbon aerogel electrodes utilized in membrane capacitive deionization. CLEAN–Soil Air Water 2023, 51, 2200396. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Li, P.; Wei, H.; Wei, K.; Chen, X. Lignin-derived carbon aerogels with high surface area for supercapacitor applications. Chem. Eng. J. 2023, 466, 143118. [Google Scholar] [CrossRef]
- Mohammed, A.E.; Abdalhalim, L.R.; Atalla, K.M.; Mohdaly, A.A.A.; Ramadan, M.F.; Abdelaliem, Y.F. Chitosan and sodium alginate nanoparticles synthesis and its application in food preservation. Rend. Lincei. Sci. Fis. Nat. 2023, 34, 415–425. [Google Scholar] [CrossRef]
- Kamel, S.; Dacrory, S.; Hesemann, P.; Bettache, N.; Ali, L.M.A.; Postel, L.; Akl, E.M.; El-Sakhawy, M. Wound Dressings Based on Sodium Alginate-Polyvinyl Alcohol-Moringa oleifera Extracts. Pharmaceutics 2023, 15, 1270. [Google Scholar] [CrossRef] [PubMed]
- Kruk, K.; Szekalska, M.; Basa, A.; Winnicka, K. The Impact of Hypromellose on Pharmaceutical Properties of Alginate Microparticles as Novel Drug Carriers for Posaconazole. Int. J. Mol. Sci. 2023, 24, 10793. [Google Scholar] [CrossRef]
- Li, X.; Xing, Y.; Xu, Y.; Deng, Q.; Zhang, K.; Shao, L. Hierarchical Nanoporous Carbon Templated and Catalyzed by the Bicontinuous Nanoporous Copper for High Performance Electrochemical Capacitors. ChemistrySelect 2019, 4, 6437–6444. [Google Scholar] [CrossRef]
- Lin, Z.; Xiang, X.; Peng, S.; Jiang, X.; Hou, L. Facile synthesis of chitosan-based carbon with rich porous structure for supercapacitor with enhanced electrochemical performance. J. Electroanal. Chem. 2018, 823, 563–572. [Google Scholar] [CrossRef]
- Shan, L.; Zhang, Y.; Xu, Y.; Gao, M.; Xu, T.; Si, C. Wood-based hierarchical porous nitrogen-doped carbon/manganese dioxide composite electrode materials for high-rate supercapacitor. Adv. Compos. Hybrid Mater. 2023, 6, 174. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Tian, J.; Li, H.; Sang, Y.; Yu, G.; Cai, H.; Liu, H.; Wong, C.P.; Umar, A. Hierarchical porous carbon aerogel derived from bagasse for high performance supercapacitor electrode. Nanoscale 2014, 6, 12120–12129. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Romanos, J.; Beckner, M.; Rash, T.; Firlej, L.; Kuchta, B.; Yu, P.; Suppes, G.; Wexler, C.; Pfeifer, P. Nanospace engineering of KOH activated carbon. Nanotechnology 2011, 23, 015401. [Google Scholar] [CrossRef] [PubMed]
- Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework. Nat. Chem. 2016, 8, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Niu, Q.; Gao, K.; Tang, Q.; Wang, L.; Han, L.; Fang, H.; Zhang, Y.; Wang, S.; Wang, L. Large-size graphene-like porous carbon nanosheets with controllable N-doped surface derived from sugarcane bagasse pith/chitosan for high performance supercapacitors. Carbon 2017, 123, 290–298. [Google Scholar] [CrossRef]
- Chen, K.; Liu, J.; Bian, H.; Wei, J.; Wang, W.; Shao, Z. Ingenious preparation of N/NiOx co-doped hierarchical porous carbon nanosheets derived from chitosan nanofibers for high-performance supercapacitors. Nanotechnology 2020, 31, 335713. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, Y.; Yan, X.; Zhang, W.; Zhang, M.; Huang, X.; Pan, J.; Shahnavaz, Z.; Moradian, J.M. Porous CoO/carbon foam composites synthesized by solvothermal method for supercapacitor and enhanced microwave absorption applications. Diam. Relat. Mater. 2023, 138, 110270. [Google Scholar] [CrossRef]
