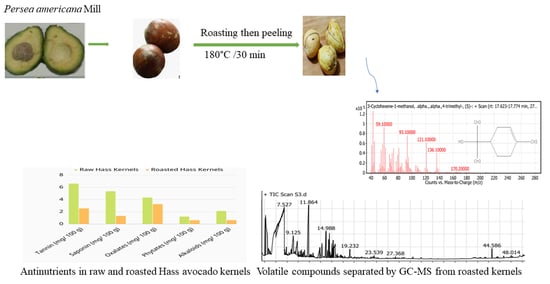
Effect of Roasting Hass Avocado Kernels on Nutritional Value and Volatile Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Hass Avocado Kernels
2.1.2. Chemicals and Reagents
2.2. Methods
2.2.1. Roasting Process
2.2.2. Chemical Analysis
2.2.3. Preparation of Aqueous Extracts
2.2.4. Determination of the Total Phenolic Content
2.2.5. Determination of the Total Flavonoid Content
2.2.6. Extraction and Analysis of Radical DPPH Scavenging Activity
2.2.7. Determination of the Antinutrients
2.2.8. The Browning Index
2.2.9. Colour Measurements
2.2.10. Estimation of Volatile Compounds
2.2.11. Statistical Analysis
3. Results
3.1. Chemical Composition
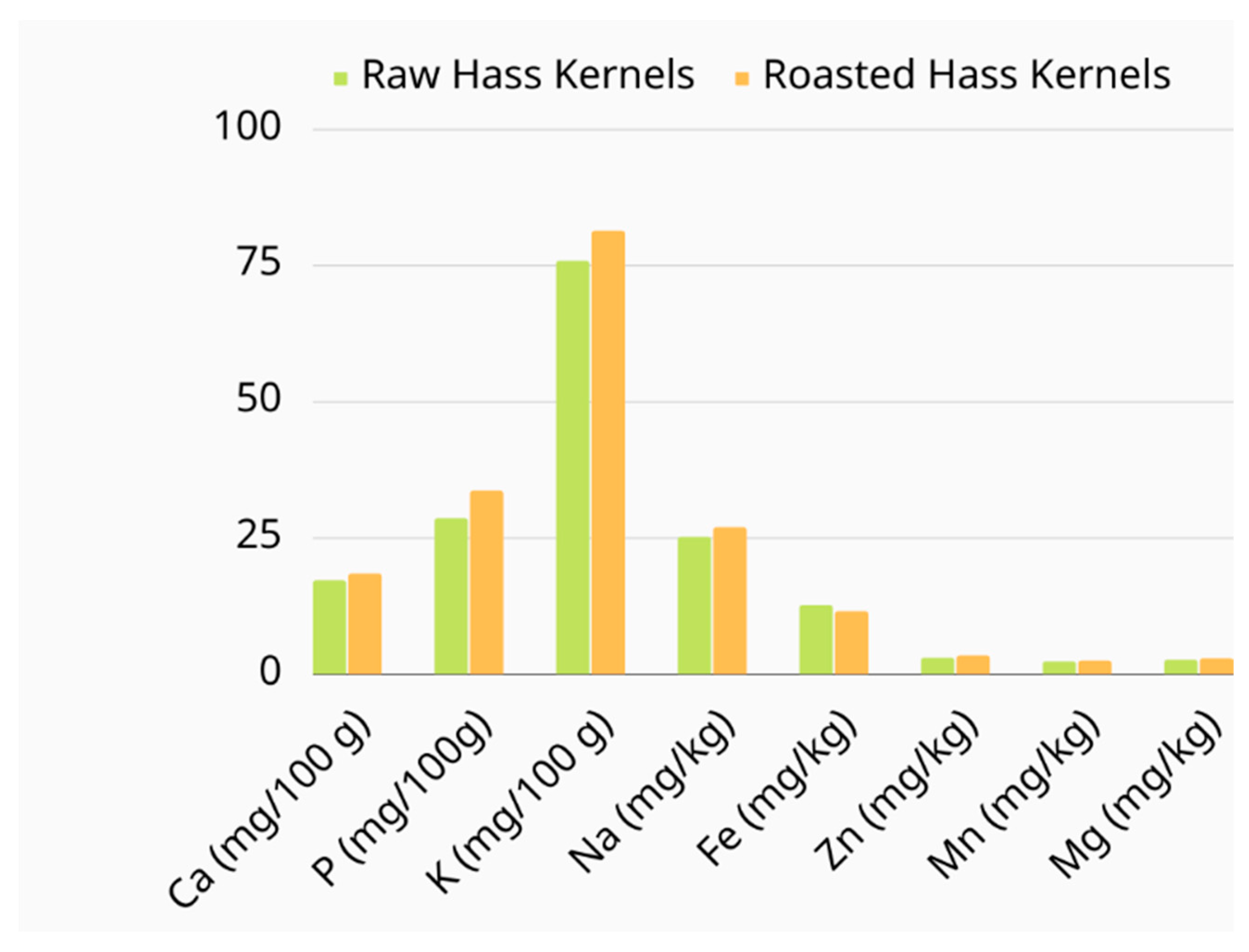
3.2. Mineral Content
3.3. Antinutrients
3.4. Colour Characteristics
3.5. Volatile Compounds
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Godfray, H.C.J.; Garnett, T. Food security and sustainable intensification. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2014, 369, 20120273. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S.; Low, J.; Tan, J.C.W.; Foo, S.Y.; Leo, C.H. Valorization of avocado seeds with antioxidant capacity using pressurized hot water extraction. Sci. Rep. 2022, 12, 13036. [Google Scholar] [CrossRef] [PubMed]
- Corrales-García, J.E.; del Rosario García-Mateos, M.; Martínez-López, E.; Barrientos-Priego, A.F.; Ybarra-Moncada, M.C.; Ibarra-Estrada, E.; Méndez-Zúñiga, S.M.; Becerra-Morales, D. Anthocyanin and oil contents, fatty acids profiles and antioxidant activity of Mexican landrace avocado fruits. Plant Foods Hum. Nutr. 2019, 74, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Rozan, M.A.; Boriy, E.G.; Bayomy, H.M. Chemical composition, bioactive compounds and antioxidant activity of six avocado cultivars Persea americana Mill. (Lauraceae) grown in Egypt. Emir. J. Food Agric. 2021, 33, 815–826. [Google Scholar]
- Rozan, M.; Alamri, E.; Bayomy, H. Fermented Hass avocado kernel: Nutritional properties and use in the manufacture of biscuits. Saudi J. Biol. Sci. 2022, 29, 103295. [Google Scholar] [CrossRef]
- Natania, K.; Wijaya, E. Optimization of roasting temperature and time of the durian seed (Durio zibethinus L.) as coffee substitution and its flavour profile. Food Res. 2022, 6, 279–287. [Google Scholar] [CrossRef]
- Jrad, Z.; Oussaief, O.; El-Hatmi, H.; Bouaziz, M.A. Fortification of goat yogurt with roasted date seeds (Phoenix dactylifera L.) powder: Impact on nutritional, technological, phenolic profile, antioxidant and sensory properties. J. Food Meas. Charact. 2022, 16, 4675–4686. [Google Scholar] [CrossRef]
- Guo, S.; Na Jom, K.; Ge, Y. Influence of roasting condition on flavor profile of sunflower seeds: A flavoromics approach. Sci. Rep. 2019, 9, 11295. [Google Scholar] [CrossRef]
- Alamri, E.; Rozan, M.; Bayomy, H. A study of chemical Composition, Antioxidants, and volatile compounds in roasted Arabic coffee. Saudi J. Biol. Sci. 2022, 29, 3133–3139. [Google Scholar] [CrossRef]
- Nicoli, M.C.; Anese, M.; Parpinel, M. Influence of processing on the antioxidant properties of fruit and vegetables. Trends Food Sci. Technol. 1999, 10, 94–100. [Google Scholar] [CrossRef]
- Ertaş, N.; Aslan, M. Antioxidant and physicochemical properties of cookies containing raw and roasted hemp flour. Acta Sci. Pol. Technol. Aliment. 2020, 19, 177–184. [Google Scholar] [PubMed]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 20th ed.; AOAC International: Washington, DC, USA, 2016. [Google Scholar]
- Kumaran, A.; Karunakaran, R.J. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006, 97, 109–114. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Alighiri, D.; Nuzulina, K.; Rodhiyah, M.; Drastisianti, A. Optimization of condition extraction in quantification of total flavonoid content in the seeds of the Arummanis (Mangifera indica L.) mango from Indonesia. J. Phys. Conf. Ser. 2019, 1321, 022041. [Google Scholar]
- Hwang, E.S.; Do-Thi, N. Effects of extraction and processing methods on antioxidant compound contents and radical scavenging activities of laver (Porphyra tenera). Prev. Nutr. Food Sci. 2014, 19, 40–48. [Google Scholar] [CrossRef]
- Tamilselvi, N.; Krishnamoorthy, P.; Dhamotharan, R.; Arumugam, P.; Sagadevan, E. Analysis of total phenols, total tannins and screening of phytocomponents in Indigofera aspalathoides (Shivanar Vembu) Vahl EX DC. J. Chem. Pharm. Res. 2012, 4, 3259–3262. [Google Scholar]
- Brunner, J.H. Direct spectrophotometric determination of saponin. Anal. Chem. 1984, 34, 1314–1326. [Google Scholar]
- Nwinuka, N.M.; Ibeh, G.O.; Ekeke, G.I. Proximate composition and levels of some toxicants in four commonly consumed spices. J. Appl. Sci. Environ. Manag. 2005, 9, 150–155. [Google Scholar]
- Vaintraub, I.A.; Lapteva, N.A. Colorimetric determination of phytate in unpurified extracts of seeds and the products of their processing. Anal. Biochem. 1988, 175, 227–230. [Google Scholar] [CrossRef]
- Mulder-Krieger, T.; Verpoorte, R.; Water, A.; Gessel, M.; Oeveren, B.C.J.A.; Svendsen, A.B. Identification of the alkaloids and anthraquinones in Cinchona ledgeriana callus cultures. Planta Med. 1982, 46, 19–24. [Google Scholar] [CrossRef]