- Shang, M.; Zhang, J.; Liu, X.; Liu, Y.; Guo, S.; Yu, S.; Filatov, S.; Yi, X. N, S self-doped hollow-sphere porous carbon derived from puffball spores for high performance supercapacitors. Appl. Surf. Sci. 2020, 542, 148697. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, Q.; Liu, R. Blueberry-Peel-Derived Porous Carbon for High-Performance Supercapacitors: The Effect of N-Doping and Activation. Chemistryselect 2020, 5, 1029–1036. [Google Scholar] [CrossRef]
- Vivekchand, S.R.C.; Rout, C.S.; Subrahmanyam, K.S.; Govindaraj, A.; Rao, C.N.R. Graphene-based electrochemical supercapacitors. J. Chem. Sci. 2008, 120, 9–13. [Google Scholar] [CrossRef]
- Yan, J.; Wei, T.; Shao, B.; Ma, F.; Fan, Z.; Zhang, M.; Zheng, C.; Shang, Y.; Qian, W.; Wei, F. Electrochemical properties of graphene nanosheet/carbon black composites as electrodes for supercapacitors. Carbon 2010, 48, 1731–1737. [Google Scholar] [CrossRef]
- Thomberg, T.; Tooming, T.; Romann, T.; Palm, R.; Jänes, A.; Lust, E. High Power Density Supercapacitors Based on the Carbon Dioxide Activated D-Glucose Derived Carbon Electrodes and Acetonitrile Electrolyte. J. Electrochem. Soc. 2013, 160, A1834–A1841. [Google Scholar] [CrossRef]
- Enterría, M.; Martín-Jimeno, F.; Suárez-García, F.; Paredes, J.; Pereira, M.; Martins, J.; Martínez-Alonso, A.; Tascón, J.; Figueiredo, J. Effect of nanostructure on the supercapacitor performance of activated carbon xerogels obtained from hydrothermally carbonized glucose-graphene oxide hybrids. Carbon 2016, 105, 474–483. [Google Scholar] [CrossRef]






| Sample ID | Smicro (m2 g−1) | Smeso (m2 g−1) | Vmicro (m3 g−1) | Vmeso (m3 g−1) |
|---|---|---|---|---|
| 500 °C carbonization | 25.5 | 38.5 | 0.014 | 0.074 |
| 600 °C carbonization | 107.5 | 34.5 | 0.057 | 0.051 |
| 700 °C carbonization | 145.3 | 31.6 | 0.077 | 0.048 |
| 800 °C carbonization | 491.6 | 226.3 | 0.263 | 0.225 |
| 700 °C activation | 524.1 | 1167.2 | 0.283 | 1.701 |
| 800 °C activation | 735.4 | 1315.2 | 0.413 | 1.847 |
| 900 °C activation | 534.2 | 1309.4 | 0.316 | 1.803 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Lai, L.; Wu, F.; Xie, W.; Liu, J. Hierarchical Porous Carbon Aerogel Derived from Sodium Alginate for High Performance Electrochemical Capacitor Electrode. Processes 2023, 11, 3355. https://doi.org/10.3390/pr11123355
Li X, Lai L, Wu F, Xie W, Liu J. Hierarchical Porous Carbon Aerogel Derived from Sodium Alginate for High Performance Electrochemical Capacitor Electrode. Processes. 2023; 11(12):3355. https://doi.org/10.3390/pr11123355
Chicago/Turabian StyleLi, Xuequan, Liting Lai, Fangdi Wu, Wenju Xie, and Junshao Liu. 2023. "Hierarchical Porous Carbon Aerogel Derived from Sodium Alginate for High Performance Electrochemical Capacitor Electrode" Processes 11, no. 12: 3355. https://doi.org/10.3390/pr11123355