- Chung, H.S.; Kim, J.K.; Moon, K.D.; Youn, K.S. Changes in color parameters of corn kernels during roasting. Food Sci. Biotechnol. 2014, 23, 1829–1835. [Google Scholar] [CrossRef]
- Commission Internationale de l’ Eclairage. Recommendations on Uniform Color Spaces—Color Difference Equations, Psychometric Color Terms; C.I.E Publication No. 15, Suppl. 2(E-1.3.1), 1971/(TC-1-3); Bureau Central de la CIE: Paris, France, 1986. [Google Scholar]
- Wang, X.; Li, Y.; Liu, Q.; Tan, X.; Xie, X.; Xia, Q.; Zhao, P. GC/MS-based metabolomics analysis reveals active fatty acids biosynthesis in the Filippi’s gland of the silkworm, Bombyx mori, during silk spinning. Insect Biochem. Mol. Biol. 2019, 105, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Onyango, C.; Noetzold, H.; Bley, T.; Henle, T. Proximate composition and digestibility of fermented and extruded uji from maize–finger millet blend. LWT 2004, 37, 827–832. [Google Scholar] [CrossRef]
- Hosseini Bai, S.; Darby, I.; Nevenimo, T.; Hannet, G.; Hannet, D.; Poienou, M.; Grant, E.; Brooks, P.; Walton, D.; Randall, B.; et al. Effects of roasting on kernel peroxide value, free fatty acid, fatty acid composition and crude protein content. PLoS ONE 2017, 12, e0184279. [Google Scholar] [CrossRef]
- Oboh, G.; Ademiluyi, A.O.; Akindahunsi, A.A. The effect of roasting on the nutritional and antioxidant properties of yellow and white maize varieties. Int. J. Food Sci. Technol. 2010, 45, 1236–1242. [Google Scholar] [CrossRef]
- Ahmed, I.A.M.; Al Juhaimi, F.Y.; Osman, M.A.; Al Maiman, S.A.; Hassan, A.B.; Alqah, H.A.; Ghafoor, K. Effect of oven roasting treatment on the antioxidant activity, phenolic compounds, fatty acids, minerals, and protein profile of Samh (Mesembryanthemum forsskalei Hochst) seeds. LWT 2020, 131, 109825. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Seabra, R.M.; Oliveira, B.P. Effects of roasting on hazelnut lipids. J. Agric. Food Chem. 2006, 54, 1315–1321. [Google Scholar] [CrossRef]
- Vasconcelos, A.L.S.; Franca, A.S.; Gloria, M.B.A.; Mendonça, J.C. A comparative study of chemical attributes and levels of amines in defective green and roasted coffee beans. Food Chem. 2007, 101, 26–32. [Google Scholar] [CrossRef]
- Win, M.M.; Abdul-Hamid, A.; Baharin, B.S.; Anwar, F.; Saari, N. Effects of roasting on phenolics composition and antioxidant activity of peanut (Arachis hypogaea L.) kernel flour. Eur. Food Res. Technol. 2011, 233, 599. [Google Scholar] [CrossRef]
- Ghafoor, K.; Aljuhaimi, F.; Özcan, M.M.; Uslu, N.; Hussain, S.; Babiker, E.E.; Fadimu, G. Effects of roasting on bioactive compounds, fatty acid, and mineral composition of chia seed and oil. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Pușcaș, A.; Tanislav, A.E.; Marc, R.A.; Mureșan, V.; Mureșan, A.E.; Pall, E.; Cerbu, C. Cytotoxicity Evaluation and Antioxidant Activity of a Novel Drink Based on Roasted Avocado Seed Powder. Plants 2022, 11, 1083. [Google Scholar] [CrossRef] [PubMed]
- Dumanović, J.; Nepovimova, E.; Natić, M.; Kuča, K.; Jaćević, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; Galván D’Alessandro, L.; Manrique, G.D. Effect of roasting conditions on the antioxidant compounds of quinoa seeds. Int. J. Food Sci. Technol. 2016, 51, 1018–1025. [Google Scholar] [CrossRef]
- El Anany, A.M. Nutritional composition, antinutritional factors, bioactive compounds and antioxidant activity of guava seeds (Psidium myrtaceae) as affected by roasting processes. J. Food Sci. Technol. 2015, 52, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Christian, A.G. Fluted pumpkin (Telfairia occidentalis Hook F.) seed: A nutritional assessments. Electron. J. Env. Agric. Food Chem. 2007, 6, 1787–1793. [Google Scholar]
- Aljuhaimi, F.; Özcan, M.M. Influence of oven and microwave roasting on bioproperties, phenolic compounds, fatty acid composition, and mineral contents of nongerminated peanut and germinated peanut kernel and oils. J. Food Process. Preserv. 2018, 42, e13462. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Lopez, H.W.; Leenhardt, F.; Coudray, C.; Remesy, C. Minerals and phytic acid interactions: Is it a real problem for human nutrition? Int. J. Food Sci. 2002, 37, 727–739. [Google Scholar] [CrossRef]
- Blaabjerg, K.; Carlsson, N.G.; Hansen-Møller, J.; Poulsen, H.D. Effect of heat-treatment, phytase, xylanase and soaking time on inositol phosphate degradation in vitro in wheat, soybean meal and rapeseed cake. Anim. Feed Sci. Technol. 2010, 162, 123–134. [Google Scholar] [CrossRef]
- Bueno-Borges, L.B.; Sartim, M.A.; Gil, C.C.; Sampaio, S.V.; Rodrigues, P.H.V.; Regitano-d’Arce, M.A.B. Sacha inchi seeds from sub-tropical cultivation: Effects of roasting on antinutrients, antioxidant capacity and oxidative stability. J. Food Sci. Technol. 2018, 55, 4159–4166. [Google Scholar] [CrossRef]
- Khan, N.; Zaman, R.; Elahi, M. Effect of heat treatments on the phytic acid content of maize products. J. Sci. Food Agric. 1991, 54, 153–156. [Google Scholar] [CrossRef]
- Ibhaze, G.A. Influence of hydrothermal treatment duration on the nutritional quality of avocado pear (Persia americana) seed meal for livestock feeding. Anim. Res. Int. 2017, 14, 2759–2763. [Google Scholar]
- Raigar, R.K.; Dalbhagat, C.G.; Mishra, H.N. Effect of pilot scale roasting on color and textural attributes of soybean kernels. J. Food Process. Preserv. 2020, 44, e14883. [Google Scholar] [CrossRef]
- Hurtado-Fernández, E.; Pacchiarotta, T.; Mayboroda, O.A.; Fernández-Gutiérrez, A.; Carrasco-Pancorbo, A. Metabolomic analysis of avocado fruits by GC-APCI-TOF MS: Effects of ripening degrees and fruit varieties. Anal. Bioanal. Chem. 2015, 407, 547–555. [Google Scholar] [CrossRef]
- Younis, I.Y.; Khattab, A.R.; Selim, N.M.; Sobeh, M.; Elhawary, S.S.; Bishbishy, M.H.E. Metabolomics-based profiling of 4 avocado varieties using HPLC–MS/MS and GC/MS and evaluation of their antidiabetic activity. Sci. Rep. 2022, 12, 4966. [Google Scholar] [CrossRef] [PubMed]
- Toci, A.T.; Azevedo, D.A.; Farah, A. Effect of roasting speed on the volatile composition of coffees with different cup quality. Food Res. Int. 2020, 137, 109546. [Google Scholar] [CrossRef]
- Crews, C.; Castle, L. A review of the occurrence, formation and analysis of furan in heat-processed foods. Trends Food Sci. Technol. 2007, 18, 365–372. [Google Scholar] [CrossRef]
- Ge, Y.; Li, K.; Xie, C.; Xu, Y.; Shi, C.; Hang, F.; Doherty, W.O.S. Formation of Volatile and Aroma Compounds during the Dehydration of Membrane-Clarified Sugarcane Juice to Non-Centrifugal Sugar. Foods 2021, 10, 1561. [Google Scholar] [CrossRef]
- Yaylayan, V.A.; Keyhani, A. Elucidation of the mechanism of pyrrole formation during thermal degradation of 13C-labeled l-serines. Food chem. 2001, 74, 1–9. [Google Scholar] [CrossRef]


| Chemical Composition | HKF | RHKF (180 °C/30 min) |
|---|---|---|
| Moisture % | 12.33 ± 0.45 a | 6.54 ± 0.35 b |
| Crude protein %, db | 5.31 ± 0.13 a | 5.04 ± 0.2 b |
| Oil extract %, db | 3.94 ± 0.1 b | 4.22 ± 0.1 a |
| Crude fibre %, db | 7.31 ± 0.34 a | 7.46 ± 0.24 a |
| Ash %, db | 2.19 ± 0.1 b | 2.46 ± 0.1 a |
| Carbohydrates %, db | 68.92 ± 0.67 b | 74.28 ± 0.74 a |
| Total Phenolics (mg GAE/g) | 22.93 ± 0.41 b | 23.82 ± 0.66 a |
| Flavonoids (mg QE/g) | 1.58 ± 0.07 a | 0.89 ± 0.05 b |
| DPPH (μ mol TE/g) | 152.3 ± 2.36 b | 181.7 ± 4.1 a |
| Degree | HKF | RHKF (180 °C/30 min) | |
|---|---|---|---|
| Browning Index (420 nm) | 0.016 ± 0.001 b | 0.058 ± 0.001 a | |
| Colour parameters | L * | 67.02 ± 0.1 a | 47.31 ± 0.17 b |
| a * | 7.12 ± 0.03 b | 7.58 ± 0.04 a | |
| b * | 22.2 ± 0.1 a | 14.87 ± 0.05 b | |
| No. | Compound | Molecular Formula | Odour Description * | % Content | |
|---|---|---|---|---|---|
| HKF | RHKF | ||||
| A | Saturated aliphatic hydrocarbons (SAH) | 10.62 | 5.29 | ||
| 1 | Undecane | C11H24 | Gasoline-like to odourless | 1.42 | 1.86 |
| 2 | 2,6,10-trimethyldodecane | C15H32 | NF | 1.08 | 0.52 |
| 3 | Tridecane | C13H28 | Hydrocarbon odour | 1.25 | ND |
| 4 | Tetradecane | C14H30 | Mild waxy | 1.54 | 0.36 |
| 5 | Pentadecane | C15H32 | NF | 5.33 | 1.31 |
| 6 | Docosane | C22H46 | Odourless | ND | 1.24 |
| B | Unsaturated aliphatic hydrocarbons (UAH) | 5.25 | 6.16 | ||
| 7 | 4-Methyl-1,4-heptadiene | C8H14 | NF | ND | 0.46 |
| 8 | 1-Ethylcyclohexene | C8H14 | NF | ND | 1.85 |
| 9 | 1-Ethyl-5-methylcyclopentene | C8H14 | NF | ND | 1.04 |
| 10 | Dodec-5-yne | C12H22 | NF | 0.63 | ND |
| 11 | 3-[(E)-hex-1-enyl] cyclohexene | C12H20 | NF | 0.95 | ND |
| 12 | (E)-Pentadec-3-ene | C15H30 | NF | 1.2 | ND |
| 13 | Nonadec-1-ene | C19H38 | NF | 2.47 | 1.32 |
| 14 | 4′-Ethyl-4-pentyl-1,1′-bi(cyclohexan)-3-ene | C19H34 | NF | ND | 1.49 |
| C | Terpenes | 2.48 | 0.64 | ||
| 15 | α-Cubebene | C15H24 | Herbal, waxy | 0.25 | ND |
| 16 | β-Copaene | C15H24 | 0.36 | ND | |
| 17 | Caryophyllene oxide | C15H24O | 1.87 | 0.64 | |
| D | Alcohols | 10.13 | 11.61 | ||
| 18 | 2-Methylbutan-1-ol | C5H12O | Cooked, roasted | ND | 5.89 |
| 19 | (E)-Hex-2-en-1-ol | C6H12O | Bitter, green | 7.34 | ND |
| 20 | 8-Azabicyclo[3.2.1]oct-6-en-3-ol, 8-methyl- | C8H13NO | NF | ND | 2.85 |
| 21 | p-Menth-1-ene-9-ol | C10H18O | Fruity, herbal | 0.65 | ND |
| 22 | 13-Tetradece-11-yn-1-ol | C14H24O | NF | ND | 0.63 |
| 23 | 1-Phenylhexan-1-ol | C12H18O | NF | ND | 1.59 |
| 24 | α-Santalol | C15H24O | Deep sweet sandalwood, woody | ND | 0.29 |
| 25 | Phytol | C20H40O | Faint floral | 2.14 | 0.36 |
| E | Aldehydes | 22.86 | 5.3 | ||
| 26 | Propanal | C3H6O | Green grass, fruity | 1.96 | ND |
| 27 | Pentanal | C5H10O | Fruity nutty | 0.58 | ND |
| 28 | (Z)-Hex-3-enal | C6H10O | Green, grassy | 2.09 | ND |
| 29 | 2-Ethylbutanal | C6H12O | Fruity, varnish, bitter, aldehydic | 3.68 | 2.11 |
| 30 | 2-Hexenal | C6H12O | Grassy, herbal | 1.16 | 0.22 |
| 31 | (E)-Hept-2-enal | C7H12O | Somewhat fatty, green | 5.36 | 1.11 |
| 32 | 1-Ethylpyrrole-2-carbaldehyde | C7H9NO | Burnt, roasted, smoky | ND | 0.87 |
| 33 | (E)-non-2-enal | C9H16O | Fatty, green, violet aroma | 2.79 | ND |
| 34 | 2-Phenylbut-2-enal | C10H10O | Cocoa-like, roasted, woody | ND | 0.81 |
| 35 | Cyclohex-3-ene-1-carbaldehyde | C7H10O | Fruity, aromatic, sweetish | 4.92 | ND |
| 36 | (Z)-Hexadec-11-enal | C16H30O | Waxy | 0.32 | 0.18 |
| F | Ketones | 12.17 | 6.68 | ||
| 37 | 1-(3,4-Dihydro-2H-pyrrol-5-yl) ethenone | C6H9NO | Roasted, popcorn-like, popcorn toasted, grain malty | ND | 2.27 |
| 38 | Octane-2,3-dione | C8H14O2 | Roasted, fruity nutty | ND | 1.36 |
| 39 | 6-Methylhept-5-en-2-one | C8H14O | Fatty, green citrus-like odour | ND | 0.25 |
| 40 | Decan-2-one | C10H20O | Fatty, peachy | 4.67 | 0.38 |
| 41 | 3-But-3-enylcyclohexan-1-one | C10H16O | NF | ND | 0.51 |
| 42 | Dodec-11-en-2-one | C12H22O | NF | 0.85 | ND |
| 43 | 7-Acetyl-3,3-dimethylbicyclo[4.1.0]heptan-2-one | C11H16O2 | NF | ND | 0.33 |
| 44 | Cycloheptadecanone | C17H32O | Musky, animal | 0.78 | ND |
| 45 | Pentadecan-2-one | C15H30O | Floral, fresh, jasmine, celery | 1.39 | 0.25 |
| 46 | 1-Hydroxy-4-methoxy-3,3-dimethyl-1,3-dihydro-2H-indol-2-one | C11H13NO3 | NF | 0.19 | ND |
| 47 | Cyclopentane-1,2-dione | C5H6O2 | NF | ND | 0.22 |
| 48 | 3,4-Dihydro-2H-thiopyran-3-one | C5H8OS | Unpleasant | 4.23 | ND |
| 49 | Dodecahydropyrido[1,2-b] isoquinolin-6-one | C13H21NO | NF | 0.06 | ND |
| 50 | 9-(Oxan-2-yloxy) nonan-2-one | C14H26O3 | NF | ND | 0.46 |
| 51 | Vestitenone | C12H18O | NF | ND | 0.65 |
| G | Esters | 27.53 | 20.36 | ||
| 52 | Methyl 2-hydroxyacetate | C3H6O3 | Sweet fruity | 1.16 | 0.7 |
| 53 | Methyl heptanoate | C8H16O2 | Fruity, orris | 4.12 | 1.12 |
| 54 | Methyl 2-norbornanecarboxylate | C9H14O2 | NF | ND | 1.59 |
| 55 | 3-Methyl-2-buten-1-yl trichloroacetate | C7H9Cl3O2 | NF | ND | 0.73 |
| 56 | Ethyl nonanoate | C11H22O | Fruity, fatty | 2.25 | ND |
| 57 | Methyl undec-10-enoate | C12H22O2 | Oily | ND | 0.84 |
| 58 | 3,7,11,Trimethyl-8,10-dodecedienylacetate | C17H30O2 | NF | ND | 6.28 |
| 59 | Decyl butanoate | C14H28O2 | Sweet, fruity, waxy slightly rosy | 0.7 | ND |
| 60 | Undec-10-enyl pentanoate | C16H30O2 | NF | ND | 0.47 |
| 61 | Ethyl 6,8-difluoro-4-hydroxy-3-quinolinecarboxylate | C12H9F2NO3 | NF | 0.96 | ND |
| 62 | Methyl hexadecanoate | C17H34O2 | Fatty, oily, waxy | 1.76 | 1.96 |
| 63 | 11,13-Dimethyl-12-tetradecen-1-ol acetate | C18H34O2 | NF | 0.47 | ND |
| 64 | [(E)-8-Methyltetradec-9-enyl] acetate | C17H32O2 | NF | 0.83 | ND |
| 65 | Methyl lineoleate | C19H34O2 | Oily, fatty, woody | 1.37 | 2.46 |
| 66 | Methyl oleate | C19H36O2 | Mild fatty | 2.74 | 0.78 |
| 67 | Methyl stearate | C19H38O2 | Oily waxy | 1.36 | 0.33 |
| 68 | 6-{[(2E)-2-Methyl-2-butenoyl] amino} hexyl (2E)-2-methyl-2-butenoate | C16H27NO3 | NF | ND | 0.88 |
| 69 | But-3-enyl pentadecyl carbonate | C20H38O3 | NF | 0.83 | 0.42 |
| 70 | methyl icosa-8,11,14,17-tetraenoate | C21H34O2 | NF | 4.11 | 1.8 |
| 71 | Fumaric acid, cyclohex-3-enylmethyl heptadecyl ester | C28H48O4 | NF | 1.14 | ND |
| 72 | Didodecyl succinate | C28H54O4 | NF | 3.73 | ND |
| H | Oxetanes | 2.31 | 1.71 | ||
| 73 | 2-Ethyloxetane | C5H10O | NF | 2.31 | 1.71 |
| I | Pyrazines | 0 | 14.92 | ||
| 74 | 2- Methyl-pyrazine | C5H6N2 | Nutty, cocoa-like | ND | 3.62 |
| 75 | 2,3,5-Trimethylpyrazine | C7H10N2 | Roasted nut, baked potato | ND | 2.26 |
| 76 | 3-Ethyl-2,5-dimethylpyrazine | C8H12N2 | Roasted potato, cocoa-like, nutty | ND | 2.08 |
| 77 | 2-Ethyl-3,5-dimethylpyrazine | C8H12O2 | Roasted, toasted nut, chocolaty, sweet woody | ND | 2.18 |
| 78 | 2-Isoamylpyrazine | C9H14N2 | Roasted | ND | 1.36 |
| 79 | 2-Methoxy-3-(2-methylpropyl) pyrazine | C9H14N2O | Green bell pepper, green pea-like | ND | 1.75 |
| 80 | 2,5-Dimethyl-3-(2-methylpropyl) pyrazine | C10H16N2 | Nutty, roasted | ND | 1.67 |
| J | Pyrroles | 0 | 3.81 | ||
| 81 | 1H-Pyrrole | C4H5N | Nutty, sweet | ND | 0.48 |
| 82 | 1-Ethylpyrrole | C6H9N | Roasted, burnt | ND | 1.79 |
| 83 | 2-Methyl-1H-pyrrole | C5H7N | Burnt, roasted | ND | 1.54 |
| K | Furans | 2.02 | 6.59 | ||
| 84 | 1-(Furan-2-yl) ethenone | C6H6O2 | Sweet, almondy, nutty, coffee-like | ND | 1.03 |
| 85 | 2-[(E)-Pent-2-enyl] furan | C9H12O | Roasted | ND | 1.6 |
| 86 | 2-Heptylfuran | C11H18O | Nutty, coffee-like | 0.93 | 2.19 |
| 87 | 2-Decylfuran | C14H24O | Spicy, aldehydic, fatty | 1.09 | 1.77 |
| L | Amines | 0.84 | 3.36 | ||
| 88 | Piperazine | C4H10N2 | Ammoniacal | ND | 3.36 |
| 89 | Tricyclo[4.3.1.1(3,8)]undecan-1-amine | C11H19N | NF | 0.53 | ND |
| 90 | 5H-Dibenzo[a,d]cyclohepten-5-amine | C15H13N | NF | 0.31 | ND |
| M | Imines | 0 | 0.86 | ||
| 91 | (2E)-N-(Allyloxy)-4,7,7-trimethylbicyclo [2.2.1] heptan-2-imine | C13H21NO | NF | ND | 0.86 |
| N | Thiazines | 0 | 0.97 | ||
| 92 | 3,4-Dimethyl-2-phenylthiomorpholine | C12H17NS | NF | ND | 0.97 |
| O | Oximes | 0 | 4.99 | ||
| 93 | 2-Decanone, O-methyloxime | C11H23NO | NF | ND | 3.73 |
| 94 | 2-Tridecanone, O-methyloxime | C14H29NO | NF | ND | 1.26 |
| Sum of the identified compounds | 96.21 | 93.25 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bayomy, H.M.; Alamri, E.S.; Rozan, M.A. Effect of Roasting Hass Avocado Kernels on Nutritional Value and Volatile Compounds. Processes 2023, 11, 377. https://doi.org/10.3390/pr11020377
Bayomy HM, Alamri ES, Rozan MA. Effect of Roasting Hass Avocado Kernels on Nutritional Value and Volatile Compounds. Processes. 2023; 11(2):377. https://doi.org/10.3390/pr11020377
Chicago/Turabian StyleBayomy, Hala M., Eman S. Alamri, and Mahmoud A. Rozan. 2023. "Effect of Roasting Hass Avocado Kernels on Nutritional Value and Volatile Compounds" Processes 11, no. 2: 377. https://doi.org/10.3390/pr11020377
APA StyleBayomy, H. M., Alamri, E. S., & Rozan, M. A. (2023). Effect of Roasting Hass Avocado Kernels on Nutritional Value and Volatile Compounds. Processes, 11(2), 377. https://doi.org/10.3390/pr11020377